To the Editor
Acute decompensated heart failure (ADHF) is the most common indication for hospital admission, particularly in the elderly, yet the identification of those with impending decompensation using conventional clinical methods is unreliable and frequently leaves insufficient lag time for therapeutic interventions (1). Exhaled breath constitutes a complex mixture of hundreds of volatile organic compounds (VOCs) that could potentially be used as a safe and noninvasive method of diagnostic and therapeutic monitoring (2). Previous research studies have identified elevated acetone, pentane, and nitric oxide levels in exhaled breath in the setting of HF correlated with disease severity (3-5). Selected ion-flow tube mass-spectrometry (SIFT-MS) combines a fast flow tube technique with quantitative mass spectrometry that is ideally suited for exhaled breath analysis because it allows for the analysis of small and humid samples without the need for cumbersome sample preparation or calibration (6). Scan times are relatively brief, thus facilitating high throughput and serial comparisons. Using this technology, we conducted a prospective, single-center cohort study to assess the feasibility of exhaled breath analysis to identify patients admitted for ADHF. The study protocol was approved by the Cleveland Clinic Institutional Review Board. We recruited 25 consecutive patients admitted with ADHF as their primary diagnosis (mean left ventricular ejection fraction 27 ± 13%, median N-terminal pro–B-type natriuretic peptide level 954 pg/ml) and a control group of 16 subjects admitted with non-ADHF cardiovascular diagnoses and who had no clinical evidence of systemic or venous congestion at the time of enrollment. Indications for hospitalization in the control group included unstable angina or non–ST-segment elevation myocardial infarction (6 of 16), conduction disorders (3 of 16), hypertensive emergency (3 of 16), atrial tachyarrhythmia (2 of 16), or stable angina (2 of 16). All analyses were performed using JMP Pro 9.0 (SAS Institute, Cary, North Carolina). As expected, there were significant (p < 0.01) baseline differences in the frequency of hypertension (54% vs. 100%) and baseline estimated glomerular filtration rate (68 ± 43 ml/min/1.73 m2 vs. 102 ± 44 ml/min/1.73 m2), which were significantly worse in the ADHF versus control group. Nevertheless, there were no significant differences between groups in age, body mass index, or several comorbidities (i.e., diabetes mellitus, chronic obstructive pulmonary disease, active smoking) theorized to result in alterations in the exhaled metabolome.
After written informed consent was obtained, exhaled breath samples were collected within 24 h of hospital admission and following an 8-h fast and before the administration of morning pharmacotherapy. Samples were collected after tap water mouth rinse to minimize and standardize the contribution of the aerodigestive tract. Patients provided a single exhaled vital capacity into a sterile mouthpiece while attempting to maintain an exhaled pressure of 15 millibars. All breath analyses were performed within 2 h of collection using a VOICE200 SIFT-MS instrument (Syft Technologies, Christchurch, New Zealand). Pre-specified quantitative assessment of acetone and pentane was performed using the SIFT-MS technique. Sample collection was well tolerated even among patients with disease severity warranting intensive care unit admission and invasive hemodynamic monitoring. Confirming previous reports (3,4), we observed increased exhaled acetone (median [interquartile range]: 811 [256 to 1974] ppb vs. 187 [115 to 572] ppb, p = 0.01) and pentane (40 [20 to 74] ppb vs. 22 [14 to 36] ppb, p = 0.03) levels in ADHF versus control groups. In addition, mass scanning of ion products for H3O+, O2+, and NO+ from 14 to 200 atomic mass units was performed, and stepwise variable selection was used to identify 5 ion peaks that were incorporated into a canonical discriminant analysis model that successfully distinguished ADHF from control patients (−2 log likelihood 0.038; Wilks’ Lambda 0.102 [p < 0.0001]) (Fig. 1). This “breathprint” was then tested in an independent validation cohort of 36 consecutive ADHF subjects with identical enrollment criteria to the derivation cohort. The discriminant analysis model correctly classified all subjects with no misclassifications (Fig. 1).
Figure 1. Canonical Discriminant Analysis: ADHF Versus Non-HF Controls.
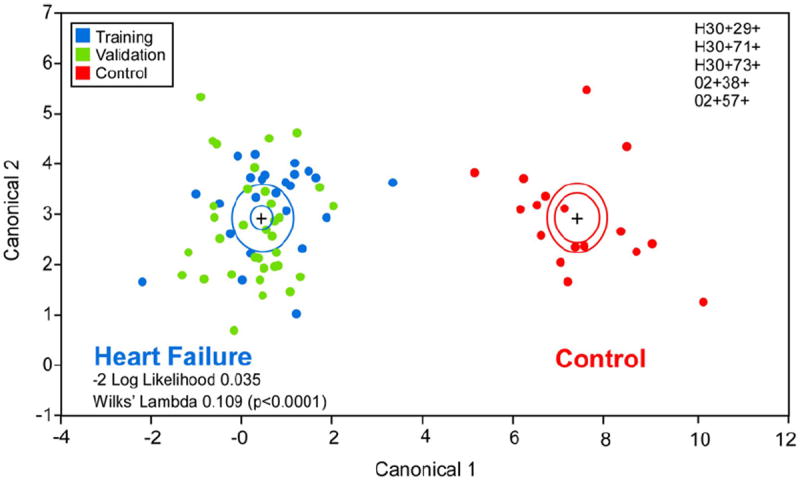
Canonical discriminant analysis using 5 selected mass scanning ion peaks was performed in a training cohort of 25 acute decompensated heart failure (ADHF) subjects (blue) and 16 controls (red). This ADHF “breathprint” was then used to classify an independent validation cohort of 36 ADHF subjects (green) with no misclassifications.
The exhaled metabolome is a temporally dynamic complex mixture, and therefore breath analysis is highly susceptible to the confounding effects of timing and context of sample collection. In addition, methods of metabolomic data analysis (e.g., data reduction techniques) are still being formalized and will need to be refined to facilitate reproducibility and generalizability of future studies. Results of this study are limited by the small sample size, and larger prospective studies are needed to validate these results. Nevertheless, our findings demonstrate the feasibility of single exhaled breath analysis in ADHF. In addition, we validated the previously reported alterations in acetone and pentane VOC concentrations in ADHF, and provided pilot evidence to support the hypothesis that a unique ADHF breathprint exists using SIFT-MS technology. Once a specific VOC or panel of VOCs is identified, highly sensitive and specific solid-state sensors can be integrated into portable detectors. Like conventionally available exhaled breath sensors, the promise of this technology lies in the potential for point-of-care and ambulatory monitoring and screening. Future studies in exhaled breath metabolomics are needed to accelerate progress in the field of cardiovascular medicine.
Acknowledgments
This research is supported by BRCP 08-049 Third Frontier Program grant from the Ohio Department of Development (Dr. Dweik), and National Institutes of Health grants R01HL103931 (Dr. Tang) and P20HL113452 (Dr. Tang), HL107147 (Dr. Dweik), HL081064 (Dr. Dweik), HL103453 (Dr. Dweik), HL109250 (Dr. Dweik), and RR026231 (Dr. Dweik). Dr. Tang has received institutional financial support from Abbott Laboratories for an investigator-initiated study. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Samara MA, Tang WH. Device monitoring strategies in acute heart failure syndromes. Heart Fail Rev. 2011;16:491–502. doi: 10.1007/s10741-011-9236-4. [DOI] [PubMed] [Google Scholar]
- 2.Cikach FS, Jr, Dweik RA. Cardiovascular biomarkers in exhaled breath. Prog Cardiovasc Dis. 2012;55:34–43. doi: 10.1016/j.pcad.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcondes-Braga FG, Gutz IG, Batista GL, et al. Exhaled acetone as a new biomaker of heart failure severity. Chest. 2012;142:457–66. doi: 10.1378/chest.11-2892. [DOI] [PubMed] [Google Scholar]
- 4.Sobtoka PA, Brottman MD, Weitz Z, Birnbaum AJ, Skosey JL, Zarling EJ. Elevated breath pentane in heart failure reduced by free radical scavenger. Free Radic Biol Med. 1995;18:377–9. doi: 10.1016/0891-5849(93)90145-k. [DOI] [PubMed] [Google Scholar]
- 5.Schuster A, Thakur A, Wang Z, Borowski AG, Thomas JD, Tang WH. Increased exhaled nitric oxide levels after exercise in patients with chronic systolic heart failure with pulmonary venous hypertension. J Card Fail. 2012;18:799–803. doi: 10.1016/j.cardfail.2012.08.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith D, Spanel P. Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis. Mass Spectrom Rev. 2005;24:661–700. doi: 10.1002/mas.20033. [DOI] [PubMed] [Google Scholar]


