Abstract
Oligodendrocytes play a fundamental supportive role in the mammalian central nervous system (CNS) as the myelinating-glial cells. Disruption of fast axonal transport mechanisms can occur as a consequence of mature oligodendrocyte loss following spinal cord injury, stroke, or due to neuroinflammatory conditions, such as multiple sclerosis. As a result of the limited remyelination ability in the CNS after injury or disease, human embryonic stem cells (hESCs) may prove to be a promising option for the generation and replacement of mature oligodendrocytes. Moreover, hESC-derived oligodendrocytes may be experimentally utilized to unravel fundamental questions of oligodendrocyte development, along with their therapeutic potential through growth factor support of axons and neurons. However, an intensive characterization and examination of hESC-derived oligodendrocytes prior to preclinical or clinical trials is required to facilitate greater success in their integration following cellular replacement therapy (CRT). Currently, the protocols utilized to derive oligodendrocytes from hESCs consist of significant variations in culture style, time-length of differentiation, and the provision of growth factors in culture. Further, these differing protocols also report disparate patterns in the expression of oligodendroglial markers by these derived oligodendrocytes, throughout their differentiation in culture. We have comprehensively reviewed the published protocols describing the derivation of oligodendrocytes from hESCs and the studies that examine their efficacy to remyelinate, along with the fundamental issues of their safety as a viable CRT. Additionally, this review will highlight particular issues of concern and suggestions for troubleshooting to provide investigators critical information for the future improvement of establishing in vitro hESC-derived oligodendrocytes.
Introduction
Stem cell biology holds enormous potential for the treatment of numerous chronic and inherited diseases. Human pluripotent stem cells (hPSCs) are an inexhaustible material with the potential to differentiate into all three germ layers. The hPSC landscape has rapidly evolved, utilizing human embryonic stem cells (hESCs) derived from the inner cell mass of developing blastocysts, and/or human-induced pluripotent stem cells (hiPSCs) produced by reprogramming specific somatic cell types.
The derivation of specific lineages and regional identities from hPSCs represents a coveted goal that is particularly relevant when considering cell replacement therapies (CRT) for patients suffering from neurological disorders. The potential of hPSCs has been shown with the deployment of differentiated neural precursors with tantalizing results in animal models of spinal cord injury (SCI) [1–3], Huntington's disease [4–6] and Parkinson's disease [7,8]. Germane to this is the comprehensive elucidation of molecular and cellular events that govern neural differentiation.
Prior to the advent of hPSCs, mammalian neural development was predominantly investigated in the rodent central nervous system (CNS), where the identification of oligodendroglial lineage stages, gene regulators of cell identity and signaling pathways that control differentiation, survival, and migration, have all been revealed [9–14]. These studies have provided a foundation for mouse embryonic stem cells (mESC) oligodendrocyte differentiation protocols [15–19], although, these have generally failed to produce pure oligodendroglial lineage populations [15–19].
More recently, hPSC protocols have capitalized on this existing knowledge with nine protocols developed to date [20–28]. While mESC studies have provided a sound foundation for hPSC oligodendrocyte differentiation protocols, there is considerable variation across protocols developed for each species with regards to media components, time requirements, and the demarcation of developmental stages [21,29]. Further, xenogenic-free and fully defined systems are an eventual necessity for hPSC-based protocols to fulfill stringent requirements for human clinical applications [27]. This review, for the first time, compiles all reported hPSC oligodendrocyte differentiation protocols established to date (Table 1). Protocols are canvassed and compared in three stages, (1) hPSCs to neural progenitors (NPs), (2) NPs to oligodendrocyte precursors (OPCs), and (3) OPCs to oligodendrocytes, with particular emphasis placed on issues of concern and troubleshooting suggestions for improving future outcomes. This review is additionally presented in a fashion mindful of future in vivo applications, aiming to provide a comprehensive and organized overview for future investigators.
Table 1.
Existing Human Pluripotent Stem Cells to Oligodendrocyte Differentiation Protocols
| Protocol | Cell lines utilized | Sorting step required | Time (days) | Xeno free | hPSC maintenancea |
|---|---|---|---|---|---|
| Nistor et al. [20] | H7 | No | 42 | No | Matrigel |
| Izrael et al. [21] | H13, H16 | No | 49 | No | MEFs |
| Kang et al. [22] | SNUhES1 | No | 40 | No | MEFs |
| Gil et al. [23] | Miz, hES4, hES6 | No | 25 | No | MEFs |
| Hu et al. [24] | H1, H9, H14 | No | 98 | No | MEFs |
| Hu et al. [25] | BG02 | No | 58 | No | HFFs |
| Kerr et al. [26] | H1 | No | 41 | No | MEFs |
| Sundberg et al. [27] | HS360, HS362 | Yes (NG2) | 91 | Yes | HFFs |
| Sundberg et al. [28] | Regea 06/040, Regea 08/023 | Yes (NG2) | 77 | Yes | HFFs |
The feeder layer used to maintain the hESC lines before the start of oligodendrocyte differentiation culture.
MEFs, mouse embryonic fibroblasts; HFF, human foreskin fibroblasts.
From Pluripotency to Neural Precursors
Neurospheres, embryoid bodies, or monolayers?
The guided differentiation of hPSCs to NPs is the first milestone on the road to oligodendrocyte commitment. NPs with a propensity for oligodendroglial differentiation are typically PAX6 and Nestin positive [27,30], and are frequently produced in culture from three-dimensional spherical structures either as constituents of neurospheres or embryoid bodies (EBs) (Table 2). Specifically, the NP structures formed in response to robust neural cues within neural conducive environments are typically referred to as neurospheres, whereas systems facilitating the spontaneous differentiation of hESCs, forming all three embryonic layers (ectoderm, mesoderm, and endoderm), are termed EBs [31]. Alternatively, a monolayer of NPs may be generated to avoid the limitations of a three-dimensional system, notably the variable nutrient/growth factor diffusion through large multicellular structures, and typically involves extracellular matrices, such as laminin, poly-d-lysine, and matrigel (Table 2) [25].
Table 2.
Differentiation Protocols for the Conversion of Human Pluripotent Stem Cells to Embryoid Bodies, then to Neural Progenitors
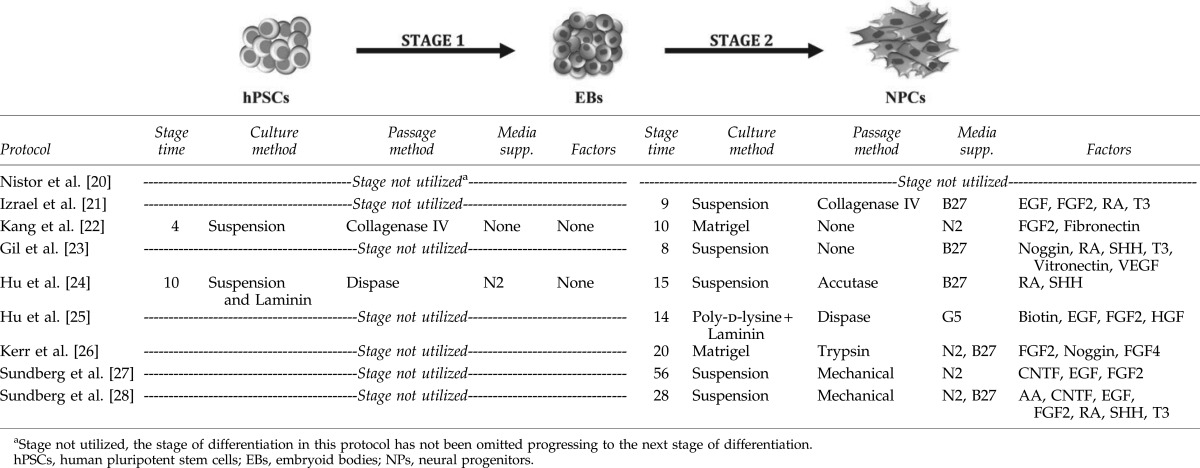 |
It is believed that neural differentiation via a three-dimensional structure supports cell-to-cell contact to enhance the survival, differentiation, and proliferation abilities of NPs [29,32]. In fact, recent data support the notion of a community effect in a three-dimensional structure, with the expression of Nestin and PAX6 in NPs, derived from EBs grown in culture for 14–25 days [22,24]. Neurosphere facilitated protocols are comparable to those utilizing EBs, with Sundberg et al., and Nistor et al., achieving PAX6 and Nestin-enriched cultures within similar culture timeframes [20,27]. Intriguingly, the generation of NPs through either EBs or neurospheres, have been shown to increase the expression of more mature cell-specific transcript, such as OLIG2 and SOX10 [24,27], suggesting oligodendroglial lineage specification. Alternatively, the derivation of neurospheres in suspension culture or by using monolayer culture, may limit the spontaneous differentiation of pluripotent cells to undesired lineages, as monolayer and neurosphere conditions encourage neural differentiation, possibly as a consequence of a “community effect” providing a robust neurogenic environment.
Although it remains a sole example, Hu et al., have described the derivation of NPs that express Nestin and PAX6 using monolayer conditions after 14 days of culture [25]. Similar outcomes such as the expression of the NP markers Nestin, and PAX6, are attainable and the temporal variation at such an early stage appears to be of little downstream consequence. Until additional monolayer protocols are reported it remains difficult to prefer monolayer or three-dimensional systems for oligodendrocyte differentiation.
NPs and their role in OPC differentiation
Oligodendrocytes arise from NP pools with distinct origins and thus varied transcription factor identities throughout embryonic and postnatal development of the mammalian CNS [33–36]. For instance, during the development of the mouse forebrain, an “early wave” of oligodendrocytes initially differentiate ventrally from NKX2.1 progenitors of the telencephalic medial ganglionic eminence (MGE) and ventral anterior entopeduncular area (AEP) regions, which disappear and may be supplanted in postnatal development by oligodendrocytes from Emx1-expressing cortical tissue [33–36]. Despite significant variation in the origin of oligodendrocytes, there is no rationale for functional differences of these post-mitotic cells, since a distinct lack of any one of the reported three waves of oligodendrocyte generation, seems to have no effect on CNS OPC generation and development by compensation through the other two waves [33,35,36]. The data would suggest that oligodendrocytes derived from Emx1-expressing NPs could play a role during postnatal rodent neurodevelopment, the period of greatest myelination, however, evidence is lacking in relation to this theory during human neurodevelopment.
Another marker that may serve as a similar discriminating factor, functioning as a preferential marker for oligodendrocytic differentiation from NPs is, NKX2.1. This is a homeodomain transcription factor that plays a crucial role in the embryonic development of the mammalian telencephalon [37–39]. Mainly, the early NPs within the MGE and AEP regions of the telencephalon have been shown to express NKX2.1 at E12.5 in mouse embryos [33,35]. Such NKX2.1 expressing NPs have been reported to give rise to OLIG2-positive cells that can mature to give rise to both interneurons and oligodendrocytes [37,40]. In the human fetal telencephalon by midgestation, NKX2.1 has been observed in cells of the basal ganglia, near DLX2-positive and PDGFRα-expressing OPCs, in the neighboring GE-SVZ [34]. These data may well implicate the NKX2.1 homeodomain transcription factor as a later marker for OPC generation. Although NKX2.1 expression may govern oligodendrocytic differentiation in vivo, the protocols defined in this review describing the hESC-derivation of OPCs, do not examine this marker in such a context, which could be aided with the development of an NKX2.1+/GFP reporter line that has been shown to generate OPCs from an NKX2.1+ fraction [41]. In addition, if the derived oligodendrocytes from NKX2.1-positive NPs can integrate within the CNS, this cell line may also serve as a valuable research tool to unravel fundamental biological questions of developmentally regulated myelination and remyelination following injury or disease.
Extraneous signals for the differentiation to NPs and their maintenance
A chief hurdle confronting stem cell biology is the standardization of culture systems in the quest to obtain homogeneous populations of NPs. This is a particular problem when culturing specific hESC lines, which demonstrate substantial variation in the transcriptional profiles of generated NPs [42]. Such differences are suggested to be a result of the extracellular signaling factors utilized within the specified culture conditions.
Since OPCs are derived from NPs, it stands to reason that the first biological response must be to promote gliogenesis and antagonize neuronogenesis. Intuitively, it has been shown that the shift from neuronogenesis to gliogenesis is a time and growth factor-dependent mechanism [43]. The switch from neuronogenesis to gliogenesis occurs by the downregulation of neuronal transcription factors and the upregulation of oligodendroglial transcription factors [44]. For example, OLIG2-expressing NPs were shown to differentiate into motor neurons and oligodendrocytes in the ventral spinal cord and into interneurons and oligodendrocytes in the forebrain [45–47]. Contrary to these findings, oligodendrocytes have been reported to arise from other OLIG2-negative NPs [48]. These data may imply that oligodendrocyte populations are derived from different NPs. Thus, a critical determinant of generating oligodendrocytes from hESCs may actually be reliant on optimizing the culture time and the growth factors (and their concentrations) utilized, to generate hESC-derived NPs that are able to differentiate into oligodendrocytes with defined transcription factor signatures.
Nonspecific early neurotrophic factors for NP survival and differentiation
Basic fibroblast growth factor (bFGF) is a widely utilized growth factor for the derivation of NPs and is included in numerous protocols for the patterning of cells to NPs and pre-OPCs. Its widespread utility stems from its known role in the promotion of cell proliferation [43,49,50]. In addition, FGF2 can increase the proliferation of OLIG2-expressing cells, while concomitantly blocking their differentiation to motor neurons [49]. However, the role of bFGF may be dispensable, since Hu et al., and Gil et al., have reported the differentiation of hESCs into NPs and then OPCs without bFGF supplementation [23,24]. In light of evidence suggesting FGF signaling (particularly FGF2), can repress the expression of the transcription factor Nkx2.2, thereby, preventing differentiation to OPCs [49], it can be argued that indeed its inclusion during the generation of oligodendroglial lineage cells should be debated further.
Both ciliary neurotrophic factor (CNTF) and epidermal growth factor (EGF) are factors also known to induce the proliferation and differentiation toward a neural lineage [27]. It is documented that EGF is crucial for early neural patterning in several regions of the developing CNS [51,52]. Moreover, EGF is important for the proliferation and migration of endogenous NPs, during embryogenesis along with the early postnatal period [51,52]. Although EGF has been utilized in several key hESC-differentiation articles, it may not be critical for the derivation of NPs in such growth factor-dependent culture conditions [20,21,25,27,28]. Recently, Sundberg et al., have reported the first use of CNTF for the generation of NPs prior to OPC specification [27], as will be discussed in section “Oligodendroglial neurotrophic factors for OPC differentiation and survival” of this review. While these growth factors are being used in various reported oligodendrocyte differentiation protocols, it seems that bFGF and EGF are predominantly utilized to derive NPs that have the ability to eventually differentiate into oligodendrocytes.
In mammalian cell development, the binding of specific signaling factors to membrane-bound receptors, along with the interaction of these receptors with ligands from the surrounding environment are proposed to be the central mechanisms governing proliferation, migration, and differentiation. Consequently, the use of CNS cell-specific extracellular matrix (ECM) is a prominent tool to promote differentiation toward NPs. A study by Ma et al., has compared the five different ECMs; laminin, matrigel, fibronectin, type-1-collagen, and poly-d-lysine, to direct the differentiation of hESC to the neural lineage [53]. The study concluded that laminin was the most effective ECM to induce their differentiation [53]. Indeed, laminin is one of the most important ECMs in the neural stem cell niche and is itself a major component of matrigel.
Nistor et al., Kang et al., and Kerr et al., have all used matrigel as an ECM during the differentiation into NPs, while the Sundberg et al., and Hu et al., groups have either used a combination of laminin, collagen IV, and nidogen-1, or a combination of laminin and poly-l-lysine, respectively [20,22,25–28]. However, the use of ECMs is not essential during hPSC to NP culture stages as Gil et al., and Izrael et al., have not incorporated ECMs within their protocols [21,23]. Matrigel appears to be the most effective ECM for the derivation of NPs by generating larger numbers of precursors that adopt the glial lineage. Protocols that utilize matrigel create two issues that stem from the xenogeneic nature of this substrate: (1) one issue relates to the difficulty in applying cells exposed to xenogeneic material for clinical applications; and (2) concerns the variability in substrate batches. Thus, further studies are required to resolve whether a more appropriate ECM for hESC-derived glial-committed NP differentiation can be developed, which is also feasible for transplantation studies.
Common neural factors for the generation of glial lineages
There are several common reagents, which are utilized throughout the various protocols during the derivation of oligodendroglial cells or alternately can be added in combination with the promotion NPs from hESCs. These include, retinoic acid (RA), sonic hedgehog (SHH), thyroid hormone (T3), and noggin, all integral signaling molecules with purported abilities to promote glial progenitors from NPs.
It has been reported that SHH may be essential for the initial production and specification of oligodendrocytes in the ventral forebrain, all-be-it within the mouse [54]. In this specific study, it was shown that, SHH is required for the specification and production of oligodendrocytes in the lateral gangilionic eminence (LGE) and MGE in the telencephalon [54]. These investigators also showed that, in the NKX2.1 knockout mouse, where SHH signaling is lost, oligodendrocytes in the LGE and MGE were reduced or absent, respectively [54]. In addition, there was a ventral to dorsal shift in the production of oligodendrocytes, which is not dependent on SHH signaling [54]. Therefore, the in vivo data identify SHH as an integral patterning regulator during development, possibly leading to oligodendrogliogenesis but may well be dispensable during the maturation of these cells in vivo.
Similarly, another neural differentiation factor, RA, is a well-known signaling molecule that supports neural specification [23,55]. RA may also be important for the organization of the ventral and dorsal axis of the neural tube, and the anterior spinal cord and posterior hindbrain during development [56]. In addition, it has been shown that RA can induce neuroectodermal, while inhibiting mesodermal differentiation in mouse ESCs, achieved through blockade of the nodal-signaling pathway [57]. Thus, RA is commonly utilized to promote the differentiation of hESC toward NPs that have the potential to ultimately give rise to OPCs.
Sundberg et al., Hu et al., and Gil et al., have all recently reported that RA and SHH enhance the derivation of NPs that later generate OPCs [23,24,28]. The upregulation of SHH expression is important to sequentially activate genes essential for OPC specification such as SOX10, OLIG2, and Nkx2.2 [40,54,55]. It is thought that SHH may be critical for the switch between neuronogenesis into oligodendrogenesis, however, its role is restricted to early developmental phases and does not extend to the maturation of pro-myelinating oligodendrocytes [37,44,58].
The use of noggin has been shown to broadly act by inhibiting bone morphogenetic proteins (BMPs) to suppress mesendodermal lineage patterning, promoting neural differentiation of hPSCs [23,26,43,59], Izrael et al., have shown that noggin also plays a role in the lineage specification of OPCs, potentiating the maturation toward oligodendrocytes [21]. Further, adding noggin at these early stages of hPSC development decreased noggin-mediated maturation, supporting the differentiation toward a glial lineage [21].
Another integral oligodendroglial differentiation member, T3, is a thoroughly characterized endocrine hormone. T3 has been reported to play a role in the survival and proliferation of pre-OPCs, but as a corollary, may promote the differentiation into mature oligodendrocytes [27]. Importantly, T3 regulates the timing of the differentiation into oligodendrocytes [60,61]. It has been shown that T3 induces the maturation of oligodendrocytes by activating the PI3K/Akt and also MAPK/ERK signaling pathways [62]. Sundberg et al., and Izrael et al., have both used T3 to induce the differentiation of NPs from hESCs. However, the NPs that were derived were similar to those obtained in other protocols, which excluded the use of T3 in culture [21,28], suggesting a subsidiary developmental role for this important hormone. Moreover, Sundberg et al. have shown that the combinatorial addition of T3, RA, and SHH to their hESC cultures, throughout the first 4 weeks of differentiation, decreased the required culture duration by 50% in the derivation of oligodendrocytes [28]. Although many oligodenroglial-influencing growth factors have been used to derive NPs, SHH, RA, and noggin seem to be all critical reagents at the initial stages of the differentiation protocol. However, as will be highlighted in the subsequent section of this review, T3 may well be more important, in the later stages of OPC differentiation.
The Identity of OPCs
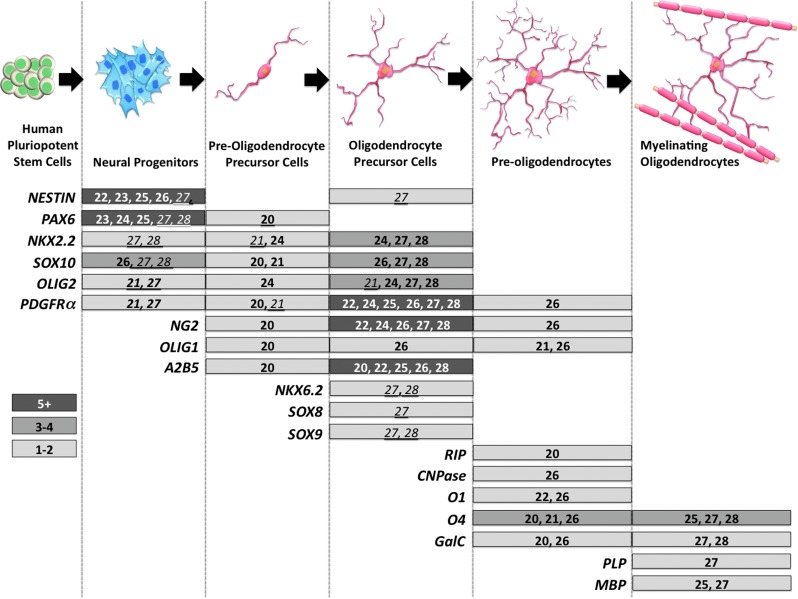
During mammalian neurodevelopment, oligodendrocytes, like their neural counterparts, are generated from neuroepithelial precursors, which line the ventricles and therefore are defined as the ventricular zone (VZ) precursors [63]. However, prior to their differentiation into post-mitotic mature myelinating oligodendrocytes, which mainly occurs after birth in rodents, there exists the generation of OPCs within the VZ with numerous morphological transitory subtypes (see Fig. 1) [64]. These OPC subtypes may be defined as pre-OPCs (originally identified as bipotential, oligodendrocyte-type-2 astrocyte glial progenitors), true OPCs (having the potential to migrate, proliferate, and only generate oligodendrocytes), and pro-myelinating OPCs (having the potential to differentiate into myelinating oligodendrocytes) (Fig. 1) [63,65,66]. What is consistent along the ontogenic pathway of lineage commitment is the array of markers used at specific stages of oligodendrocyte development, which may well serve as signatures of OPC subtypes (Fig. 1). Of course a major limitation in the definition of these OPC subtypes, using various biochemical markers, may well be that they have been primarily defined for rodent research and so it is now a responsibility of hESC neurobiologists to demonstrate the relevance of these markers in human oligodendrocyte differentiation.
FIG. 1.
Schematic of the progressive differentiation of human pluripotent stem cells (hPSCs) to myelinating oligodendrocytes. The illustration shows commonly used markers during the developmental stages of mammalian oligodendrocytes. These markers are either transcription factors that regulate neural and oligodendrocyte maturation or extracellular receptors that signal through growth factors and through the developmental extracellular matrix. All markers of oligodendroglial cells have varying expression time points, reported throughout the differentiation of oligodendrocytes derived from human embryonic stem cells (hESCs) by the protocols defined by the different research groups who have been compared in this review article. Gene expression that has been attributed to each stage has been included, with the specific references listed. References that have observed gene expression based upon immunocytochemistry or flow cytometry are shown in bold, and those observed by reverse transcription–polymerase chain reaction are italic and underlined.
The protocols described in this review utilize different panels of markers to characterize OPCs in an attempt to determine the purity of the various cell populations obtained via hESC culture, with NG2 and PDGFRα as the most commonly used markers to characterize OPCs [67], (Fig. 1). Sundberg et al., Kang et al., and Nistor et al., showed that ∼80%–88% of their hESC-derived OPCs express PDGFRα [20,22,27]. In particular, Sundberg et al., introduced a fluorescence-activated cell-sorting step to purify the NG2-positive OPC fraction that was otherwise 55% of their total population [27,28]. While beneficial, sorting stages are not likely to be critical and indeed Nistor et al., have without a sorting step, generated a population comprising 98% OPCs, as determined by NG2-positivity [20], and Hu et al., and Kang et al., have achieved a more modest 80%–81% of OPCs that were NG2-positive in their cultures [22,24].
These disparate results may be interpreted as the individual investigators definitions of what a true OPC is, with an overreliance on NG2-immunoreactivity alone. NG2-positive OPCs are known to have a high proliferative ability and can eventually generate myelinating oligodendrocytes [68]. Importantly, NG2-positively selected OPCs were negative for the pluripotency marker, OCT4, showing that these specific cells were committed to a neural cell lineage. However, there exists data suggesting that the NG2 marker can identify a population of cells that do not only generate post-mitotic oligodendroglial cells but may also identify cells capably of being driven into a neuronal or astrocytic lineage [69]. Therefore, it is necessary that highly proliferative populations generated from hESCs can yield sufficient numbers for eventual differentiation culture but the specificity of identifying true oligodendrocyte lineage cells must also be matched for the success of such protocols.
Moreover, a robust definition of these OPCs may arise from the interrogation of transcriptional markers of oligodendroglial differentiation, reported in the protocols of Sundberg et al., and Nistor et al. [20,27] as an example. These investigators noted the expression of NKX2.2 and NKX6.2 by OPCs [20,27]. While Hu et al., and Nistor et al., reported that 94% or 97% of OPCs, respectively, were labeled by using the A2B5 antibody [20,24,27]. In fact these homeobox proteins, NKX2.2 and NKX6.2, were demonstrated to control the expression of the mature oligodendrocyte marker, proteolipid protein (PLP) [70,71]. NKX6.2, in particular, is an important transcription factor for normal myelination as it binds to the promoter region of myelin binding protein (mbP) [72]. Further identification of OPC commitment has been reported as the upregulation of SOX8, SOX9, and SOX10, which play a crucial role in switching from neuronogenesis to oligodendrogenesis [27,73]. SOX10 and NKX2.2 are critical transcription factors for the terminal differentiation of oligodendrocytes and for the induction of MBP expression [73].
OLIG1 is another transcription factor that is expressed by OPCs. However, reports demonstrate a difference in localization. Kerr et al., [26] reported the cytoplasmic expression of OLIG1, while Keirstead and colleagues [1], described the nuclear expression of OLIG1. The disparity in OLIG1 localization patterns reported in these studies could be due to the differences in the stage of maturity during differentiation. OLIG1 is first expressed in the nucleus, where it interacts with SOX10 forming a complex that can activate mbp transcription by binding to elements in the 5′ region in the mbp gene [74]. It is expressed in the cytoplasm later during differentiation and its expression was shown to be essential for oligodendrocyte maturation [75]. A study by Arnett et al., on rodents showed that OLIG1 is important to repair demyelinating injuries, where its expression is translocated back into the nucleus [76]. However, investigations performed on fresh sections of human fetuses at the midgestational period, showed substantial redistribution of OLIG1 from the nucleus to the cytoplasm, within the cortical unmyelinated forebrain [77], again emphasizing the importance of nuclear localization of this transcription factor for myelination. Another study, used the nucleofector system to transfect primary mouse NPs with OLIG1 and demonstrated that 55% of the cells (almost all of those cells transfected), displayed enhanced differentiation to O4-immunopositive oligodendrocyte lineage cells. This was in contrast to untransfected cells where only 12% showed differentiation [78]. Thus, OLIG1 probably plays a crucial role in the differentiation/maturation of oligodendrocytes toward myelination.
NPs to OPCs
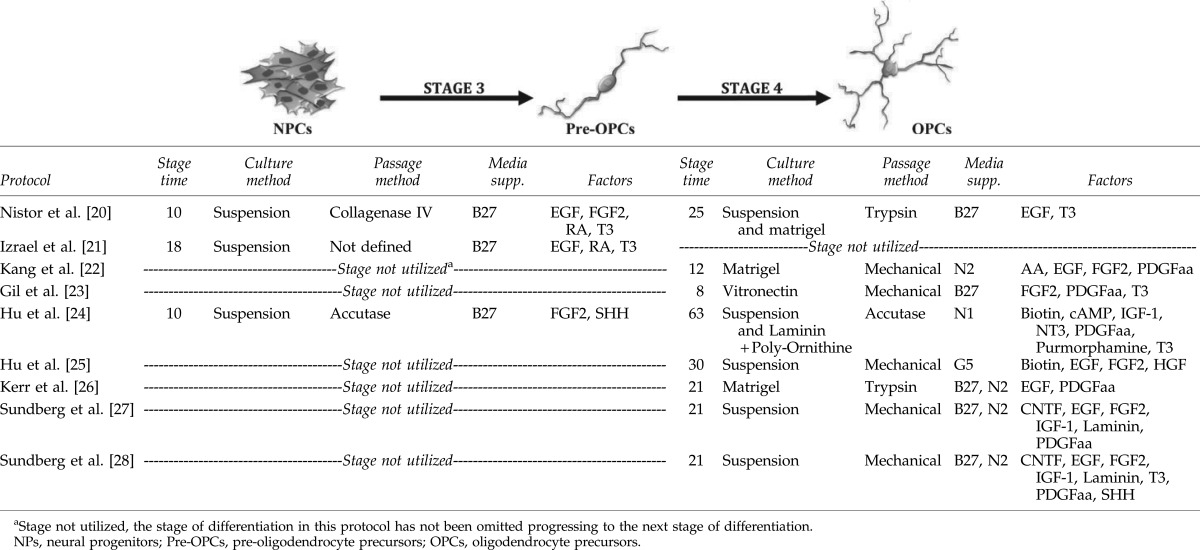
Utilizing the markers CD140a and NG2 to identify OPCs, Hu et al., were able to show that under their culture conditions, direct differentiation of NPs to pre-OPCs (expressing PAX6, NKX2.2, SOX10, and OLIG2), was then followed by the differentiation to OPCs, demonstrating a well-characterized intermediate precursor lineage (Table 2; Fig. 1) [24]. Conversely, Izrael et al. differentiated NPs to pre-OPCs, which expressed NKX2.2, SOX10, and OLIG2 (maintaining the expression of NP- along with OPC-markers) and then showed differentiation of these pre-OPCs to pro-myelinating oligodendrocytes (labeled with the O4 antibody) [21]. Despite this study's identification of an intermediate pre-OPC, other recently published protocols report direct differentiation of NPs to OPCs in one step (Table 3; Fig. 1) [22,23,26–28]. These protocols highlight considerable differences in the time taken to differentiate NPs to OPCs. Specifically, Hu et al. described the longest differentiation protocol, establishing oligodendrocytes within 73 days, while Gil et al. described the derivation of OPCs in the shortest time, with only 8 days required for differentiation [23,24]. Again, the differences in culture style, the ECMs and growth factors used in the differentiation protocols, are probably a major cause of the variation in the nature of OPC generation.
Table 3.
Differentiation Protocols for the Conversion of Neural Progenitors to Pre-Oligodendrocyte Precursors, then to Oligodendrocyte Precursors
 |
Oligodendroglial neurotrophic factors for OPC differentiation and survival
In addition to bFGF and EGF, platelet-derived growth factor (PDGF) is the growth factor most commonly used by all protocols during the differentiation of OPCs from NPs. However, provision of PDGF may not be essential, as Nistor et al. and Izrael et al. have both achieved oligodendrocytes in its absence [20,21]. EGF is a mitogen, which was shown to increase the pool of OPCs in rodents [79,80] and may well be able to replace PDGF in protocols used for oligodendrocyte differentiation from hESCs [20,21]. However, the addition of PDGF appears important in promoting differentiation to OPCs but not necessarily to mature oligodendrocytes [81], and so would seem an important inclusion during the early stages of hESC-differentiation. Supporting this contention is the evidence through careful dissection of developmental pathways, which established that PDGF could prevent premature differentiation and enhance proliferation by controlling the timing of oligodendrocyte maturation [82–84]. Such data have been well defined in developmental in vivo settings as a vital requirement for oligodendrogliogenesis and would therefore advocate for the inclusion of PDGF at strategic time points in hESC differentiation, as an adjunct to bFGF and EGF.
Both insulin-like growth factor (IGF) and T3, have also been used by Hu et al. and Sundberg et al. to support the derivation of OPCs [24,27,28]. Further, Sundberg et al. also reported the use of SHH to derive OPCs from previously generated NPs [28]. Although the addition of T3 and SHH at this stage (NPs to OPCs; Table 2) of the protocol improved differentiation outcomes, the culture time required to reach maturation was not reduced [27,28]. Similarly, with regard to the neural induction step prior to OPC specification, Sundberg et al., were able to demonstrate that through the provision of CNTF, the generation of pure OPCs from hESC-derived NPs could be achieved [27]. CNTF and IGF-1 were shown to increase the differentiation and maturation of OPCs along with the enhancement of their survival, proliferation, and differentiation in rodents [85,86]. Primarily, CNTF was shown to enhance the differentiation toward an oligodendroglial lineage from adult rat hippocampal NPs [87]. In a mechanistic study, IGF-1 was shown to induce the differentiation of adult rat hippocampal NPs to oligodendrocytes by inhibiting the BMP signaling pathway [88]. Moreover, mouse OPC studies have shown that IGF-1 increases their survival [89]. These data would advocate the benefit provided by adding IGF-1 and CNTF in the early stages of OPC differentiation from hESC-derived NPs.
Indeed, Sundberg et al. demonstrated that CNTF and IGF in combination could enhance the survival and proliferation of NG2-positive OPCs from hESCs [27]. Moreover, these growth factors increased the expression of Nkx2.2, which may be initiated through the inhibition of the BMP signaling pathway as defined in rodents [88]. Izrael et al., showed that the addition of RA in culture is also capable of inducing the expression of Nkx2.2, regulating mature oligodendroglial gene expression [21]. They also demonstrated that adding noggin to the medium is important for the upregulation of SOX10, another transcription factor defining specific oligodendrocyte maturation [21]. Although RA induces Nkx2.2 expression, it may increase the expression of BMP2, which has been documented to inhibit oligodendrogenesis [21]. Thus, noggin, as a BMP antagonist, was shown to promote oligodendrocyte differentiation by inhibiting specific BMPs [43]. One hypothesis is that noggin counters the increasing synthesis of BMPs, a result of RA stimulation, allowing OLIG2 to induce the expression of SOX10 [21].
Controlling OPC differentiation through physical interactions with ECM
Utilizing ECMs in differentiation protocols at the OPC stage is critical for the growth and survival of the generated OPCs. Recently, Hu et al. examined the effects of laminin, fibronectin, and matrigel as ECMs on the proliferation, migration, survival, and differentiation of OPCs isolated from rat spinal cords at embryonic day 15 [90]. Despite all three ECMs promoting these cell cycle events, it was noted that fibronectin was the most biologically potent [90]. However, limited evidence exists for fibronectin as a substrate when deriving OPCs from hESCs and so similar assays would need to be validated for its potential use in culture.
Different ECMs have been used for the differentiation of OPCs from the hESC-derived NPs. Nistor et al., Kang et al., and Izrael et al., have all used matrigel, whereas, Gil et al., have used vitronectin [20–23]. On the other hand, Sundberg et al., have used a combination of laminin, collagen IV, and nidogen-1 [27,28]. It is well documented that laminin was shown to direct the differentiation to a neural lineage and it plays a fundamental role in the survival, maturation, and myelination of oligodendrocytes [91]. Sundberg et al., is the only group to have described the use of laminin in suspension culture as a supplement [27,28], for the promotion of their OPC induction, possibly achieved by enhancing the interaction of surrounding cells through their surface molecules.
On the other hand, it has been reported that vitronectin, used as a soluble supplement, and combined with RA, SHH, and noggin, could increase the yield of derived oligodendrocytes [23]. As a corollary, it has been shown that vitronectin plays a significant role in the migration, proliferation, and maturation of OPCs [92]. Li et al., from the Geron Corporation, have shown that the vitronectin-derived synthetic peptide acrylate surface can be a viable alternative to matrigel in differentiation cultures, for hESC-derived OPC generation by utilizing the established Nistor et al. protocol [93]. Similar yields of PDGFRα-positive OPCs were obtained in this study, using vitronectin-derived synthetic peptide acrylate surface, in direct comparison with OPCs that were generated on matrigel [93]. However, these investigators generated a higher yield of NG2-positive cells on vitronectin-derived synthetic peptide acrylate surface than on matrigel [93], illustrating a potentiation of robust generation of OPCs with co-expression of PDGFRα. No difference in the proliferation of OPCs, whether they were cultured on matrigel or on vitronectin-derived synthetic peptide acrylate surface could be demonstrated [93]. These data now imply that an alternative source of synthetic generated ECM can now be utilized to derive excellent yields of OPCs from hESCs, replacing the problematic, complex animal-derived ECM.
OPCs to Oligodendrocytes
The differentiation of OPCs into mature oligodendrocytes is a critical step in hESC-derived cultures because maturation is a unique biological indicator of functionality and quit logically the suitability of such cells for various practical applications, which may include CRT. Kang et al., Hu et al., and Sundberg et al., have all reported the derivation of mature, myelinating oligodendrocytes in 2–4 weeks using growth factor-free medium (Table 4) [22,24,27]. In addition to MBP and PLP, these specific studies of hESC-derived oligodendrocytes, could demonstrate GalC-, O1-, and O4-immunopositive markers on these mature cells. On the other hand, Nistor et al., Izrael et al., and Gil et al., showed the differentiation of OPCs to pre-oligodendrocytes through the expression patterns of GalC, the O4-antigen and the receptor interacting protein [20,21,23]. Sundberg et al., and Nistor et al., showed that 80%–85% of the differentiated oligodendrocytes were labeled with the O4 antibody, while Izrael et al., were able to achieve 95% O4-immunopositivity [20,21,27]. Sundberg et al., and Nistor et al., revealed that 80% and 95% of oligodendrocytes were positive for GalC, respectively [20,27]. Similarly, Kerr et al., showed that the differentiation of OPCs to pre-oligodendrocytes promoted the expression of the O1-antigen, GalC, and CNPase [26]. However, these cell populations failed to express MBP in vitro, which is essential for myelination and one of the greatest challenges confronting scientists investigating remyelination treatments.
Table 4.
Differentiation Protocols for the Conversion of Oligodendrocyte Precursors to Pre-Oligodendrocytes, then to Oligoderocytes
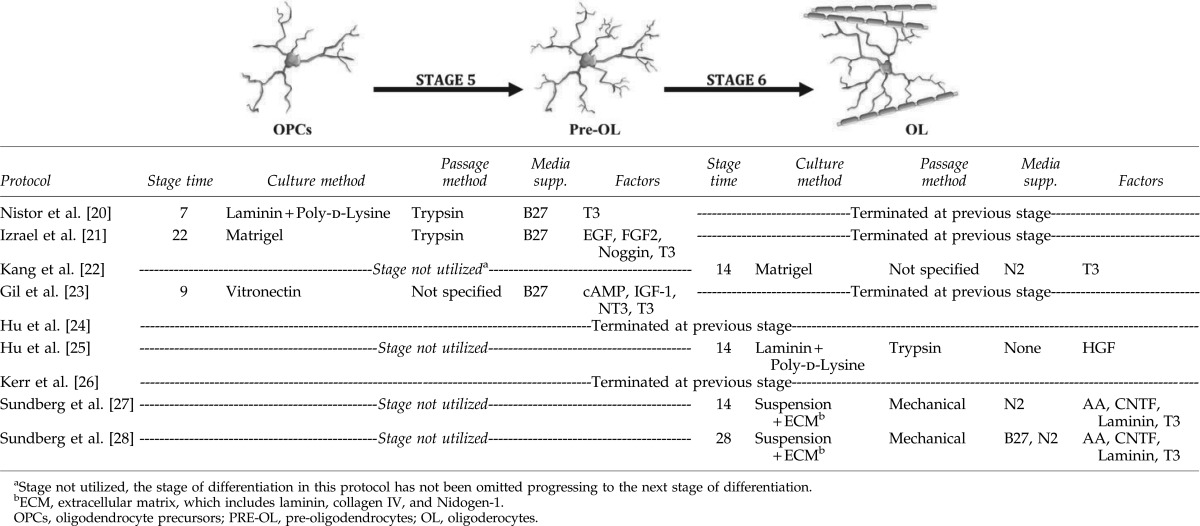 |
It should be noted that although some protocols failed to obtain mature oligodendrocytes that express MBP in vitro, the OPCs differentiated into mature MBP-positive oligodendrocytes in vivo following transplantation into the shiverer model of dysmyelination [20,21,49]. This could be a result of the different extracellular environments, such as axonally-derived factors, that can induce the differentiation [94]. Therefore, it stands to reason that a determination of the opportune time for oligodendrocyte transplantation is established for each neurological disease paradigm to facilitate successful engraftment. Alternatively, we must be better at modifying the diseased or injured tissue milieu to enhance the viability and promote repair in the CNS post-transplantation with hESC-derived oligodendrocytes.
Similarly, it is critical to examine the myelinating ability of the derived oligodendrocytes in vitro or in vivo. For instance, Sundberg et al., and Kang et al., showed the myelinating ability of derived oligodendrocytes in vitro by coculturing with neurons [22,27]. Conversely, Izrael et al., Hu et al., and Nistor et al., have all shown the myelination potential of hESC-derived oligodendrocytes in vivo following the transplantation of OPCs into a shiverer mouse model [20,21,49]. The transplanted oligodendrocytes myelinated bare axons, at variable anatomical sites. Moreover, Izrael et al. demonstrated that treating OPCs with noggin prior to transplantation enhanced the maturation and myelination in vivo [21]. Importantly, no teratomas were reported in these studies. This experimental dysmyelinated environment is substantially less hostile than that encountered in the injured or diseased CNS, both in acute and chronic neurological conditions. Therefore, modulating the tissue environment prior to transplantation must be an edict for future studies.
The Differentiation of Oligodendrocytes from hiPSCs
Recent advances in somatic cell reprogramming have uncovered that hiPSCs may well serve as an attractive therapeutic tool in regenerative medicine. The negligible immunoreactivity upon differentiation of iPSCs presents a unique opportunity for future CRT applications, where immunosuppression may not be required. However, this specific stream of research is very much in its embryonic stages with only two studies recently reporting the use of hiPSCs to generate oligodendrocytes [95,96].
Recent data generated by Ogawa et al., examined the differentiation of two hiPSC lines (201B7 and 253G1) to oligodendrocyte lineages using either the Nistor et al., (using EGF as a mitogen) or Kang et al. (using PDGF as a mitogen) protocols [20,22,95]. These investigators reported that they were not able to generate premyelinating oligodendrocytes, as defined by O4 antibody immunoreactivity. However, rare O4-positive cells (less than 0.01%), were derived from the 253G1 hiPSC line, by using the Nistor et al. protocol [95]. The authors suggested that the absence of O4 antibody-positive premyelinating oligodendrocytes could be due to the hiPSC lines, which were generated from adult fibroblasts, due to the inherent epigenetic differences. However, it would seem that the logical next step is that all hiPSC lines be rigorously evaluated with the inclusion of hESC lines as controls to appropriately evaluate the efficacies of the OPC differentiation protocols on these cells.
Pouya et al., recently showed that the differentiation of hiPSCs (hiPSC1 and hiPSC8) lines established from adult fibroblasts, differentiated in tandem with one hESC line (Royan H6 line) using the hESC-derived OPC protocol of Nistor et al. [20,96]. Through immunocytochemical analysis, the authors documented that ∼90% of OPCs could be generated in culture using the hiPSC1 line, based on expression profiles that included OLIG2, SOX10, PDGFRα, and NG2. Moreover, Pouya et al. demonstrated the generation of ∼20% MBP-positive oligodendrocytes by the end of the differentiation stage from the hiPSCs [96]. Although the authors stated that they have achieved mature oligodendrocytes in culture following their differentiation from hiPSCs and hESCs, the data did not show that the hESC-derived cells were MBP-positive [96]. Further, few neurons and astrocytes existed within these cultures by the end of the hESC-differentiation protocol. Conversely, there were few neurons but no astrocytes present in culture by the end of the hiPSC differentiation stage [96]. Such disparities may reside in the inherent biological differences between individual cell lines or between hESCs and hiPSCs themselves and require future elucidation.
The first reported generation of OPCs from hiPSCs [97], was in fact optimized based on protocols for hESC-derived oligodendrocytes [21,24,97]. Wang et al. were able to generate OLIG2/NKX2.2/SOX10/PDGFRα-positive OPCs from three different hiPSC lines (C14 and C27; from human fibroblast and K04; from human keratinocyte) along with the concordant differentiation of a specific hESC line (H9) [97]. The derived OPCs were similar to those derived from hESC in previous studies, demonstrating the ability to myelinate in an in vitro coculture (in coculture with neurons) along with in vivo myelination of dysmyelinated axons of shiverer mice following transplantation. However, these “OPCs” were able to differentiate to both oligodendrocytes and astrocytes. The data illustrated by Wang et al. estimated that 50% of the cell culture population where indeed GFAP-positive astrocytes, while a fraction of the cells (4%–11%) were O4 immunoreactive and MBP-positive oligodendrocytes [97]. In addition, after in vivo transplantation within the shiverer mouse model, many of the transplanted OPCs differentiated to astrocytes [97]. Thus, it is a fundamental issue if these cell types were to be utilized as a CRT following SCI, since the majority of these cells could become incorporated within the glial scar.
Despite the variability of the results generated from the different hPSC lines used in this study, Wang et al. were able to establish that the most protracted protocol used to generate OPCs and oligodendrocytes from hiPSCs (requiring differentiation culture for ∼150 days and 200 days, respectively) was the most effective in promoting OPC maturation [97]. The derived OPCs displayed a greater myelination potential than OPCs isolated from human fetal brain tissue in vitro and in vivo [97]. Importantly, no evidence of tumorigenesis was reported after transplanting OPCs-derived from hiPSCs to shiverer mice [97]. Therefore, these results may suggest that hiPSC-derived OPCs require extended growth defined culture conditions at present and further work may identify such cells as excellent candidates for autologous CRT in acquired myelin diseases.
Prospective studies in the field could in fact focus on deriving highly pure homogenous populations of OPCs from hiPSCs, along with hESCs, that have the potential to differentiate to oligodendrocytes, exclusively. Further, while iPSC-derived terminally differentiated cells are believed to elicit little immunoreactivity upon autologous transplantation, the reprogramming techniques used to generate hiPSCs may pose safety concerns particularly where reprogramming involves retroviral miss-expression [98]. Emergent reprogramming techniques involving mRNA, episomal and non-integrative viral transduction methods are in development and may circumvent these issues, providing a safe cellular product for CRT.
Issues of Concern and Troubleshooting
Variations within interline differentiation
The limited protocols that have been reported for oligodendrocyte differentiation from hESCs could demonstrate highly pure cultures of OPCs, along with the generation of small numbers of mature oligodendrocytes. This has been facilitated by an increased understanding of the oligodendrocyte differentiation pathway and the critical constituents within these cultures, such as PDGF, thyroid hormone, and the growth factor signaling pathways imperative in the regulation of cell cycle events at the various stages of culture [27,99]. As further protocols are reported, it is clear both major and minor differences exist between them, confounding efforts to generate a consensus for the differentiation process and to resolve the most appropriate method for transplantation purposes. However, these protocols have been useful in defining methodologies for the derivation of oligodendroglial lineage cells. Principally, the difference may lie in the hESC lines used during differentiation in culture and may contribute to the variation in derived cell yields, with different stages of oligodendrogenesis. This raises the contention that the efficiency of deriving mature oligodendrocytes will vary according to the hESC lines, a notion that aligns with existing evidence of neuronal interline differentiation variability [100–104].
The prominent protocols recently defined by Sundberg et al., examine the oligodendroglial differentiation efficacy of their protocol on two different hESC lines, 08/023REGEA and 06/040REGEA [27,28]. Using these two different hESC lines gave 50%–70% purity of NG2-positive oligodendrocytes, suggesting that even a standardized protocol will generate significantly varied results that could substantially effect mature oligodendrocyte yield. Similarly, Hu et al., Izrael et al., and Gil et al., all reported the use of two cell lines or more to examine their protocols [21,23,24] but others persist with the use of one cell line [20,22,25,26] rendering incongruent results. It would thus seem crucial and logical to evaluate the various protocols reported using different cell lines in different laboratories, primarily as an attempt to establish parallels in their oligodendroglial differentiation potential.
The coculture addiction and time-dependent development
Most hESC lines, which have been utilized in the currently discussed protocols, rely on embryonic fibroblast cultures for pluripotent maintenance. The majority of original research reports the use of mouse embryonic fibroblasts (MEFs) [21–24,26]. However, Sundberg et al., [27,28] and Hu et al., [25], have both used human foreskin fibroblasts in their protocols for the purpose of establishing xeno-free conditions, to satisfy the requirements for clinical application.
Second, the length of time to conduct these protocols varies significantly. The time-dependency of the hESC differentiation culture may again affect the maturation of oligodendrocytes. For example, Hu et al., have established the most protracted protocol (14 weeks), achieving 80% oligodendrocytes, while Nistor et al., and Kerr et al., have described protocols that are six weeks in duration, deriving 90%–95% oligodendrocytes [20,24,26,49]. The generation of oligodendrocytes from hESC cultures over different time frames may be a result of the complex nature of gliogenesis and the heterogeneity of oligodendrocytes generated [33,35,36,48]. Indeed, understanding the different pathways that induce the differentiation of oligodendrocytes from hESCs would improve our understanding of in vivo mechanisms for gliogenesis. For instance, by defining the master gene regulators and growth factors that are vital in the regulation of varied differentiation pathways, a detailed understanding of the ontogeny of heterogeneic oligodendroglial populations may be resolved. Such information will provide clarity in transplantation studies with a direct translational impact. The differences among the growth factors, which to date have been used in the various in vitro protocols, probably play a major role in the variation in the time-dependent culture assays that have been reported.
It is now established that the length of hESC culture time is a fundamental factor because it may well replicate the developmental ontogeny toward functional oligodendrocytes. On the other hand, it is important that the differentiation process mimics normal oligodendrogliogenesis in vivo because the derived mature oligodendrocytes must be able to form a functional myelin membrane. The early induction of the maturation of OPCs would probably fail to obtain normally functioning mature oligodendrocytes that have the ability to myelinate. Although Sundberg et al., and Hu et al., have described the most protracted protocol (ie, 3 months), the benefits of using such protocols are that they resemble the time required for the early appearance of OPCs in human embryos, which arrive at the end of the first trimester [24,27,28,49]. In addition to the developmental regulation of oligodendrogliogensis, the appropriate growth factors used in the protocols and their concentrations play a critical role in the function of mature oligodendrocytes.
Population purity and lighting up targets
It is an essential step to have a high purity of oligodendrocytes by the end of the differentiation protocols, avoiding any contamination with other cell types or undifferentiated cells, which could result in tumor formation following the transplantation of these cells. It is difficult to assess overall oligodendrocyte purity derived from hESCs, demonstrated from the various published protocols, as each independent laboratory has used different individual, or a combination of markers, to characterize these differentiated oligodendrocytes. In general, the average purity of oligodendrocytes was estimated to be ∼90% (80%–95%), with variations in maturity and myelinating ability as a number of protocols show the differentiation of hESCs to pre-oligodendrocytes or OPCs, but not to mature MBP-positive oligodendrocytes [20–28].
It is difficult to identify oligodendrocyte populations derived from hESC based on a single marker, since there exists vast heterogeneity in cellular phenotypes with the real “gold standard” being the identification of mature and predictive myelination capacity. Oligodendrocytes co-express many different surface markers and transcription factors at strategic stages during their development [33,34,48]. Further, neural lineage cells, other than oligodendrocytes, express a number of markers, such as NG2 [68,105], posing a major problem in the identification of derived OPCs. In addition, oligodendrocytes have been shown to arise from different NPs in rodents [35–37,44]. Thus, it is logical to identify the populations of oligodendrocytes according to a panel of markers based on different time points and stages of maturity, rather than using single differentiation markers. Importantly, oligodendrocytes should be negative for undifferentiated pluripotency markers, such as OCT3/4 and Nanog. With one exception, all the defined protocols have reported that the derivation of high yields of mature oligodendrocytes, are in fact negative for pluripotency markers [20–22,24–28]. However, the study by Gil et al., found that undifferentiated cell populations persist within their culture systems [23]. This population of cells may retain the potential to differentiate into any cell type and proliferate indefinitely, with the potential to form teratomas and exclude these cells as a viable option for transplantation.
If OPCs are to serve as a viable transplantation option, it is crucial to have a population with a high proliferative potential (at specific stages throughout the differentiation), providing large numbers of expanded cells for different clinical and experimental applications. Due to the prolonged lengths of the protocols, it would be preferential to freeze a stock of the OPC population to be used when needed. The major difficulty that faces the differentiation of OPCs from hESCs is the variation in the results obtained in different laboratories when repeating a defined protocol. As already discussed, this is mainly due to the differences in the hESC lines and MEFs that are used but may also relate to the limited description of these protocols throughout the various publications. Therefore, a consensus is promptly required allowing other research groups to replicate hESC-derived oligodendrocytes ab initio.
Key issues of protocol reproducibility
One of the major limitations in deriving oligodendroglial lineage cells from the large repertoire of hESC lines, is the successful replication of results using published protocols, from the various international laboratories. The protocol published by the Nistor et al. group, is the only protocol to generate OPCs from hESCs that has been replicated by different research groups [20]. This particular protocol was independently reported on three occasions to successfully derive oligodendroglial lineage cells, all laboratories achieving similar yields by using the same cell lines (H1 and H7) [99,107,108]. In addition, Kerr et al. [26] have optimized their protocol based on that published by Nistor and co-workers [20] with some modifications, although they have achieved similar results.
On the other hand, the other reported protocols have been utilizing alternate hESC lines, have to date not been replicated in different laboratories. Therefore, it would seem that the protocol of Nistor and colleagues [20], at this very early stage, may well be an excellent starting point for any laboratory wishing to attempt oligodendroglial lineage differentiation from their particular hESC lines. Again, there may be a limitation with this protocol, since these investigators primarily include xeno-derived reagents such as matrigel, which may be problematic for independent laboratories generating OPCs with the intention to enter into translational research. Thus, future studies to optimize the published protocols are now warranted to generate homogeneous transplantable OPCs that could be safely used under controlled clinical trial guidelines.
Applications of Oligodendrocytes Derived from hESCs
In vitro oligodendrocyte differentiation from hESCs may have many promising applications, however, the use of such cells in transplantation paradigms for CNS trauma and disease is still very much in its infancy. Idealistically, the differentiation from hESCs may present an infinite supply of cells for replacement therapy, drug screening, and models of injury or disease. In addition, their differentiation may serve as a model of early oligodendrogliogenesis in humans. Indeed, the differentiation of oligodendrocytes from hESCs has provided the opportunity to study the development of oligodendrocytes in humans because access to human embryos is extremely limited and most studies have been extrapolated from animal embryos. For instance, Letzen et al., showed miRNA profiles during different stages of oligodendrocyte differentiation from hESCs, which possibly presents key markers of oligodendrocyte maturation in vivo [109]. Further, Chaerkady et al., demonstrated several novel proteins, such as fatty acid-binding protein and thrombospondin-1, which are probably involved in oligodendrocyte differentiation and maturation, by presenting an extensive proteomic profiling of oligodendrocytes derived from hESCs [110]. It is through these studies that a comprehensive understanding of molecular and cellular regulators of oligodendrocyte development will be achieved, providing investigators with the most powerful selection criteria for transplantation studies.
Transplantation
During the pathological sequelae following neurotrauma, the widespread apoptosis of oligodendrocytes occurs at the site of injury and some distance rostral and caudal from the traumatic epicenter, leading to prodigious demyelination, axonal degeneration, and subsequent neurological dysfunction [3,26]. Therefore, OPCs are believed to be an attractive therapeutic target to limit the demyelination and degeneration of axons, along with the potential of enhancing remyelination, neuronal functionality, and survival.
There are several aspects that should be considered in choosing a candidate cell type for CRT, such as availability, proliferative potential, and most importantly, safety. In spinal cord injuries, demyelination has been reported to continue up to 450 days post-injury depending on the nature and level of the injury, along with the age of the individual [111]. To remyelinate denuded axons and limit the decline in neurological function after SCI, the transplantation of hESC-derived OPCs has been proposed as a promising therapy to repair the CNS lesions. However, the time of transplantation has been identified as playing a vital role in the efficacy of any such CRT [1].
It has been reported by Keirstead et al. that upon the transplantation of OPCs derived from hESCs, 7 days after a thoracic SCI performed on adult rats, remyelination could be achieved [1]. The benefit attributed to this outcome could be demonstrated as an improved locomotor performance, defined according to the Basso Beattie and Bresnahan (BBB) scale [1]. However, the transplantation of the OPCs, 10 months after injury, showed no enhancement of remyelination or improvement in locomotor function [1]. Nevertheless, the survival rates of the transplanted OPCs were demonstrated to be similar in both cases. Therefore, OPC transplantation during the early stages after SCI may be essential for the efficacy of such CRT. However, the rationale of this outcome is yet to be defined. Moreover, Keirstead et al. demonstrated that the transplanted OPCs differentiate in vivo into oligodendrocytes almost exclusively [1]. On the other hand, Erceg et al., showed that by following the same protocol, some of the transplanted OPCs derived from hESCs, differentiated into neurons [108]. These variations could be due to the heterogeneity and purity of the transplanted OPCs (with Erceg et al., using the H9-eGFP cell line) [108]. Similarly, Clouiter et al., showed an enhancement in locomotor function and remyelination after OPC-transplantation, 7 days after injury [112]. Conversely, after mild SCI using the New York University impactor, with undetectable demyelination, only a limited therapeutic effect with transplanted OPCs could be demonstrated [112]. Despite the reported successes of these OPC transplantation studies, what is clearly lacking is a more robust documentation of the in vivo results, followed over a greater length of time (ie, during chronic injury or disease time scales and not only in the acute setting).
Studying the fate of these cells over a more protracted period has been highlighted in a separate non-trauma model of demyelination, where the transplantation of OPCs into a viral model of demyelination (Theiler's virus), could demonstrate a decrease in demyelination and an enhancement in remyelination [113]. However, the engrafted cells did not survive beyond 2 weeks even when the animals were treated with anti-inflammatory agents. This may well be a common issue in other models and human diseases such as multiple sclerosis (MS), where the aggressive neuroinflammation limits the survival and differentiation of transplanted OPCs [114,115]. On the other hand, it is reported that inflammatory cytokines, such as tumor necrosis factor-α, may be important for OPC migration, possibly enhancing the remyelination process. So, the use of immunosuppressant therapies in MS patients during the transplantation of OPCs derived from hESCs, may not be an ideal adjunct therapeutic option, since these may limit the migratory potential of OPCs thereby prohibiting remyelination [116].
One of the main concerns related to OPCs derived from hESCs is immune rejection because their transplantation is a form of allogeneic transplantation. Nonetheless, Okamura et al., showed that the derived OPCs retained some of the unique immunological characteristics of hESCs [107]. They demonstrated that the derived OPCs are weakly immunogenic and exhibited significant resistance to natural killer cells. Thus, immune suppression is a necessity, as it is possible that such transplantation will evoke a deleterious immune reaction. Alternatively, hiPSC technology could circumvent this concern.
Despite the potential pathological implications of using hESC-derived OPCs as CRT in SCI, promising results have been reported, resulting in the engagement of a larger-scale translational phase I trial. Particularly, Keirstead et al. sponsored by the Geron Company have led to the first FDA-approved clinical trial on SCI patients, who were selected on the basis of a thoracic level (T3 to T11) contusion at least 7 and no greater than 14 days post-injury, manifest as ASIA impairment scale A. However, there are concerns with regards to this clinical trial as the safety of the transplanted cells is not fully understood, a consequence of the limited definition of the fundamental biological principles governing the hESC-derived OPCs. Recently, the Geron-led clinical phase trial was suspended. Further, limited published data exist in the literature on OPCs derived from hESCs and their potential as a CRT in animal models. Moreover, different xenogeneic materials are currently being included in these protocols with significant safety concerns upon transplantation. It may well be that modification of the current protocols through a more comprehensive understanding of culture conditions, time dependency, and intrinsic molecular regulators of hESC-derived OPCs will pave the way for a whole new series of successful clinical phase trials for transplantation. Moreover, the derivation of mature oligodendrocytes would provide an opportunity to study myelin and myelin formation. Therefore, an appropriate and efficient production of OPCs from hESCs is a critical step in conducting accurate investigations and credible studies of OPC survival, migration, and remyelination ability. Crucially, the achievement of this goal will offer an ideal platform for studying the differentiation of oligodendrocytes and OPCs from hiPSC lines, and remove the ethical issues regarding the use of hESCs. Additionally, OPCs derived from hiPSC lines could be autologously transplanted, as the hiPSC lines could be established from the same patient who may require the transplantation.
Conclusion
The evolution of adult and embryonic pluripotential cell differentiation methods, with the aim of deriving mature functional cell types and eventual integration in various tissues of the body, have generated considerable excitement in the field of regenerative medicine. However, there is a distinct lack of data focusing on deriving functional oligodendrocytes from hESC lines that may hold significant promise as an infinite source of immature and mature cells for repair of pathological lesions occurring within the CNS in acquired disorders such as MS, genetic disorders such as the leukodystrophies, along with CNS trauma. The major challenge that faces scientists in the quest for identifying the most appropriate hESC line, culture time and growth conditions for the derivation of these cells, is without a doubt the vast heterogeneity of the cells during their in vitro culture. This fundamental issue in the field limits the potential of these cells as CRT. However, through a deeper understanding of the molecular regulators of oligodendrocyte differentiation, as is currently being uncovered through the advent of conditional mutant mouse models during CNS development, we now are becoming aware of the complexities of oligodendrocyte development and what it may take for the use of stem cell research as a therapeutic tool.
Funding Information
W.F.A. was funded by Taif University postgraduate scholarship and S.P. was supported by the National Multiple Sclerosis Society (USA) project grant #RG4398A1/1.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Keirstead HS. Nistor G. Bernal G. Totoiu M. Cloutier F. Sharp K. Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;19:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu B. Sun L. Li P. Tian M. Luo Y. Ren X. Transplantation of oligodendrocyte precursor cells improves myelination and promotes functional recovery after spinal cord injury. Injury. 2012;6:794–801. doi: 10.1016/j.injury.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Faulkner J. Keirstead HS. Human embryonic stem cell-derived oligodendrocyte progenitors for the treatment of spinal cord injury. Transpl Immunol. 2005;2:131–142. doi: 10.1016/j.trim.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Aubry L. Bugi A. Lefort N. Rousseau F. Peschanski M. Perrier AL. Striatal progenitors derived from human ES cells mature into DARPP32 neurons in vitro and in quinolinic acid-lesioned rats. Proc Natl Acad Sci U S A. 2008;43:16707–16712. doi: 10.1073/pnas.0808488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vazey EM. Dottori M. Jamshidi P. Tomas D. Pera MF. Horne M. Connor B. Comparison of transplant efficiency between spontaneously derived and noggin-primed human embryonic stem cell neural precursors in the quinolinic acid rat model of Huntington's disease. Cell Transplant. 2010;8:1055–1062. doi: 10.3727/096368910X494632. [DOI] [PubMed] [Google Scholar]
- 6.Ma L. Hu B. Liu Y. Vermilyea SC. Liu H. Gao L. Sun Y. Zhang X. Zhang SC. Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell Stem Cell. 2012;4:455–464. doi: 10.1016/j.stem.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kriks S. Shim JW. Piao J. Ganat YM. Wakeman DR. Xie Z. Carrillo-Reid L. Auyeung G. Antonacci C, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;7378:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkeby A. Grealish S. Wolf DA. Nelander J. Wood J. Lundblad M. Lindvall O. Parmar M. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 2012;6:703–714. doi: 10.1016/j.celrep.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Raff MC. Lillien LE. Richardson WD. Burne JF. Noble MD. Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature. 1988;6173:562–565. doi: 10.1038/333562a0. [DOI] [PubMed] [Google Scholar]
- 10.McKinnon RD. Matsui T. Dubois-Dalcq M. Aaronson SA. FGF modulates the PDGF-driven pathway of oligodendrocyte development. Neuron. 1990;5:603–614. doi: 10.1016/0896-6273(90)90215-2. [DOI] [PubMed] [Google Scholar]
- 11.Barres BA. Burne JF. Holtmann B. Thoenen H. Sendtner M. Raff MC. Ciliary neurotrophic factor enhances the rate of oligodendrocyte generation. Mol Cell Neurosci. 1996;2–3:146–156. doi: 10.1006/mcne.1996.0053. [DOI] [PubMed] [Google Scholar]
- 12.Barres BA. Lazar MA. Raff MC. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;5:1097–1108. doi: 10.1242/dev.120.5.1097. [DOI] [PubMed] [Google Scholar]
- 13.Nishiyama A. Lin XH. Giese N. Heldin CH. Stallcup WB. Interaction between NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells is required for optimal response to PDGF. J Neurosci Res. 1996;3:315–330. doi: 10.1002/(SICI)1097-4547(19960201)43:3<315::AID-JNR6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 14.Bansal R. Warrington AE. Gard AL. Ranscht B. Pfeiffer SE. Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mAb used in the analysis of oligodendrocyte development. J Neurosci Res. 1989;4:548–557. doi: 10.1002/jnr.490240413. [DOI] [PubMed] [Google Scholar]
- 15.Liu S. Qu Y. Stewart TJ. Howard MJ. Chakrabortty S. Holekamp TF. McDonald JW. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc Natl Acad Sci U S A. 2000;11:6126–6131. doi: 10.1073/pnas.97.11.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brustle O. Jones KN. Learish RD. Karram K. Choudhary K. Wiestler OD. Duncan ID. McKay RD. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;5428:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- 17.Sadowski D. Kiel ME. Apicella M. Arriola AG. Chen CP. McKinnon RD. Teratogenic potential in cultures optimized for oligodendrocyte development from mouse embryonic stem cells. Stem Cells Dev. 2010;9:1343–1353. doi: 10.1089/scd.2009.0520. [DOI] [PubMed] [Google Scholar]
- 18.Jiang P. Selvaraj V. Deng W. Differentiation of embryonic stem cells into oligodendrocyte precursors. J Vis Exp. 2010;39 doi: 10.3791/1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C. Daugherty D. Jiang P. Deng W. Oligodendrocyte progenitor cells derived from mouse embryonic stem cells give rise to type-1 and type-2 astrocytes in vitro. Neurosci Lett. 2012;2:180–185. doi: 10.1016/j.neulet.2012.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nistor GI. Totoiu MO. Haque N. Carpenter MK. Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;3:385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- 21.Izrael M. Zhang P. Kaufman R. Shinder V. Ella R. Amit M. Itskovitz-Eldor J. Chebath J. Revel M. Human oligodendrocytes derived from embryonic stem cells: effect of noggin on phenotypic differentiation in vitro and on myelination in vivo. Mol Cell Neurosci. 2007;3:310–323. doi: 10.1016/j.mcn.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Kang SM. Cho MS. Seo H. Yoon CJ. Oh SK. Choi YM. Kim DW. Efficient induction of oligodendrocytes from human embryonic stem cells. Stem Cells. 2007;2:419–424. doi: 10.1634/stemcells.2005-0482. [DOI] [PubMed] [Google Scholar]
- 23.Gil JE. Woo DH. Shim JH. Kim SE. You HJ. Park SH. Paek SH. Kim SK. Kim JH. Vitronectin promotes oligodendrocyte differentiation during neurogenesis of human embryonic stem cells. FEBS Lett. 2009;3:561–567. doi: 10.1016/j.febslet.2008.12.061. [DOI] [PubMed] [Google Scholar]
- 24.Hu BY. Du ZW. Zhang SC. Differentiation of human oligodendrocytes from pluripotent stem cells. Nat Protoc. 2009;11:1614–1622. doi: 10.1038/nprot.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu Z. Li T. Zhang X. Chen Y. Hepatocyte growth factor enhances the generation of high-purity oligodendrocytes from human embryonic stem cells. Differentiation. 2009;2–3:177–184. doi: 10.1016/j.diff.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Kerr CL. Letzen BS. Hill CM. Agrawal G. Thakor NV. Sterneckert JL. Gearhart JD. All AH. Efficient differentiation of human embryonic stem cells into oligodendrocyte progenitors for application in a rat contusion model of spinal cord injury. Int J Neurosci. 2010;4:305–313. doi: 10.3109/00207450903585290. [DOI] [PubMed] [Google Scholar]
- 27.Sundberg M. Skottman H. Suuronen R. Narkilahti S. Production and isolation of NG2+ oligodendrocyte precursors from human embryonic stem cells in defined serum-free medium. Stem Cell Res. 2010;2:91–103. doi: 10.1016/j.scr.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Sundberg M. Hyysalo A. Skottman H. Shin S. Vemuri M. Suuronen R. Narkilahti S. A xeno-free culturing protocol for pluripotent stem cell-derived oligodendrocyte precursor cell production. Regen Med. 2011;4:449–460. doi: 10.2217/rme.11.36. [DOI] [PubMed] [Google Scholar]
- 29.Hong S. Kang UJ. Isacson O. Kim KS. Neural precursors derived from human embryonic stem cells maintain long-term proliferation without losing the potential to differentiate into all three neural lineages, including dopaminergic neurons. J Neurochem. 2008;2:316–324. doi: 10.1111/j.1471-4159.2007.04952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez-Lopez S. Wiskow O. Favaro R. Nicolis SK. Price DJ. Pollard SM. Smith A. Sox2 and Pax6 maintain the proliferative and developmental potential of gliogenic neural stem cells In vitro. Glia. 2011;11:1588–1599. doi: 10.1002/glia.21201. [DOI] [PubMed] [Google Scholar]
- 31.Shevde NK. Mael AA. Techniques in embryoid body formation from human pluripotent stem cells. Methods Mol Biol. 2013:535–546. doi: 10.1007/978-1-62703-128-8_33. [DOI] [PubMed] [Google Scholar]
- 32.Campos LS. Neurospheres: insights into neural stem cell biology. J Neurosci Res. 2004;6:761–769. doi: 10.1002/jnr.20333. [DOI] [PubMed] [Google Scholar]
- 33.Richardson WD. Kessaris N. Pringle N. Oligodendrocyte wars. Nat Rev Neurosci. 2006;1:11–18. doi: 10.1038/nrn1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rakic S. Zecevic N. Early oligodendrocyte progenitor cells in the human fetal telencephalon. Glia. 2003;2:117–127. doi: 10.1002/glia.10140. [DOI] [PubMed] [Google Scholar]
- 35.Ventura RE. Goldman JE. Telencephalic oligodendrocytes battle it out. Nat Neurosci. 2006;2:153–154. doi: 10.1038/nn0206-153. [DOI] [PubMed] [Google Scholar]
- 36.Kessaris N. Fogarty M. Iannarelli P. Grist M. Wegner M. Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;2:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qi Y. Stapp D. Qiu M. Origin and molecular specification of oligodendrocytes in the telencephalon. Trends Neurosci. 2002;5:223–225. doi: 10.1016/s0166-2236(02)02145-8. [DOI] [PubMed] [Google Scholar]
- 38.Flandin P. Kimura S. Rubenstein JL. The progenitor zone of the ventral medial ganglionic eminence requires Nkx2-1 to generate most of the globus pallidus but few neocortical interneurons. J Neurosci. 2010;8:2812–2823. doi: 10.1523/JNEUROSCI.4228-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corbin JG. Rutlin M. Gaiano N. Fishell G. Combinatorial function of the homeodomain proteins Nkx2.1 and Gsh2 in ventral telencephalic patterning. Development. 2003;20:4895–4906. doi: 10.1242/dev.00717. [DOI] [PubMed] [Google Scholar]
- 40.Petryniak MA. Potter GB. Rowitch DH. Rubenstein JL. Dlx1 and Dlx2 control neuronal versus oligodendroglial cell fate acquisition in the developing forebrain. Neuron. 2007;3:417–433. doi: 10.1016/j.neuron.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goulburn AL. Alden D. Davis RP. Micallef SJ. Ng ES. Yu QC. Lim SM. Soh CL. Elliott DA, et al. A targeted NKX2.1 human embryonic stem cell reporter line enables identification of human basal forebrain derivatives. Stem Cells. 2011;3:462–473. doi: 10.1002/stem.587. [DOI] [PubMed] [Google Scholar]
- 42.Kozubenko N. Turnovcova K. Kapcalova M. Butenko O. Anderova M. Rusnakova V. Kubista M. Hampl A. Jendelova P. Sykova E. Analysis of in vitro and in vivo characteristics of human embryonic stem cell-derived neural precursors. Cell Transplant. 2010;4:471–486. doi: 10.3727/096368909X484707. [DOI] [PubMed] [Google Scholar]
- 43.Itsykson P. Ilouz N. Turetsky T. Goldstein RS. Pera MF. Fishbein I. Segal M. Reubinoff BE. Derivation of neural precursors from human embryonic stem cells in the presence of noggin. Mol Cell Neurosci. 2005;1:24–36. doi: 10.1016/j.mcn.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Rowitch DH. Kriegstein AR. Developmental genetics of vertebrate glial-cell specification. Nature. 2010;7321:214–222. doi: 10.1038/nature09611. [DOI] [PubMed] [Google Scholar]
- 45.Richardson WD. Smith HK. Sun T. Pringle NP. Hall A. Woodruff R. Oligodendrocyte lineage and the motor neuron connection. Glia. 2000;2:136–142. doi: 10.1002/(sici)1098-1136(20000115)29:2<136::aid-glia6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 46.Takebayashi H. Nabeshima Y. Yoshida S. Chisaka O. Ikenaka K. The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr Biol. 2002;13:1157–1163. doi: 10.1016/s0960-9822(02)00926-0. [DOI] [PubMed] [Google Scholar]
- 47.Miyoshi G. Butt SJ. Takebayashi H. Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci. 2007;29:7786–7798. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kitada M. Rowitch DH. Transcription factor co-expression patterns indicate heterogeneity of oligodendroglial subpopulations in adult spinal cord. Glia. 2006;1:35–46. doi: 10.1002/glia.20354. [DOI] [PubMed] [Google Scholar]
- 49.Hu BY. Du ZW. Li XJ. Ayala M. Zhang SC. Human oligodendrocytes from embryonic stem cells: conserved SHH signaling networks and divergent FGF effects. Development. 2009;9:1443–1452. doi: 10.1242/dev.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Axell MZ. Zlateva S. Curtis M. A method for rapid derivation and propagation of neural progenitors from human embryonic stem cells. J Neurosci Methods. 2009;2:275–284. doi: 10.1016/j.jneumeth.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 51.Doetsch F. Petreanu L. Caille I. Garcia-Verdugo JM. Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;6:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 52.Martens DJ. Seaberg RM. van der Kooy D. In vivo infusions of exogenous growth factors into the fourth ventricle of the adult mouse brain increase the proliferation of neural progenitors around the fourth ventricle and the central canal of the spinal cord. Eur J Neurosci. 2002;6:1045–1057. doi: 10.1046/j.1460-9568.2002.02181.x. [DOI] [PubMed] [Google Scholar]
- 53.Ma W. Tavakoli T. Derby E. Serebryakova Y. Rao MS. Mattson MP. Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev Biol. 2008;8:90. doi: 10.1186/1471-213X-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nery S. Wichterle H. Fishell G. Sonic hedgehog contributes to oligodendrocyte specification in the mammalian forebrain. Development. 2001;4:527–540. doi: 10.1242/dev.128.4.527. [DOI] [PubMed] [Google Scholar]
- 55.Wilson PG. Stice SS. Development and differentiation of neural rosettes derived from human embryonic stem cells. Stem Cell Rev. 2006;1:67–77. doi: 10.1007/s12015-006-0011-1. [DOI] [PubMed] [Google Scholar]
- 56.Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. 2007;10:755–765. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- 57.Engberg N. Kahn M. Petersen DR. Hansson M. Serup P. Retinoic acid synthesis promotes development of neural progenitors from mouse embryonic stem cells by suppressing endogenous, Wnt-dependent nodal signaling. Stem Cells. 2010;9:1498–1509. doi: 10.1002/stem.479. [DOI] [PubMed] [Google Scholar]
- 58.Danesin C. Agius E. Escalas N. Ai X. Emerson C. Cochard P. Soula C. Ventral neural progenitors switch toward an oligodendroglial fate in response to increased Sonic hedgehog (Shh) activity: involvement of Sulfatase 1 in modulating Shh signaling in the ventral spinal cord. J Neurosci. 2006;19:5037–5048. doi: 10.1523/JNEUROSCI.0715-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bastida MF. Sheth R. Ros MA. A BMP-Shh negative-feedback loop restricts Shh expression during limb development. Development. 2009;22:3779–3789. doi: 10.1242/dev.036418. [DOI] [PubMed] [Google Scholar]
- 60.Temple S. Raff MC. Clonal analysis of oligodendrocyte development in culture: evidence for a developmental clock that counts cell divisions. Cell. 1986;5:773–779. doi: 10.1016/0092-8674(86)90843-3. [DOI] [PubMed] [Google Scholar]
- 61.Ibarrola N. Mayer-Proschel M. Rodriguez-Pena A. Noble M. Evidence for the existence of at least two timing mechanisms that contribute to oligodendrocyte generation in vitro. Dev Biol. 1996;1:1–21. doi: 10.1006/dbio.1996.0280. [DOI] [PubMed] [Google Scholar]
- 62.Liu X. Li Y. Zhang Y. Lu Y. Guo W. Liu P. Zhou J. Xiang Z. He C. SHP-2 promotes the maturation of oligodendrocyte precursor cells through Akt and ERK1/2 signaling in vitro. PLoS One. 2011;6:e21058. doi: 10.1371/journal.pone.0021058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Noble M. Proschel C. Mayer-Proschel M. Getting a GR(i)P on oligodendrocyte development. Dev Biol. 2004;1:33–52. doi: 10.1016/j.ydbio.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 64.Yu WP. Collarini EJ. Pringle NP. Richardson WD. Embryonic expression of myelin genes: evidence for a focal source of oligodendrocyte precursors in the ventricular zone of the neural tube. Neuron. 1994;6:1353–1362. doi: 10.1016/0896-6273(94)90450-2. [DOI] [PubMed] [Google Scholar]
- 65.Gregori N. Proschel C. Noble M. Mayer-Proschel M. The tripotential glial-restricted precursor (GRP) cell and glial development in the spinal cord: generation of bipotential oligodendrocyte-type-2 astrocyte progenitor cells and dorsal-ventral differences in GRP cell function. J Neurosci. 2002;1:248–256. doi: 10.1523/JNEUROSCI.22-01-00248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002;6:451–467. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 67.Wilson HC. Scolding NJ. Raine CS. Co-expression of PDGF alpha receptor and NG2 by oligodendrocyte precursors in human CNS and multiple sclerosis lesions. J Neuroimmunol. 2006;1–2:162–173. doi: 10.1016/j.jneuroim.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 68.Boda E. Vigano F. Rosa P. Fumagalli M. Labat-Gest V. Tempia F. Abbracchio MP. Dimou L. Buffo A. The GPR17 receptor in NG2 expressing cells: focus on in vivocell maturation and participation in acute trauma and chronic damage. Glia. 2011;12:1958–1973. doi: 10.1002/glia.21237. [DOI] [PubMed] [Google Scholar]
- 69.Zhu X. Zuo H. Maher BJ. Serwanski DR. LoTurco JJ. Lu QR. Nishiyama A. Olig2-dependent developmental fate switch of NG2 cells. Development. 2012;13:2299–2307. doi: 10.1242/dev.078873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qi Y. Cai J. Wu Y. Wu R. Lee J. Fu H. Rao M. Sussel L. Rubenstein J. Qiu M. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development. 2001;14:2723–2733. doi: 10.1242/dev.128.14.2723. [DOI] [PubMed] [Google Scholar]
- 71.Awatramani R. Beesley J. Yang H. Jiang H. Cambi F. Grinspan J. Garbern J. Kamholz J. Gtx, an oligodendrocyte-specific homeodomain protein, has repressor activity. J Neurosci Res. 2000;4:376–387. doi: 10.1002/1097-4547(20000815)61:4<376::AID-JNR4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 72.Southwood C. He C. Garbern J. Kamholz J. Arroyo E. Gow A. CNS myelin paranodes require Nkx6-2 homeoprotein transcriptional activity for normal structure. J Neurosci. 2004;50:11215–11225. doi: 10.1523/JNEUROSCI.3479-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stolt CC. Rehberg S. Ader M. Lommes P. Riethmacher D. Schachner M. Bartsch U. Wegner M. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 2002;2:165–170. doi: 10.1101/gad.215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li H. Lu Y. Smith HK. Richardson WD. Olig1 and Sox10 interact synergistically to drive myelin basic protein transcription in oligodendrocytes. J Neurosci. 2007;52:14375–14382. doi: 10.1523/JNEUROSCI.4456-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ligon KL. Kesari S. Kitada M. Sun T. Arnett HA. Alberta JA. Anderson DJ. Stiles CD. Rowitch DH. Development of NG2 neural progenitor cells requires Olig gene function. Proc Natl Acad Sci U S A. 2006;20:7853–7858. doi: 10.1073/pnas.0511001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arnett HA. Fancy SP. Alberta JA. Zhao C. Plant SR. Kaing S. Raine CS. Rowitch DH. Franklin RJ. Stiles CD. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science. 2004;5704:2111–2115. doi: 10.1126/science.1103709. [DOI] [PubMed] [Google Scholar]
- 77.Jakovcevski I. Zecevic N. Olig transcription factors are expressed in oligodendrocyte and neuronal cells in human fetal CNS. J Neurosci. 2005;44:10064–10073. doi: 10.1523/JNEUROSCI.2324-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Balasubramaniyan V. Timmer N. Kust B. Boddeke E. Copray S. Transient expression of Olig1 initiates the differentiation of neural stem cells into oligodendrocyte progenitor cells. Stem Cells. 2004;6:878–882. doi: 10.1634/stemcells.22-6-878. [DOI] [PubMed] [Google Scholar]
- 79.Benoit BO. Savarese T. Joly M. Engstrom CM. Pang L. Reilly J. Recht LD. Ross AH. Quesenberry PJ. Neurotrophin channeling of neural progenitor cell differentiation. J Neurobiol. 2001;4:265–280. [PubMed] [Google Scholar]
- 80.Craig CG. Tropepe V. Morshead CM. Reynolds BA. Weiss S. van der Kooy D. In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J Neurosci. 1996;8:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Plemel JR. Chojnacki A. Sparling JS. Liu J. Plunet W. Duncan GJ. Park SE. Weiss S. Tetzlaff W. Platelet-derived growth factor-responsive neural precursors give rise to myelinating oligodendrocytes after transplantation into the spinal cords of contused rats and dysmyelinated mice. Glia. 2011;12:1891–1910. doi: 10.1002/glia.21232. [DOI] [PubMed] [Google Scholar]
- 82.Rao RC. Boyd J. Padmanabhan R. Chenoweth JG. McKay RD. Efficient serum-free derivation of oligodendrocyte precursors from neural stem cell-enriched cultures. Stem Cells. 2009;1:116–125. doi: 10.1634/stemcells.2007-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hu JG. Fu SL. Wang YX. Li Y. Jiang XY. Wang XF. Qiu MS. Lu PH. Xu XM. Platelet-derived growth factor-AA mediates oligodendrocyte lineage differentiation through activation of extracellular signal-regulated kinase signaling pathway. Neuroscience. 2008;1:138–147. doi: 10.1016/j.neuroscience.2007.10.050. [DOI] [PubMed] [Google Scholar]
- 84.Hu JG. Wang YX. Wang HJ. Bao MS. Wang ZH. Ge X. Wang FC. Zhou JS. Lu HZ. PDGF-AA mediates B104CM-induced oligodendrocyte precursor cell differentiation of embryonic neural stem cells through Erk, PI3K, and p38 signaling. J Mol Neurosci. 2012;3:644–653. doi: 10.1007/s12031-011-9652-x. [DOI] [PubMed] [Google Scholar]
- 85.Stankoff B. Aigrot MS. Noel F. Wattilliaux A. Zalc B. Lubetzki C. Ciliary neurotrophic factor (CNTF) enhances myelin formation: a novel role for CNTF and CNTF-related molecules. J Neurosci. 2002;21:9221–9227. doi: 10.1523/JNEUROSCI.22-21-09221.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carson MJ. Behringer RR. Brinster RL. McMorris FA. Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron. 1993;4:729–740. doi: 10.1016/0896-6273(93)90173-o. [DOI] [PubMed] [Google Scholar]
- 87.Rivera FJ. Kandasamy M. Couillard-Despres S. Caioni M. Sanchez R. Huber C. Weidner N. Bogdahn U. Aigner L. Oligodendrogenesis of adult neural progenitors: differential effects of ciliary neurotrophic factor and mesenchymal stem cell derived factors. J Neurochem. 2008;3:832–843. doi: 10.1111/j.1471-4159.2008.05674.x. [DOI] [PubMed] [Google Scholar]
- 88.Hsieh J. Aimone JB. Kaspar BK. Kuwabara T. Nakashima K. Gage FH. IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J Cell Biol. 2004;1:111–122. doi: 10.1083/jcb.200308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zaka M. Rafi MA. Rao HZ. Luzi P. Wenger DA. Insulin-like growth factor-1 provides protection against psychosine-induced apoptosis in cultured mouse oligodendrocyte progenitor cells using primarily the PI3K/Akt pathway. Mol Cell Neurosci. 2005;3:398–407. doi: 10.1016/j.mcn.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 90.Hu J. Deng L. Wang X. Xu XM. Effects of extracellular matrix molecules on the growth properties of oligodendrocyte progenitor cells in vitro. J Neurosci Res. 2009;13:2854–2862. doi: 10.1002/jnr.22111. [DOI] [PubMed] [Google Scholar]
- 91.O'Meara RW. Michalski JP. Kothary R. Integrin signaling in oligodendrocytes and its importance in CNS myelination. J Signal Transduct. 2011:354091. doi: 10.1155/2011/354091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blaschuk KL. Frost EE. ffrench-Constant C. The regulation of proliferation and differentiation in oligodendrocyte progenitor cells by alphaV integrins. Development. 2000;9:1961–1969. doi: 10.1242/dev.127.9.1961. [DOI] [PubMed] [Google Scholar]
- 93.Li Y. Gautam A. Yang J. Qiu L. Melkoumian Z. Weber J. Telukuntla L. Srivastava R. Whiteley EM. Brandenberger R. Differentiation of oligodendrocyte progenitor cells from human embryonic stem cells on vitronectin-derived synthetic peptide acrylate surface. Stem Cells Dev. 2013;22:1497–1505. doi: 10.1089/scd.2012.0508. [DOI] [PubMed] [Google Scholar]
- 94.Calaora V. Rogister B. Bismuth K. Murray K. Brandt H. Leprince P. Marchionni M. Dubois-Dalcq M. Neuregulin signaling regulates neural precursor growth and the generation of oligodendrocytes in vitro. J Neurosci. 2001;13:4740–4751. doi: 10.1523/JNEUROSCI.21-13-04740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ogawa S. Tokumoto Y. Miyake J. Nagamune T. Induction of oligodendrocyte differentiation from adult human fibroblast-derived induced pluripotent stem cells. In Vitro Cell Dev Biol Anim. 2011;7:464–469. doi: 10.1007/s11626-011-9435-2. [DOI] [PubMed] [Google Scholar]
- 96.Pouya A. Satarian L. Kiani S. Javan M. Baharvand H. Human induced pluripotent stem cells differentiation into oligodendrocyte progenitors and transplantation in a rat model of optic chiasm demyelination. PLoS One. 2011;11:e27925. doi: 10.1371/journal.pone.0027925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang S. Bates J. Li X. Schanz S. Chandler-Militello D. Levine C. Maherali N. Studer L. et al K. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013;2:252–264. doi: 10.1016/j.stem.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Araki R. Uda M. Hoki Y. Sunayama M. Nakamura M. Ando S. Sugiura M. Ideno H. Shimada A. Nifuji A. Abe M. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013 doi: 10.1038/nature11807. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 99.Zhang YW. Denham J. Thies RS. Oligodendrocyte progenitor cells derived from human embryonic stem cells express neurotrophic factors. Stem Cells Dev. 2006;6:943–952. doi: 10.1089/scd.2006.15.943. [DOI] [PubMed] [Google Scholar]
- 100.Tokumoto Y. Ogawa S. Nagamune T. Miyake J. Comparison of efficiency of terminal differentiation of oligodendrocytes from induced pluripotent stem cells versus embryonic stem cells in vitro. J Biosci Bioeng. 2010;6:622–628. doi: 10.1016/j.jbiosc.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 101.Hu BY. Weick JP. Yu J. Ma LX. Zhang XQ. Thomson JA. Zhang SC. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A. 2010;9:4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tavakoli T. Xu X. Derby E. Serebryakova Y. Reid Y. Rao MS. Mattson MP. Ma W. Self-renewal and differentiation capabilities are variable between human embryonic stem cell lines I3, I6 and BG01V. BMC Cell Biol. 2009;10:44. doi: 10.1186/1471-2121-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Osafune K. Caron L. Borowiak M. Martinez RJ. Fitz-Gerald CS. Sato Y. Cowan CA. Chien KR. Melton DA. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol. 2008;3:313–315. doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]
- 104.Wu H. Xu J. Pang ZP. Ge W. Kim KJ. Blanchi B. Chen C. Sudhof TC. Sun YE. Integrative genomic and functional analyses reveal neuronal subtype differentiation bias in human embryonic stem cell lines. Proc Natl Acad Sci U S A. 2007;34:13821–13826. doi: 10.1073/pnas.0706199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aguirre A. Gallo V. Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone. J Neurosci. 2004;46:10530–10541. doi: 10.1523/JNEUROSCI.3572-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rivers LE. Young KM. Rizzi M. Jamen F. Psachoulia K. Wade A. Kessaris N. Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;12:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Okamura RM. Lebkowski J. Au M. Priest CA. Denham J. Majumdar AS. Immunological properties of human embryonic stem cell-derived oligodendrocyte progenitor cells. J Neuroimmunol. 2007;1–2:134–144. doi: 10.1016/j.jneuroim.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 108.Erceg S. Ronaghi M. Oria M. Rosello MG. Arago MA. Lopez MG. Radojevic I. Moreno-Manzano V. Rodriguez-Jimenez FJ, et al. Transplanted oligodendrocytes and motoneuron progenitors generated from human embryonic stem cells promote locomotor recovery after spinal cord transection. Stem Cells. 2010;9:1541–1549. doi: 10.1002/stem.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Letzen BS. Liu C. Thakor NV. Gearhart JD. All AH. Kerr CL. MicroRNA expression profiling of oligodendrocyte differentiation from human embryonic stem cells. PLoS One. 2010;5:e10480. doi: 10.1371/journal.pone.0010480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chaerkady R. Letzen B. Renuse S. Sahasrabuddhe NA. Kumar P. All AH. Thakor NV. Delanghe B. Gearhart JD. Pandey A. Kerr CL. Quantitative temporal proteomic analysis of human embryonic stem cell differentiation into oligodendrocyte progenitor cells. Proteomics. 2011;20:4007–4020. doi: 10.1002/pmic.201100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Totoiu MO. Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol. 2005;4:373–383. doi: 10.1002/cne.20517. [DOI] [PubMed] [Google Scholar]
- 112.Cloutier F. Siegenthaler MM. Nistor G. Keirstead HS. Transplantation of human embryonic stem cell-derived oligodendrocyte progenitors into rat spinal cord injuries does not cause harm. Regen Med. 2006;4:469–479. doi: 10.2217/17460751.1.4.469. [DOI] [PubMed] [Google Scholar]
- 113.Hatch MN. Schaumburg CS. Lane TE. Keirstead HS. Endogenous remyelination is induced by transplant rejection in a viral model of multiple sclerosis. J Neuroimmunol. 2009;1–2:74–81. doi: 10.1016/j.jneuroim.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 114.Fancy SP. Harrington EP. Yuen TJ. Silbereis JC. Zhao C. Baranzini SE. Bruce CC. Otero JJ. Huang EJ, et al. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat Neurosci. 2011;8:1009–1016. doi: 10.1038/nn.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fancy SP. Glasgow SM. Finley M. Rowitch DH. Deneen B. Evidence that nuclear factor IA inhibits repair after white matter injury. Ann Neurol. 2012 doi: 10.1002/ana.23590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Arnett HA. Mason J. Marino M. Suzuki K. Matsushima GK. Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;11:1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]