Abstract
d-chiro-Inositol (DCI) and pinitol (1D-3-O-methyl-chiro-inositol) are distinctive inositols reported to possess insulin-mimetic properties. DCI-containing compounds were abundant in common laboratory animal feed. By GC-MS of 6 M-HCl hydrolysates, Purina Laboratory Rodent Diet 5001 (diet 5001) contained 0.23% total DCI by weight with most found in the Lucerne and soy meal components. In contrast, only traces of l-chiro-inositol were observed. The DCI moiety was present in a water-soluble non-ionic form of which most was shown to be pinitol. To measure the absorption of dietary inositols, rats were fed diet 5001 in a balance study or given purified pinitol or [2H6]DCI. More than 98% of the total DCI fed to rats as diet 5001, purified pinitol or [2H6]DCI was absorbed from the gastrointestinal tract. Rats chronically on diet 5001 consumed 921 μmol total DCI/kg body weight pet d but excreted less than 5.3% in the stool and urine, suggesting that the bulk was metabolized. The levels of pinitol or DCI in plasma, stool, or urine remained relatively stable in mice fed Purina PicoLab® Rodent Diet 20 5053 over a 5-week period, whereas these values declined to very low levels in mice fed a pinitol/DCI-deficient chemically-defined diet. To test whether DCI was synthesized or converted from myo-inositol, mice were treated with heavy water or [2H6]myo-inositol. DCI was neither synthesized endogenously from 2H-labelled water nor converted from [2H6]myo-inositol. DCI and pinitol in rodents appear to be derived solely from the diet.
Keywords: Pinitol, Insulin, Mass spectrometry, Defined diet
Introduction
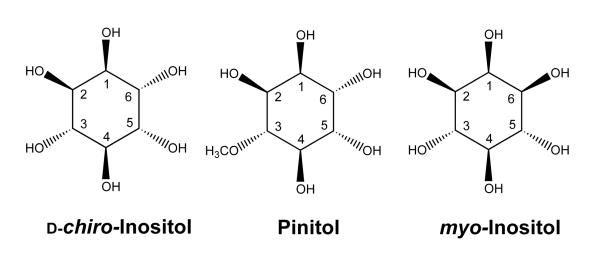
The mechanism of insulin action is still incompletely understood and the subject of active investigation. After insulin binds to its cell surface receptor, many metabolic pathways are activated. Interest in these secondary signaling systems has resulted in the isolation of certain inositol phosphoglycan molecules which mimic some actions of insulin, especially those related to glucose oxidation and lipid metabolism(1-5). Interestingly, the inositol present in some of these putative insulin mediator preparations was predominantly or solely d-chiro-inositol (DCI) (Fig. 1), an epimer of myo-inositol (MI), which is the predominant mammalian inositol(6,7). DCI differs from MI only in the orientation of a single hydroxyl group (labelled 6 in both DCI and MI). It is this hydroxyl group by which MI is attached to phosphatidic acid in phosphatidylinositol. The prefix “chiro” refers to the fact that this inositol has optically-active D and L enantiomers. chiro- Inositol also has been identified in preparations derived from the phosphatidylinositol anchors of membrane proteins(8-10). Both the chemical structure and the occurrence of DCI in insulin signaling molecules suggest that it may have a physiological role in mammals. Pinitol (1D-3-O-methyl-chiro-inositol) is structurally related to DCI, having a methoxyl group at position 3 (Fig. 1). Pinitol was originally described in 1855 as a component of the sugar pine tree(11). It is also a prominent component of animal forage legumes(12) and is found in beans including soybeans(13,14).
Fig. 1.
Structures of inositols: d-chiro-inositol (DCI); pinitol; and myo-inositol. DCI is numbered as recommended by the International Union of Pure and Applied Chemistry(34). Pinitol and myo-inositol according to the convention for DCI.
Diabetic patients (both type 1 and type 2) have increased urinary excretion of DCI, the magnitude of which correlates with plasma glucose and glycated hemoglobin levels(15). Pima Indians with type 2 diabetes have low levels of muscle chiro-inositol(16). A similar abnormality in chiro-inositol excretion is found in diabetic db/db mice and streptozotocin diabetic rats(17). Although not all clinical trials have been positive(18), at least some insulin-resistant patients appear to have responded to treatment with DCI or pinitol with improved insulin sensitivity(19,20).
Studies of animal and human treatment with pinitol or DCI have been limited by lack of experimental control of dietary intake and incomplete knowledge of DCI metabolism. MI, a component of mixed diets(21), has been shown to be actively absorbed in the small intestine of hamsters(22) and also is synthesized from glucose(23-25). However, there is no information about DCI absorption, and previous work on the possible bioconversion of DCI from MI has not given consistent results. Inositol epimerase from bovine brain converted MI to neo- and scyllo-inositol but not to chiro-inositol(26). In cockroach fat body extracts, however, epimerization of MI to chiro-inositol was observed(27). Injection of tritiated MI into rats showed conversion of MI to chiro-inositol as judged by counts comigrating with chiro-inositol(28).
In the present study we analyzed dietary inositol contents of commercial animal diets and studied the absorption of pinitol and DCI and biosynthesis of DCI in rodents maintained on chemically-defined diets.
Materials and Methods
Materials
Diisopropylidene pinitol and l-chiro-inositol were gifts of Professor Laurens Anderson of the University of Wisconsin, Madison. Pinitol containing less than 1% DCI was prepared from its isopropylidene derivative by hydrolysis in 0.1 N HCl for 1 hour at 110°C, followed by lyophilization and recrystallization from 90% ethanol(29). DCI was purchased from Calbiochem. dl-1,2,3,4,5,6-[2H6]chiro-inositol(30) was the gift of Dr. Ken Sasaki, Connaught Centre for Biotechnology Research, Toronto, Canada. 1,2,3,4,5,6-[2H6]MI was purchased from MSD Isotopes, Montreal, Quebec. [2H3]Pinitol labelled in the methyl radical was a gift of Dr. Andrew Falshaw of Industrial Research Limited, Lower Hutt, New Zealand. Ethanol-extracted casein (C3400) was purchased from Sigma (St. Louis, MO).
Diets
Purina Laboratory Rodent Diet 5001 (5001) and Purina PicoLab Rodent Diet 20 5053 (5053) were purchased from LabDiet (Richmond, IN). Basal Diet 5755 (5755, a chemically-defined diet) was purchased from TestDiet (Richmond, IN). Components of 5001 were kindly supplied by Dr. Daniel Hopkins. The pinitol/DCI-deficient diet (Table 1) was based on 5755. The ingredients for both diets were dextrin, casein, sucrose, mineral mix, vitamin mix, cellulose, choline chloride, DL-methionine, and fat. Corn oil (5%) and lard (5%) were used in 5755 whereas only soybean oil (5%) was used in our pinitol/DCI-deficient diet. Components of 5755 were analyzed for total DCI and levels were generally very low. The original powdered cellulose contained 0.014 nMol/mg total DCI and was replaced by Avicel® cellulose (FMC Biopolymer, Philadelphia, PA), which contained non-detectable total DCI.
Table 1.
Pinitol/DCI-deficient diet formulation
| Ingredients | Source | g/kg |
|---|---|---|
| Dextrin | TestDiet* | 393.7 |
| Casein | Sigma† | 248.8 |
| Sucrose | TestDiet* | 200.0 |
| Soybean oil | Sam’s Club‡ | 50.0 |
| Cellulose | FMC Biopolymerζ | 33.2 |
| Choline chloride | TestDiet* | 2.0 |
| DL-Methione | TestDiet* | 2.0 |
| Mineral Mix∥ | TestDiet* | 50.0 |
| Vitamin Mix¶ | TestDiet* | 50.0 |
ppm, Parts per million.
Richmond, IN, USA.
St Louis, MO, USA
Head office: Bentonville, AR, USA
Philadelphia, PA, USA.
Final concentration of Mineral Mix components in the diet, as provided by the manufacturer: Ca, 0.60%; P, 0.60%; K, 0.40%; Mg, 0.07%; Na, 0.21%; Cl, 0.24%; F, 5.0; Fe, 63.0 ppm; Zn, 21.0 ppm; Mn, 65.0 ppm; Cu, 15.0 ppm; Co, 3.2 ppm; I, 0.57 ppm; Cr, 3.0 ppm; Mo, 0.82 ppm; Se, 0.23 ppm.
Final concentration of Vitamin Mix in the diet, as provided by the manufacturer: vitamin A, 22.1 lU/g; vitamin D3, 2.2 IU/g; vitamin E, 50.1 IU/kg; vitamin K, as menadione, 10.4 ppm; thiamin hydrochloride, 20.6 ppm; riboflavin, 20.0 ppm; niacin, 90.0 ppm; pantothenic acid, 55.0 ppm; folic acid, 4.0 ppm; pyridoxine, 16.5 ppm; biotin, 0.4 ppm; vitamin B12, 20.0 μg/kg; choline chloride, 1,400 ppm; ascorbic acid, 0.0 ppm.
Gas chromatography/mass spectrometry (GC/MS)
For inositol analysis in plasma and urine, deuterated internal standards for DCI, MI and pinitol were added and they were then processed by protein precipitation and anion exchange adsorption and dried as described previously(15). Urine samples were further purified by solid phase extraction. They were taken up in 0.5 mL water, applied to a 1.0-mL SupelcleanTM LC-18 SPE column (Supelco, Bellefonte, PA) previously equilibrated with 2 mL methanol, eluted with 2 mL water and lyophilized.
Both free and total inositols in feed are reported. To isolate free inositols, dried mouse stool homogenate or 50 mg aliquots of finely ground mouse chow received all three deuterated internal standards and were extracted with 0.5 mL water at room temperature for 12 hours. For total inositols, aliquots of ground mouse chow or dried stool homogenate received 0.5 mL 6N HCl with deuterated internal standards for DCI and MI and were heated at 110°C for 24 hours in tightly-sealed tubes. Total DCI contents measured by acid hydrolysis include free/complex DCI such as inositol glycans or phosphates and free/complex pinitol since acid hydrolysis converts pinitol to DCI and hydrolyzes complex inositols to free inositols. The acid hydrolyzate was separated from charred residue, dried under nitrogen at 85°C and taken up in 0.5 mL water. Samples were treated with anion exchange resins, passed over 6 mL LC-18 solid phase extraction columns, and dried.
Lyophilized samples were derivatized for GC/MS by incubation overnight with 10% pentafluoropropionyl imidazole in acetonitrile at 65°C and then separated on a 25 m × 0.25 mm I.D. Chirasil-Val fused silica capillary column with 0.16 μm film thickness (Alltech Associates, Deerfield, IL). The oven temperature was kept at 80°C for 0.5 minute, then raised at a rate of 60°C/minute to 100°C and held for 4 minutes, and again raised at a rate of 20°C/minute to a final temperature of 185°C and held for 3.5 minutes. The effluent was analyzed in an Agilent Technologies 5973 quadrupole mass spectrometer by negative ion chemical ionization (NCI) mass spectrometry using methane as the reagent gas (Hewlett-Packard, Palo Alto, CA). Selected ion monitoring was performed at m/z 573 and 576 (for natural and deuterated pinitols, respectively) and at m/z 726 and 731 (for natural and deuterated chiro-inositols and MI).
The enrichment of deuterated water in urine was determined following alkaline exchange with acetone according to the published protocol, except that hexane rather than chloroform was used in the exchange procedure(31). Exchanged acetone was separated isothermally at 60°C for 5 minutes on a 30 m × 0.25 mm I.D. DB17-MS capillary column with 0.25 μm film thickness (J&W Scientific, Folsom, CA). Selected ion monitoring was performed at m/z 58 and 59 using electron impact ionization mode (70 eV). The incorporation of deuterium from deuterated water into MI was calculated by mass isotope distribution analysis considering both deuterium and natural carbon-13 enrichment.
Metabolic balance studies in rats fed 5001 or 5755
For metabolic balance studies, male Sprague-Dawley rats weighing 400-500 g were fed 5001 for at least 1 month, then housed individually in metabolic cages and fed powdered diets consisting of either 5001 or 5755 for 1 week. Dietary consumption was measured and urine and feces were collected for 24 hours. The total amount of DCI after HCl hydrolysis was measured by NCI GC/MS, and DCI balance was computed as the difference between intake and output. Institutional and national guidelines for the care and use of animals were followed. All procedures involving animals were approved by the Washington University Animal Studies Committee.
Effect of stool bacteria on fecal total DCI levels
Equal amounts of stools from 3 rats fed 5001 were pooled and dispersed in 0.15 M NaCl to a concentration of 0.2 g/mL and MI and glucose were added to final concentrations of 30 μM and 5.5 mM, respectively. Aliquots were incubated for 24 hours at either 4°C (control for the measurements at 37°C) or 37°C in anaerobic jars, after which internal standards were added and the samples were hydrolyzed and processed by NCI GC/MS for total DCI content.
Absorption studies in rats fed 5755
Three rats weighing 427 ± 27 g were housed individually in metabolic cages and fed 5755 for 3 days. Pinitol, 51.5 μMol (10 mg), was then given orally; urine and stools were collected for the following 2 days and analyzed for total DCI. Then 25 nMol hexadeuterated DCI was given orally followed by an additional 2 days of urine and stool collection.
Inositol clearance over time in mice fed 5053 or the pinitol/DCI-deficient diet
Male C57BL/6J mice were fed 5053 for 1 week before randomly being assigned to 5053 (n=8) or the pinitol/DCI-deficient diet (n=8) for up to 5 weeks. Food intake was recorded twice a week and body weight once a week. Plasma, stool, and urine were obtained before the experimental assignment (week 0), 1, 2, and 5 weeks after the diet assignment. For each time point, urine and stool were collected over a 24-hour period in individual metabolic cages. Pinitol, DCI, and MI levels in plasma, stool, or urine were determined by GC/MS.
Measurement of inositol biosynthesis in mice fed the pinitol/DCI-deficient diet
To measure endogenous inositols (DCI and MI) synthesis, male C57BL/6J mice (n=3) were maintained on the pinitol/DCI-deficient diet for 10 weeks. Then, mice were provided with drinking water containing 20% heavy water (deuterium oxide, catalogue number: 151882, Sigma-Aldrich) for one week to label the ring hydrogens of inositols, followed by 24-hour urine collection in individual metabolic cages.
Five weeks later, d6-MI (1 mg in water) was administered by Intraperitoneal (IP) injection to each mouse (n=3, maintained on the pinitol/DCI-deficient diet for 15 weeks before IP injection) to determine whether MI is converted into DCI in vivo. Urine was collected for 24 hours each before and after injection. Urine samples were processed and GC/MS was performed as described above.
Statistical analyses
Means ± SEM for group data are reported. Two-way Repeated-Measures ANOVA was used to analyze diet, time, and interactions between diet and time with SAS Proc GLM (V9.2, SAS Institute, Cary, NC). Multiple comparisons were performed using the Tukey adjustment.
Results
Total acid-released DCI content of Purina rodent chows
Total DCI measured by GC/MS after hydrolysis with 6 N HCl at 110°C for 24 hours was a prominent component of laboratory animal chows (Table 2). Chows 5001 and 5053 each contained more than 4 nMol total DCI/mg. Diet 5001 contained 12.9 ± 1.2 nMol total DCI/mg or 0.23% by weight. DCI was present in 5001 in an amount approximately 2/3 that of MI, which was 20.2 ± 0.7 nMol/mg or 0.36% by weight following hydrolysis. Another rodent chow 5053, currently used in our animal facility, contained comparable levels of total DCI at 10.94 ± 0.45 nMol/mg (Table 2). Diet 5755 had only trace levels of total DCI and these levels were reduced even further in the pinitol/DCI deficient diet.
Table 2.
Nutrient composition of the diets*
| Diet 5001† | Diet 5053† | Diet 5755‡ | Pinitol/DCI- deficient diet |
|
|---|---|---|---|---|
| PFV (kJ/g)ζ | 13.98 | 14.24 | 17.12 | 15.95 |
| By energy (%) | ||||
| Protein | 28.0 | 23.6 | 18.6 | 23.7 |
| Fat | 12.2 | 11.9 | 22.0 | 11.9 |
| Carbohydrates | 59.8 | 64.5 | 59.3 | 64.4 |
| By weight (%) | ||||
| Protein | 23.4 | 20.0 | 19.0 | 22.5 |
| Fat | 4.5 | 4.5 | 10.0 | 5.0 |
| SFA | 1.50 | 0.84 | 2.72 | 0.74 |
| MFA | 1.58 | 1.04 | 3.31 | 1.06 |
| PUFA | 1.42 | 2.61 | 3.97 | 3.20 |
| Carbohydrates | 49.9 | 54.8 | 60.6 | 61.3 |
| Fiber | 5.3 | 4.7 | 4.3 | 4.6 |
| Inositols (nmol/mg) | ||||
| Free pinitol | ||||
| Mean | NA | 7.09 | 0.0014 | 0.0008 |
| SEM | 0.02 | 0.0003 | 0.0001 | |
| Free DCI | ||||
| Mean | NA | 0.16 | ND∥ | ND∥ |
| SEM | 0.02 | |||
| Total DCI | ||||
| Mean | 12.9 | 10.94 | 0.0018 | 0.0012 |
| SEM | 1.2 | 0.45 | 0.0001 | 0.0003 |
| Free MI | ||||
| Mean | NA | 1.96 | 0.75 | 0.62 |
| SEM | 0.09 | 0.03 | 0.01 | |
| Total MI | ||||
| Mean | 20.2 | 41.1 | 1.07 | 0.68 |
| SEM | 0.07 | 8.6 | 0.05 | 0.06 |
Diet 5001, Purina Laboratory Rodent Diet 5001; diet 5053, Purina PicoLab® Rodent Diet 20 5053; diet 5755, TestDiet® 5755 Basal Diet; DCI, d-chiro-inositol; PFV, physiological fuel energy; NA, not analysed; ND, not detectable; MI, myo-inositol.
The mouse chows and the pinitol/DCI-deficient diet (n=3, about 50 mg each) were weighed. Free (for pinitol, DCI and MI) or total (for DCI and MI) were determined as described in Materials and Methods. The rest of nutrient composition was from the manufacturers.
Purchased from LabDiet (Richmond, IN, USA)
Purchased from TestDiet (Richmond, IN, USA)
Sum of decimal fractions of protein, fat, and carbohydrates multiplied by 16.8, 37.7, and 16.8 kJ/g, respectively.
The limit of detection for DCI is 0.0003 nmol/mg.
Analyses of chow components showed that corn, wheat, meat, fish and dairy products contributed relatively little DCI (Table 3). However, total DCI was very high in the legumes alfalfa and soybean meal in which it constituted 1.3% and 1.0% of weight, respectively, or more than 50 times as much as in other chow components. l-chiro-Inositol, in contrast, was very low in all ingredients.
Table 3.
Total chiro-inositol content of components of Purina Rodent Diet 5001
| Components (nmol/mg dry weight) | d-chiro-Inositol | l-chiro-Inositol |
|---|---|---|
| Lucerne* | ||
| Mean | 74.7 | -† |
| SEM | 5.2 | - |
| Soybean meal* | ||
| Mean | 54.1 | -† |
| SEM | 4.1 | - |
| Wheat germ | 1.011 | 0.008 |
| Ground oats | 0.758 | 0.028 |
| Fish meal | 0.676 | 0.012 |
| Ground beet pulp | 0.248 | 0.003 |
| Meat meal | 0.058 | 0.004 |
| Yeast | 0.049 | 0.014 |
| Midds | 0.049 | 0.015 |
| Ground corn | 0.044 | 0.003 |
| Bleachable fat | 0.018 | 0.001 |
| Molasses | 0.010 | 0.004 |
| Whey | 0.009 | 0.001 |
Values for Lucerne and soy for 6 replicates.
l-chiro-Inositol could not be measured accurately in the presence of the large amount of d-chiro-inositol but it constituted less than 2% of d-chiro-inositol.
The molecular form of DCI in 5001 was further investigated. Finely ground chow was suspended at room temperature in 1 N HCl, then extracted and partitioned by the method of Bligh and Dyer(32) to yield aqueous and organic fractions. Less than 1% of the total DCI was found in the organic fraction; in contrast, 83% was recovered in the aqueous fraction with the remainder in the insoluble residue. Thus, substantial amounts of DCI did not appear to be present in phospholipids and DCI was present predominantly in water-soluble form. Only 6% of the total DCI was measurable without prior strong acid hydrolysis. The species containing DCI did not appear to be ionic (such as a phytate) because there was no change in the measured concentration when the sample was treated with an AG 501-X8(D) mixed bed ion exchange resin.
Metabolic balance studies in rats fed 5001 or 5755
Rats chronically fed 5001 consumed 921 μMol/kg body weight/day or 0.17 g/kg body weight/day of total DCI (Table 4). Only 0.7% of total DCI intake was found in the stool output suggesting that gastrointestinal absorption was essentially complete. Urinary output was only 4.6% of that consumed. The total excretion from the urine and stool represents 5.3% of the total DCI intake, suggesting that the majority of the dietary inositols was metabolized. Thus, total DCI balance was strongly positive at 872.5 ± 79.0 μMol/kg body weight/day.
Table 4.
Metabolic balance of total d-chiro-inositol (DCI) in rats fed Purina Rodent Diet 5001* (Mean values with their standard errors)
| Mean | SEM | |
|---|---|---|
| Rat Weight (g) | 471 | 35 |
| Chow consumed (g/d) | 33.2 | 1.6 |
| Stool produced (g/d) | 9.4 | 0.7 |
| DCI intake (μmol/kg body weight per d) | 921.0 | 84.0 |
| DCI output (μmol/kg body weight per d) | ||
| Stools | 6.2 | 0.9 |
| Urine | 42.3 | 4.2 |
| Total | 48.5 | 8.1 |
| DCI balance (μmol/kg body weight per d) | 872.5 | 79.0 |
Rats (n=3) were fed Purina Rodent Diet 5001 for at least 1 month and housed in individual metabolic cages for 1 week. Dietary consumption was measured and urine and feces were collected for 24 hours. The total amount of DCI after HCl hydrolysis was measured by negative ion chemical ionization GC-MS, and DCI balance was computed as the difference between intake and output.
Effect of stool bacteria on fecal total DCI levels
Since bacterial degradation or production of DCI in the gastrointestinal tract could have influenced these experiments, rat stool samples were fortified with MI and glucose (possible metabolic precursors of DCI) and then incubated at 37°C anaerobically for 24 hours. A large amount of gas was produced indicating active bacterial metabolism, but little effect on measured total DCI levels was seen. There was a 9.5% decrease in total DCI after 37°C incubation (10.54 ± 0.27 nMol/mL at 4°C vs. 9.85 ± 0.19 at 37°C, p=0.052), suggesting that a small amount of degradation might have occurred. Stool l-chiro-inositol was unchanged by incubation (1.11 nMol/mL ± 0.36 at 4°C vs. 1.32 ± 0.11 at 37°C). Bacterial degradation or metabolism, therefore, could not account for the quantitative reduction of total DCI in stool compared to the diet.
Absorption studies in rats fed 5755
The gastrointestinal absorption of purified pinitol was studied next (Table 5, top). Three days before the experiment, rats were switched from 5001 to 5755 low in total DCI in order to reduce excretion of total DCI to approximately 0.5 μMol/kg body weight/day. Then 10 mg pinitol (containing 51.5 μMol total DCI) was given orally and the fecal and urinary excretion of total DCI was measured. Most of the material eliminated was excreted on the first day when fecal loss was 0.9% of intake and urinary loss was 21%. These data suggest that pinitol is absorbed from the gastrointestinal tract and most is retained or metabolized. That somewhat more total DCI appeared in urine (21%) compared to 5001 feeding (4.6%, Table 4) may be due to the large single pinitol bolus administered. The day after pinitol feeding, total excretion in urine and feces was similar to baseline.
Table 5.
Gastrointestinal absorption of pinitol and [2H6]d-chiro-inositol (DCI) in rats fed Basal Diet 5755* (Mean values with their standard errors)
| Dietary intake | Fecal output | Urinary output | Total output | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| Pinitol feeding | ||||||||
| Total DCI (μmol/kg body weight per d) | ||||||||
| Pre-pinitol | 0.09 | 0.02 | 0.07 | 0.02 | 0.48 | 0.31 | 0.54 | 0.31 |
| Pinitol, Day 1 | 122 | 7.4 | 1.11 | 0.53 | 26.0 | 1.7 | 27.11 | 0.56 |
| Pinitol, Day 2 | 0.07 | 0.01 | 0.10 | 0.01 | 0.38 | 0.11 | 0.47 | 0.12 |
| [2H6]DCI Feeding Free [2H6]DCI (nmol/kg body weight per d) |
||||||||
| Day 1 | 59.1 | 3.6 | 0.15 | 0.1 | 4.0 | 0.4 | 4.2 | 0.2 |
| Day 2 | ND | 1.0 | 1.0 | 4.3 | 0.1 | 5.3 | 1.1 | |
ND, not detectable.
Three rats weighing 427 ± 27 g were housed individually in metabolic cages and fed Purina Basal Diet 5755 for 3 d. Pinitol, 51.5 μmol (10 mg), was given orally with urine and stools collected for the following 2 d, which were analyzed for total DCI. Then 25 nmol [2H6]DCI was given orally, followed by an additional 2 d of urine and stool collection. No [2H6]DCI was detectable before isotope administration.
Similar results were obtained when labelled free DCI tracer was given orally (Table 5, bottom). Absorption, estimated by recovery of hexadeutero-DCI in the stool for two days, was 98%. The urinary output was only 14% of that administered, consistent with incomplete urinary excretion.
Inositol clearance over time in mice fed 5053 or the pinitol/DCI-deficient diet
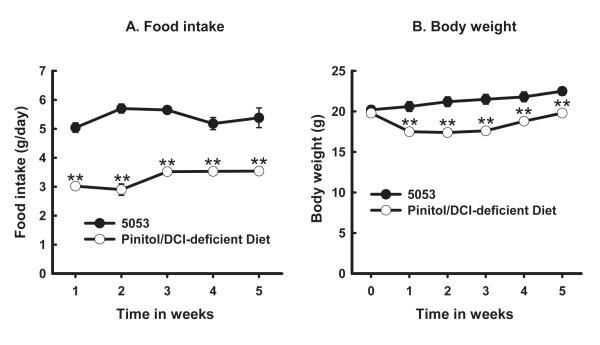
After a more defined diet was developed, we studied the clearance of inositols over a longer period of time. There were time and diet effects and interactions between time and diet effects on the food intake (Fig. 2A) and body weight (Fig. 2B). Food intakes (Fig. 2A) and body weights (Fig. 2B) were statistically different at 1, 2, 3, 4, and 5 weeks between the two diets.
Fig. 2.
Food intakes (panel A) and body weights (B) of mice fed Purina PicoLab® Rodent Diet 20 5053 (diet 5053) (solid black circles) or pinitol/DCI-deficient Diet (empty circles). Male C57BL/6J mice (n=8 for each diet) were fed 5053 or pinitol/DCI-deficient diet for 5 weeks. Food intake was measured twice a week and their average represents weekly food intake (g/day). Body weight was determined at the beginning and once a week. Effects of diet (P=0.0002), time (P<0.0001), and interactions between time and diet (P<0.0001) were statistically significant. The levels of statistical differences between 5053 and the pinitol/DCI-deficient diet at each time point are indicated by **P < 0.001.
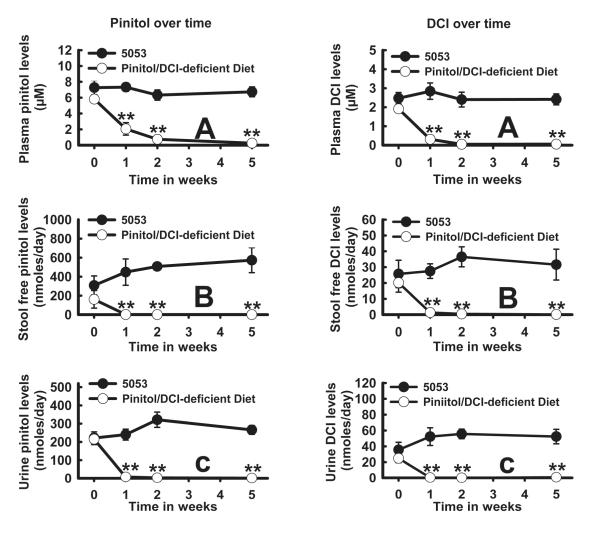
Both pinitol (Fig. 3, left panel) and DCI (Fig. 3, right panel) in plasma, stool, or urine remained relatively stable over the 5-week period for the mice fed 5053. In contrast, both pinitol and DCI in these various compartments declined rapidly to a very low level over the 5-week period in mice fed the pinitol/DCI-deficient diet. Repeated-measures ANOVA showed that there was a diet effect on pinitol and DCI concentrations in plasma, stool, or urine (Fig. 3). This indicates that the diet plays an important role in pinitol or DCI levels. A time effect was observed for all measurements except for stool pinitol, DCI, or urine DCI. A time and diet interaction was seen for all parameters except for stool DCI. The differences at weeks 1, 2, and 5 between 5053 and the pinitol/DCI-deficient diet in all inositol measurements were statistically significant (P < 0.001).
Fig. 3.
Plasma, stool, or urine inositols over time in mice fed Purina PicoLab® Rodent Diet 20 5053 (5053) (solid black circles) or Pinitol/DCI-deficient Diet (empty circles). Male C57BL/6J (n=8 for each diet) were fed 5053 or Pinitol/DCI-deficient diet for 5 weeks. Blood, urine, and stool were obtained at 0, 1, 2, and 5 weeks. Pinitol (Left panel: A. plasma; B. stool; C. urine) or DCI (right panel: A. plasma; B. stool; C. urine) was analyzed as described in Materials and Methods. The levels of statistical differences between 5053 and the pinitol/DCI-deficient diet at each time point are indicated by **P < 0.001.
Endogenous DCI/MI biosynthesis from deuterated water in mice fed the pinitol/DCI-deficient diet
To determine whether the carbon ring hydrogen atoms of DCI were synthesized endogenously from water, male C57BL/6J mice (n=3) were fed the pinitol/DCI-deficient diet for 10 weeks and received drinking water containing 20% w/w deuterium oxide for one week followed by a 24-hour urine collection while housed in metabolic cages. The resulting concentration of deuterium oxide in urine was 8.70 ± 0.52% as determined by equilibration with acetone followed by GC/MS. Neither natural nor 2H-labelled DCI could be detected in the urine (< 0.0067 nMol/mL). In contrast, deuterated MI was present in the urine and it was calculated that 34.4 ± 2.8% of urinary MI was derived by biosynthesis from body water.
Conversion of MI to DCI in mice fed the pinitol/DCI-deficient diet
Male C57BL/6J mice (n=3) were maintained on the chemically-defined pinitol/DCI-deficient diet for 15 weeks and then given 1.0 mg MI-d6 by IP injection. Following a 24-hour isotope equilibration period, urine was collected in individual metabolic cages. MI-d6 constituted 38.7 ± 11.5% of urinary MI after labeling, which indicated a large enrichment of endogenous MI pool. Ionic masses corresponding to natural DCI (m/z 726) through fully-labelled DCI (m/z 731) were scanned, but no natural DCI or DCI with any amount of deuterium labeling was detected (< 0.0067 nMol/mL).
Discussion
Total DCI levels were unexpectedly high in common laboratory animal chows and constituted 0.23% of the weight of 5001 (Table 2). The amounts consumed by animals were very large; for example, rats chronically ingested 166 mg/kg body weight/day of total DCI while on 5001 (Table 4). Thus, a potential source of DCI is abundant in laboratory animal diets, principally in the legumes alfalfa and soybeans, which are ingredients of many commercial laboratory animal chows. However, meat and other animal products contained little total DCI. l-chiro-Inositol was present only in small amounts relative to DCI (Table 3). In alfalfa and soybeans, at least 98% of the total chiro-inositol was the D-enantiomer. In the 5053 diet, only 1.5% of the DCI was present in free form, whereas 65% was found in free pinitol. Another 33.5% of DCI is in complex forms such as glycosides liberated by acid hydrolysis. In contrast to common rodent chows, 5755 and our pinitol/DCI-deficient diet had only trace amounts of total DCI (Table 2).
In the equilibrium state on 5001, there was a markedly positive total DCI balance of 872.5 μMol/kg body weight/day (Table 4). DCI must have been metabolized since only about 5.3% was recovered in the stool (0.7%) and urine (4.6%). The complete absorption (99.3%) of total DCI suggests that both free and complex forms of pinitol or DCI were absorbed from the diet. Their complete absorption from the gastrointestinal tract was further confirmed by using purified pinitol and [2H6]DCI since less than 2% of the orally administered material was recovered in the stool (Table 5). More urinary output was observed on day 1 with feeding the natural pinitol than with the gavaging of deuterated DCI, suggesting that their metabolic kinetics differed.
It has been reported previously that soil bacteria transform labelled MI into chiro- inositol(30). Although the extent of conversion was small (4% over 12 days), the possibility of bacterial synthesis or degradation of chiro-inositol in the large bowel was considered in our experiments under anaerobic incubation mimicking colonic fermentation. However, no net synthesis of DCI was found and only a small amount of DCI was degraded. Fecal bacteria do not appear, therefore, to be an important source of DCI in these animals.
Since pinitol is of plant origin(33), it is expected to come exclusively from the diet. Thus pinitol in the plasma, stool, or urine fell rapidly to a very low level over the 5-week period after switching from 5053 to the pinitol/DCI-deficient diet (Fig. 3, left panel, lines with empty dots). By contrast, pinitol levels in the same three compartments remained relatively stable when mice were fed 5053 (Fig. 3 left panel, lines with solid dots). Similarly, DCI fell rapidly to an extremely low level in plasma, stool, or urine of mice fed the pinitol/DCI-deficient diet, in contrast to relatively stable levels of these inositols in mice fed 5053 (Fig. 3, right panel). In fact, plasma, stool, or urinary levels of pinitol or DCI were not detectable in mice fed the pinitol/DCI-deficient diet for 10 weeks. These results strongly suggest that DCI in body fluids derives from diet and not from endogenous biosynthesis. Indeed, deuterated water was not incorporated into deuterated DCI, indicating no endogenous synthesis of DCI from body water hydrogen. In contrast, 34.4% of urinary MI was synthesized from 2H-labelled water, consistent with endogenous synthesis of MI in the literature(23).
Bioconversion of MI to DCI appears not to occur in mice. 2H-labelled DCI was not detected in the urine of mice 24 hours after IP injection of [2H6]MI. The injected [2H6]MI constituted 38.7 ± 11.5% of urinary MI, indicating good enrichment of the putative precursor. Inconsistent results have been obtained in the past regarding conversion of MI to DCI. Inositol epimerase from bovine brain led to conversion of MI to neo- and scyllo-inositol but not chiro-inositol(26). In cockroach fat body extracts, however, epimerization of MI to chiro-inositol was observed(27). More recently, Pak and collaborators injected 1.0 millicurie of 3H-MI intraperitoneally into rats over 3 days and found that it was converted to radioactive counts that comigrated with chiro-inositol(28). The percent conversion was very large, 8% in blood, 9% in liver and 36% in urine. However, DCI natural abundance of total inositols (sum of chiro-inositol and MI) is only 0.4% in plasma and 2.3% of urinary inositols in humans(15). Furthermore, the ratio of basal (when all mice were on 5053) urinary DCI to urinary MI was barely 10.0% in the current work. This raises the possibility that some of the counts co-migrating with chiro-inositol on paper chromatograms may not have been chiro-inositol. Furthermore, the method used did not distinguish d-from l-chiro-inositol(28).
Our time-course study in mice comparing 5053 and the pinitol/DCI-deficient diet showed little change in body weight in either group over 5 weeks. However, mice in the pinitol/DCI-deficient diet weighed less (Fig. 2B) and had less food intake (Fig. 2A) when compared with those on 5053. We speculate that this could be due to differences in MI, DCI, or other nutrients, or to reduced palatability of the pinitol/DCI-deficient diet. This experiment was performed to test whether or not DCI was synthesized endogenously rather than testing the function of DCI in the diet, which requires further research.
In conclusion, pinitol and DCI are present in large amounts in rodent chow diets and they are avidly absorbed from chow 5001. DCI was not synthesized in vivo nor converted from [2H6]MI suggesting that, like pinitol, it derives solely from the diet. Our work suggests that dietary control of inositols should be considered for future research in diabetes and insulin resistance. The chemically-defined diet 5755 and the pinitol/DCI-deficient diet described here establish a deficient baseline for future control studies, and are suitable for the determination of the effects of DCI and pinitol on glucose and lipid metabolism.
Acknowledgments
This work was supported by a NIH grant R01 DK58698 and the Washington University Mass Spectrometry Resource RR00954, Diabetes Research and Training Center DK20579 and Clinical Nutrition Research Center DK56341. The authors declare no conflicts of interest. Contribution of each author: Dr. Richard Ostlund, design and writing; Dr. Xiaobo Lin, design, performance of experiments, statistical analysis of data, and writing; Lina Ma, performance of experiments, GC/MS analysis of samples; Dr. Chaya Gopalan, performance of experiments and writing.
References
- 1.Romero G, Luttrell L, Rogol A, et al. Phosphatidylinositol-glycan anchors of membrane proteins: potential precursors of insulin mediators. Science. 1988;240:509–511. doi: 10.1126/science.3282305. [DOI] [PubMed] [Google Scholar]
- 2.Saltiel AR. Second messengers of insulin action. Diabetes Care. 1990;13:244–256. doi: 10.2337/diacare.13.3.244. [DOI] [PubMed] [Google Scholar]
- 3.Mato JM, Kelly KL, Abler A, et al. Identification of a novel insulin-sensitive glycophospholipid from H35 hepatoma cells. J Biol Chem. 1987;262:2131–2137. [PubMed] [Google Scholar]
- 4.Saltiel AR, Fox JA, Sherline P, et al. Insulin-stimulated hydrolysis of a novel glycolipid generates modulators of cAMP phosphodiesterase. Science. 1986;233:967–972. doi: 10.1126/science.3016898. [DOI] [PubMed] [Google Scholar]
- 5.Kunjara S, McLean P, Greenbaum AL, et al. Insight into the role of inositol phosphoglycans in insulin response and the regulation of glucose and lipid metabolism illustrated by the response of adipocytes from two strains of rats. Mol Genet Metab. 2008;94:263–266. doi: 10.1016/j.ymgme.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Mato JM, Kelly KL, Abler A, et al. Partial structure of an insulin-sensitive glycophospholipid. Biochem Biophys Res Commun. 1987;146:764–770. doi: 10.1016/0006-291x(87)90595-x. [DOI] [PubMed] [Google Scholar]
- 7.Larner J, Huang LC, Schwartz CF, et al. Rat liver insulin mediator which stimulates pyruvate dehydrogenase phosphate contains galactosamine and D-chiroinositol. Biochem Biophys Res Commun. 1988;151:1416–1426. doi: 10.1016/s0006-291x(88)80520-5. [DOI] [PubMed] [Google Scholar]
- 8.Futerman AH, Low MG, Ackermann KE, et al. Identification of covalently bound inositol in the hydrophobic membrane-anchoring domain of Torpedo acetylcholinesterase. Biochem Biophys Res Commun. 1985;129:312–317. doi: 10.1016/0006-291x(85)91439-1. [DOI] [PubMed] [Google Scholar]
- 9.Low MG, Futerman AH, Ackermann KE, et al. Removal of covalently bound inositol from Torpedo acetylcholinesterase and mammalian alkaline phosphatases by deamination with nitrous acid. Evidence for a common membrane-anchoring structure. Biochem J. 1987;241:615–619. doi: 10.1042/bj2410615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walter EI, Roberts WL, Rosenberry TL, et al. Structural basis for variations in the sensitivity of human decay accelerating factor to phosphatidylinositol-specific phospholipase C cleavage. J Immunol. 1990;144:1030–1036. [PubMed] [Google Scholar]
- 11.Berthelot M. Pinitol. Compt rend. 1856;41:392. [Google Scholar]
- 12.Smith AE, Phillips DV. Ocurrence of pinitol in foliage of several forage legumes species. Crop Science. 1980;20:75–77. [Google Scholar]
- 13.Phillips DV, Dougherty DE, Smith AE. Cyclitols in soybean. J Agric Food Chem. 1982;30:456–458. doi: 10.1021/jf00111a011. [DOI] [PubMed] [Google Scholar]
- 14.Streeter JG. Carbohydrates in Soybean Nodules: II. Distribution of compounds in seedling sduring the oneset of nitrogen fixation. Plant Physiol. 1980;66:471–476. doi: 10.1104/pp.66.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostlund RE, Jr., McGill JB, Herskowitz I, et al. D-chiro-inositol metabolism in diabetes mellitus. Proc Natl Acad Sci U S A. 1993;90:9988–9992. doi: 10.1073/pnas.90.21.9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennington AS, Hill CR, Craig J, et al. Low urinary chiro-inositol excretion in non-insulin-dependent diabetes mellitus. N Engl J Med. 1990;323:373–378. doi: 10.1056/NEJM199008093230603. [DOI] [PubMed] [Google Scholar]
- 17.Kawa JM, Przybylski R, Taylor CG. Urinary chiro-inositol and myo-inositol excretion is elevated in the diabetic db/db mouse and streptozotocin diabetic rat. Exp Biol Med (Maywood) 2003;228:907–914. doi: 10.1177/153537020322800806. [DOI] [PubMed] [Google Scholar]
- 18.Davis A, Christiansen M, Horowitz JF, et al. Effect of pinitol treatment on insulin action in subjects with insulin resistance. Diabetes Care. 2000;23:1000–1005. doi: 10.2337/diacare.23.7.1000. [DOI] [PubMed] [Google Scholar]
- 19.Iuorno MJ, Jakubowicz DJ, Baillargeon JP, et al. Effects of d-chiro-inositol in lean women with the polycystic ovary syndrome. Endocr Pract. 2002;8:417–423. doi: 10.4158/EP.8.6.417. [DOI] [PubMed] [Google Scholar]
- 20.Kim MJ, Yoo KH, Kim JH, et al. Effect of pinitol on glucose metabolism and adipocytokines in uncontrolled type 2 diabetes. Diabetes Res Clin Pract. 2007;77(Suppl 1):S247–251. doi: 10.1016/j.diabres.2007.01.066. [DOI] [PubMed] [Google Scholar]
- 21.Holub BJ. Metabolism and function of myo-inositol and inositol phospholipids. Annu Rev Nutr. 1986;6:563–597. doi: 10.1146/annurev.nu.06.070186.003023. [DOI] [PubMed] [Google Scholar]
- 22.Caspary WF, Crane RK. Active transport of myo-inositol in hamster small intestine. Naunyn Schmiedebergs Arch Pharmakol. 1970;266:309–310. doi: 10.1007/BF00997947. [DOI] [PubMed] [Google Scholar]
- 23.Daughaday WH, Larner J, Hartnett C. The synthesis of inositol in the immature rat and chick embryo. J Biol Chem. 1955;212:869–875. [PubMed] [Google Scholar]
- 24.Clements RS, Jr., Diethelm AG. The metabolism of myo-inositol by the human kidney. J Lab Clin Med. 1979;93:210–219. [PubMed] [Google Scholar]
- 25.Hauser G, Finelli VN. The Biosynthesis of Free and Phosphatide Myo-Inositol from Glucose by Mammalian Tissue Slices. J Biol Chem. 1963;238:3224–3228. [PubMed] [Google Scholar]
- 26.Hipps PP, Ackermann KE, Sherman WR. Inositol epimerase--inosose reductase from bovine brain. Methods Enzymol. 1982;89(Pt D):593–598. doi: 10.1016/s0076-6879(82)89102-7. [DOI] [PubMed] [Google Scholar]
- 27.Hipps PP, Sehgal RK, Holland WH, et al. Identification and partial characterization of inositol: NAD+ epimerase and inosose: NAD(P)H reductase from the fat body of the American cockroach, Periplaneta americana L. Biochemistry. 1973;12:4705–4712. doi: 10.1021/bi00747a025. [DOI] [PubMed] [Google Scholar]
- 28.Pak Y, Huang LC, Lilley KJ, et al. In vivo conversion of [3H]myoinositol to [3H]chiroinositol in rat tissues. J Biol Chem. 1992;267:16904–16910. [PubMed] [Google Scholar]
- 29.Anderson AB, MacDonald DL, Fischer HOL. The structure of pinitol. J Am Chem Soc. 1952;74:1479–1480. [Google Scholar]
- 30.Sasaki K, Balza F, Taylor IEP. Preparative-scale separation by anion-exchange chromatography of six per-C-deuterated inositol epimers produced during C-1H-C-2H exchange reactions with raney nickel in deuterium oxide. Carbohydrate Res. 1987;166:171–180. [Google Scholar]
- 31.McCabe BJ, Bederman IR, Croniger C, et al. Reproducibility of gas chromatography-mass spectrometry measurements of 2H labeling of water: application for measuring body composition in mice. Anal Biochem. 2006;350:171–176. doi: 10.1016/j.ab.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 32.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 33.Vernon DM, Bohnert HJ. A novel methyl transferase induced by osmotic stress in the facultative halophyte Mesembryanthemum crystallinum. EMBO J. 1992;11:2077–2085. doi: 10.1002/j.1460-2075.1992.tb05266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anomymous IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC) and IUPAC-IUB Commission on Biochemical Nomenclature (CBN). Nomenclature of cyclitols. Recommendations, 1973. Biochem J. 1976;153:23–31. doi: 10.1042/bj1530023. [DOI] [PMC free article] [PubMed] [Google Scholar]