Abstract
Cells mixedly infected with parainfluenza virus SV5 and vesicular stomatitis virus (VSV) yield phenotypically mixed virions, in addition to both parental types. Two types of phenotypically mixed virions have been identified: 0.6 to 1.2% of the VSV plaque formers were neutralized by SV5 antiserum, but not by VSV antiserum, suggesting the presence of a VSV genome in an SV5 envelope; 9 to 45% of the VSV plaque formers were neutralized by both antisera, indicating the presence of both SV5 and VSV antigens in their envelopes. The presence of SV5 antigen in virions with the typical bullet-shaped appearance of VSV was confirmed with ferritin-labeled anti-SV5 antibody. In contrast to standard VSV, phenotypically mixed virions adsorbed to and eluted from chicken erythrocytes, indicating that these virions contained in their envelopes SV5 hemagglutinin, and possibly neuraminidase. Thus, the VSV nucleocapsid can interact with membranes which contain SV5 proteins in the manner which leads to virus maturation, and the production of a high yield of phenotypically mixed virions with the morphology of VSV indicates that this process can function efficiently. No evidence of genetic recombination between the two viruses was found. These results raise the possibility of an evolutionary relatedness between the paramyxoviruses and the rhabdoviruses.
Full text
PDF



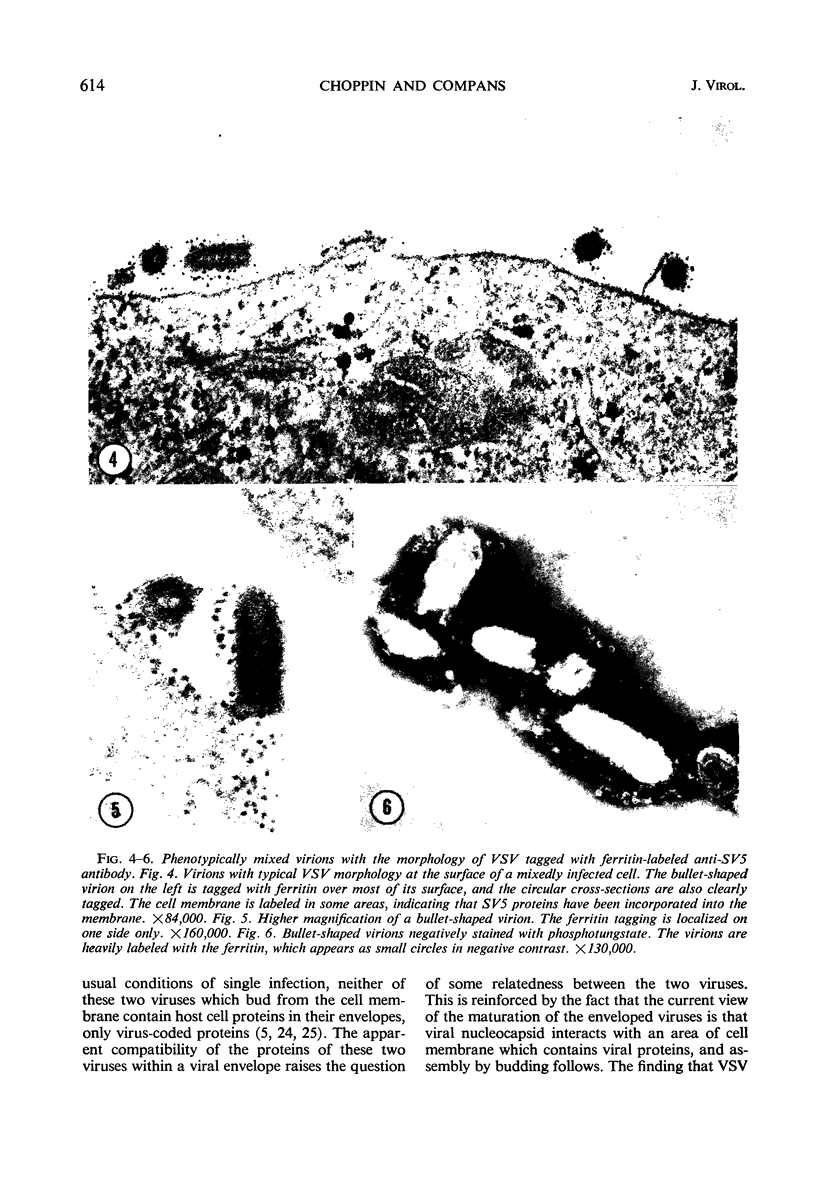


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BABLANIAN R., EGGERS H. J., TAMM I. STUDIES ON THE MECHANISM OF POLIOVIRUS-INDUCED CELL DAMAGE. I. THE RELATION BETWEEN POLIOVIRUS,-INDUCED METABOLIC AND MORPHOLOGICAL ALTERATIONS IN CULTURED CELLS. Virology. 1965 May;26:100–113. doi: 10.1016/0042-6822(65)90030-9. [DOI] [PubMed] [Google Scholar]
- Bikel I., Duesberg P. H. Proteins of Newcastle disease virus and of the viral nucleocapsid. J Virol. 1969 Oct;4(4):388–393. doi: 10.1128/jvi.4.4.388-393.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown F., Martin S. J., Cartwright B., Crick J. The ribonucleic acids of the infective and interfering components of vesicular stomatitis virus. J Gen Virol. 1967 Oct;1(4):479–486. doi: 10.1099/0022-1317-1-4-479. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Phenotypic mixing between group A arboviruses. Nature. 1966 Jun 25;210(5043):1397–1399. doi: 10.1038/2101397a0. [DOI] [PubMed] [Google Scholar]
- CHOPPIN P. W. MULTIPLICATION OF A MYXOVIRUS (SV5) WITH MINIMAL CYTOPATHIC EFFECTS AND WITHOUT INTERFERENCE. Virology. 1964 Jun;23:224–233. doi: 10.1016/0042-6822(64)90286-7. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Klenk H. D., Choppin P. W. The proteins of the parainfluenza virus SV5. 1. Separation of virion polypeptides by polyacrylamide gel electrophoresis. Virology. 1969 Nov;39(3):460–466. doi: 10.1016/0042-6822(69)90094-4. [DOI] [PubMed] [Google Scholar]
- Choppin P. W. Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology. 1969 Sep;39(1):130–134. doi: 10.1016/0042-6822(69)90354-7. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Choppin P. W. The nucleic acid of the parainfluenza virus SV5. Virology. 1968 Jun;35(2):289–296. doi: 10.1016/0042-6822(68)90269-9. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Holmes K. V., Dales S., Choppin P. W. An electron microscopic study of moderate and virulent virus-cell interactions of the parainfluenza virus SV5. Virology. 1966 Nov;30(3):411–426. doi: 10.1016/0042-6822(66)90119-x. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensminger W. D., Tamm I. The step in cellular DNA synthesis blocked by Newcastle disease or mengovirus infection. Virology. 1970 Jan;40(1):152–165. doi: 10.1016/0042-6822(70)90387-9. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Kingsbury D. W. Separation of Newcastle disease virus proteins by polyacrylamide gel electrophoresis. Virology. 1969 Apr;37(4):597–604. doi: 10.1016/0042-6822(69)90277-3. [DOI] [PubMed] [Google Scholar]
- GOTLIEB T., HIRST G. K. The experimental production of combination forms of virus. III. The formation of doubly antigenic particles from influenza A and B virus and a study of the ability of individual particles of X virus to yield two separate strains. J Exp Med. 1954 Apr 1;99(4):307–320. doi: 10.1084/jem.99.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANOFF A., HIRST G. K. Experimental production of combination forms of virus. IV. Mixed influenza A-Newcastle disease virus infections. Proc Soc Exp Biol Med. 1954 May;86(1):84–88. doi: 10.3181/00379727-86-21016. [DOI] [PubMed] [Google Scholar]
- GRANOFF A. Heterozygosis and phenotypic mixing with Newcastle disease virus. Cold Spring Harb Symp Quant Biol. 1962;27:319–326. doi: 10.1101/sqb.1962.027.001.030. [DOI] [PubMed] [Google Scholar]
- HIRST G. K., GOTLIEB T. The experimental production of combination forms of virus. I. Occurrence of combination forms after simultaneous inoculation of the allantoic sac with two distinct strains of influenza virus. J Exp Med. 1953 Jul;98(1):41–52. doi: 10.1084/jem.98.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett A. J., Schaffer F. L., Madin S. H. The separation of infectious and autointerfering particles in vesicular stomatitis virus preparations. Virology. 1967 Jan;31(1):114–119. doi: 10.1016/0042-6822(67)90014-1. [DOI] [PubMed] [Google Scholar]
- Holmes K. V., Choppin P. W. On the role of the response of the cell membrane in determining virus virulence. Contrasting effects of the parainfluenza virus SV5 in two cell types. J Exp Med. 1966 Sep 1;124(3):501–520. doi: 10.1084/jem.124.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Greenawalt J. W., Wagner R. R. Defective T particles of vesicular stomatitis virus. I. Preparation, morphology, and some biologic properties. Virology. 1966 Oct;30(2):161–172. doi: 10.1016/0042-6822(66)90092-4. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Defective T particles of vesicular stomatitis virus. II. Biologic role in homologous interference. Virology. 1966 Oct;30(2):173–181. doi: 10.1016/0042-6822(66)90093-6. [DOI] [PubMed] [Google Scholar]
- Sokol F., Schlumberger H. D., Wiktor T. J., Koprowski H. Biochemical and biophysical studies on the nucleocapsid and on the RNA of rabies virus. Virology. 1969 Aug;38(4):651–665. doi: 10.1016/0042-6822(69)90184-6. [DOI] [PubMed] [Google Scholar]
- TAMM I. Influenza virus-erythrocyte interaction. I. Reversible reaction between Lee virus and cat erythrocytes. J Immunol. 1954 Sep;73(3):180–189. [PubMed] [Google Scholar]
- Wagner R. R., Schnaitman T. A., Snyder R. M. Structural proteins of vesicular stomatitis viruses. J Virol. 1969 Apr;3(4):395–403. doi: 10.1128/jvi.3.4.395-403.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Schnaitman T. C., Snyder R. M., Schnaitman C. A. Protein composition of the structural components of vesicular stomatitis virus. J Virol. 1969 Jun;3(6):611–618. doi: 10.1128/jvi.3.6.611-618.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]