Abstract
Background
Accurate estimation of life expectancy is essential to offering appropriate care to men with early-stage prostate cancer, but mortality risks associated with comorbidity are poorly defined.
Objective
To determine the effect of age, comorbidity, and tumor risk on other-cause and prostate cancer–specific mortality in men with early-stage disease.
Design
Prospective cohort study.
Setting
A nationally representative, population-based cohort.
Patients
3183 men with nonmetastatic prostate cancer at diagnosis.
Measurements
Baseline self-reported comorbidity (scored as a count of 12 major comorbid conditions), tumor characteristics, initial treatment, and overall and disease-specific mortality through 14 years of follow-up. Survival analyses that accounted for competing risks were performed.
Results
Fourteen-year cumulative other-cause mortality rates were 24%, 33%, 46%, and 57% for men with 0, 1, 2, and 3 or more comorbid conditions, respectively. For men diagnosed at age 65 years, subhazard ratios for other-cause mortality among those with 1, 2, or 3 or more comorbid conditions (vs. none) were 1.2 (95% CI, 1.0 to 1.4), 1.7 (CI, 1.4 to 2.0), and 2.4 (CI, 2.0 to 2.8), respectively. Among men with 3 or more comorbid conditions, 10-year other-cause mortality rates were 26%, 40%, and 71% for those aged 60 years or younger, 61 to 74 years, and 75 years or older at diagnosis, respectively. Prostate cancer–specific mortality was minimal in patients with low-risk (3%) and intermediate-risk (7%) disease but appreciable in those with high-risk disease (18%) and did not vary by number of comorbid conditions (10% to 11% in all groups).
Limitation
Comorbid conditions were self-reported.
Conclusion
Older men with multiple major comorbid conditions are at high risk for other-cause mortality within 10 years of diagnosis and should consider this information when deciding between conservative management and aggressive treatment for low- or intermediate-risk prostate cancer.
Primary Funding Source
National Cancer Institute.
Men with a new diagnosis of clinically localized prostate cancer are faced with many treatment options that range from no initial therapy (watchful waiting or active surveillance) to aggressive therapy with surgery and radiation. The first question a newly diagnosed man should consider is whether immediate aggressive treatment is necessary. Because the survival benefits of aggressive treatment for low- and intermediate-risk disease are delayed for 8 to 10 years (1), clinical guidelines recommend that men with a life expectancy of less than 10 years be spared the morbidity and expense associated with such treatment (2, 3).
Despite the recognized importance of estimating life expectancy in medical decision making for men with prostate cancer, physicians are poor judges of prognosis (4); this often leads to inappropriate treatment decisions. Recent retrospective data have shown that men with Charlson scores of 3 or greater are treated aggressively with surgery or radiation more often than not, despite a 70% other-cause mortality rate at 8 years after diagnosis (5). This practice may be due to several reasons. First, the long-term risk for other-cause mortality associated with different ages and comorbidity states is unclear. The current data on risk for other-cause mortality associated with comorbidity are from institutional case series (6–9) or populations receiving only 1 type of treatment (10); to our knowledge, there has been no true population-based assessment of risk for other-cause mortality associated with comorbidity in U.S. patients with prostate cancer. Second, the current instruments for determining risk for other-cause mortality are prohibitively cumbersome to use in the clinical setting. Finally, few data simultaneously integrate risk assessment for cancer-specific and other-cause mortality and include relevant variables, such as age, comorbidity, and tumor risk, to predict both.
In this study, we sought to characterize the association of comorbidity, age, and tumor features with long-term other-cause and disease-specific mortality in a large, population-based sample of men with clinically localized prostate cancer. We restricted our comorbidity assessment to a count of 12 major conditions to allow for easy translation to the clinical setting. We hoped to identify groups of men with a high risk for other-cause mortality and low risk for prostate cancer–specific mortality so that they might better understand the risks and benefits of various therapeutic strategies and make a truly informed decision about treatment.
Methods
Study Participants
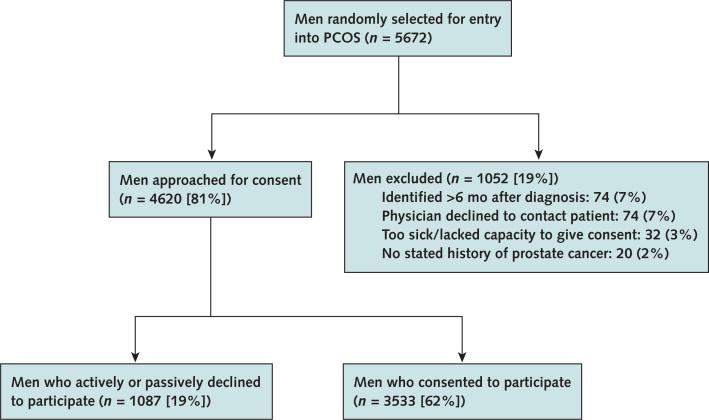
The Prostate Cancer Outcomes Study (PCOS) involved a population-based cohort of men diagnosed with prostate cancer, as ascertained from the National Cancer Institute Surveillance, Epidemiology and End Results (SEER) program. Details of PCOS have been published previously (11). In brief, the study included men diagnosed with prostate cancer between 1 October 1994 and 31 October 1995 who resided in an area covered by 1 of 6 SEER tumor registries: Connecticut; Utah; New Mexico; and the metropolitan areas of Atlanta, Georgia; Los Angeles County, California; and King County (Seattle), Washington. All men aged 39 to 89 years were eligible except in King County, where inclusion was limited to men aged 60 to 89 years. Patients were identified within 6 months of diagnosis by using a rapid case ascertainment system. Population attrition is reported in the Appendix Figure (available at www.annals.org). A total of 3533 (62%) eligible men completed the survey at 6 or 12 months. The institutional review board of each participating institution approved the study.
For the current analysis, we included all men in PCOS with nonmetastatic prostate cancer at diagnosis. We excluded men with nodal or distant metastases, those without information on comorbid conditions at diagnosis, and those diagnosed incidentally at the time of cystoprostatectomy. Our final analytic sample comprised 3183 men.
Data Collection
All patients in this analysis completed a baseline survey within 6 months of diagnosis that assessed sociodemographic and clinical information (including presence or absence of comorbid conditions) as well as self-reported function and quality of life.
Medical Record Data
Medical records of all participants were reviewed at 1 and 5 years after diagnosis. Abstractors obtained demographic information; clinical symptoms; diagnostic examinations; tumor-related information (diagnostic prostate-specific antigen [PSA] level, Gleason score, and clinical stage); and primary treatment type, which was defined as aggressive (surgery, external-beam radiation therapy, or brachytherapy) or nonaggressive (androgen deprivation therapy or watchful waiting). In addition, information on tumor characteristics, primary treatment, and vital status was collected from the SEER registries.
Comorbidity
We used a modified version of the Charlson comorbidity index (12) to assess comorbidity. In the current analysis, comorbidity was expressed as a count of the following 12 major conditions at the time of diagnosis: diabetes, bleeding gastrointestinal ulcer, chronic lung disease, congestive heart failure, stroke, myocardial infarction, angina or chest pain, cirrhosis or liver disease, arthritis, inflammatory bowel disease, hypertension, and depression. Patients answered “yes” or “no” for each of these comorbid conditions on the baseline survey. Responses were not validated by chart review.
Tumor Characteristics
We stratified tumors by clinical and pathologic features by using the widely accepted D'Amico criteria, which use diagnostic PSA level, Gleason score, and clinical stage at diagnosis to predict risk for progression, overall mortality, and cancer-specific mortality. Tumors were classified as low (PSA level <10 μg/L, clinical stage ≤T2a, and Gleason score ≤6), intermediate (PSA level of 10 to 20 μg/L, clinical stage T2b, or Gleason score of 7), or high (PSA level >20 μg/L, clinical stage ≥T2c, or Gleason score ≥8) risk (13, 14). Tumors were categorized into a higher risk stratum if they had at least one of the characteristics of that stratum.
Vital Status
Vital status and underlying cause of death were determined through 14 years after diagnosis by using SEER data.
Statistical Analysis
We first grouped patients by number of comorbid conditions (0, 1, 2, or ≥3) and compared baseline characteristics by using the analysis of variance for continuous variables and the chi-square test for categorical variables. Cumulative incidence rates were computed for overall, prostate cancer–specific, and other-cause mortality.
Other-cause mortality was modeled with the proportional subdistribution hazards regression described by Fine and Gray (15), with prostate cancer death treated as a competing risk. A competing event eliminates the possibility of the primary event of interest, and treating it as a censored observation would violate the noninformative censoring assumption of the Cox proportional hazards model. When competing risks are modeled, the conclusion may be based on a cause-specific or subdistributional hazard. As summarized by Dignam and colleagues (16), both approaches are valid and informative and the choice often depends on questions of interest. We chose to use the Fine and Gray model, which applies regression modeling directly on a cumulative incidence function and allows for estimation of the effect of covariates on this function. We included number of comorbid conditions, age, race, SEER site, D'Amico tumor risk, and treatment type as covariates. An interaction term between age and number of comorbid conditions was included to account for age-specific effects of the latter on survival. Prostate cancer–specific mortality was modeled using a similar approach. We checked the proportional hazards assumption by using scaled Schoenfeld residuals (17), as suggested by Fine and Gray (15). We found weak evidence of violation of the assumption on the categorized comorbid condition counts for other-cause mortality (P = 0.043); however, closer graphical examination of the Schoenfeld residuals revealed that the proportionality seems to hold except for at the end of the study period (≥13 years), where data were scarce. A similar trend was found for D'Amico tumor risk (P = 0.021); only 94 patients were categorized into the unknown group that caused assumption violation. For prostate cancer mortality, the proportional hazards assumption was likely to be violated for comorbid condition counts (P < 0.001), as well as age, SEER site, tumor risk, and treatment type; however, for this secondary end point, we still present average sub-hazard ratios (18) to denote its relationship with number of comorbid conditions.
To determine whether aggressive treatment was associated with better prostate cancer–specific survival among men with more comorbid conditions, we further analyzed the potential interaction between treatment (aggressive or nonaggressive) and comorbidity. An α level of 0.05 was used to denote statistical significance, and all tests were 2-sided. Statistical analyses were performed using R 2.14 (19), with the cmprsk package (20) for Fine and Gray modeling.
Role of the Funding Source
The National Cancer Institute, National Institutes of Health, provided funding for the study. The funding source had no role in the design, conduct, or analysis of the study or the decision to publish the manuscript.
Results
Baseline characteristics of the sample after stratification by number of comorbid conditions are shown in the Table. Older men, African American men, and those with higher tumor risk tended to have more comorbid conditions. Those with higher numbers of comorbid conditions tended to receive aggressive treatment less often than those with lower numbers. However, 256 of 419 men (61%) with 3 or more comorbid conditions were treated aggressively for clinically localized disease. Counts of specific comorbid conditions across comorbidity groups are reported in Appendix Table 1 (available at www.annals.org).
Table.
Patient Characteristics, by Number of Comorbid Conditions*
| Characteristic | No Comorbid Conditions (n = 1221) | 1 Comorbid Condition (n = 1020) | 2 Comorbid Conditions (n = 523) | ≥3 Comorbid Conditions (n = 419) | P Value† |
|---|---|---|---|---|---|
| Age, n (%) | <0.001 | ||||
| ≤55 y | 203 (57) | 101 (29) | 29 (8) | 21 (6) | |
| 56–65 y | 446 (42) | 341 (32) | 165 (15) | 113 (11) | |
| 66–75 y | 436 (34) | 418 (32) | 243 (19) | 203 (16) | |
| ≥76 y | 136 (29) | 160 (34) | 86 (19) | 82 (18) | |
| Race, n (%) | <0.001 | ||||
| Non-Hispanic white | 885 (40) | 716 (32) | 326 (15) | 278 (13) | |
| African American | 169 (31) | 162 (30) | 121 (22) | 88 (16) | |
| Hispanic | 167 (38) | 142 (32) | 76 (17) | 53 (12) | |
| Median PSA level at diagnosis (IQR), μg/L | 8.3 (5.5–16.1) | 8.4 (5.6–16.3) | 8.6 (5.7–16.4) | 10.4 (5.8–20.7) | 0.051 |
| Clinical stage, n (%) | 0.74 | ||||
| T1 | 340 (40) | 271 (32) | 143 (17) | 99 (12) | |
| T1/T2 | 367 (37) | 321 (33) | 152 (15) | 144 (14) | |
| T2 | 467 (38) | 382 (32) | 204 (17) | 156 (13) | |
| T3 | 47 (34) | 46 (34) | 24 (17) | 20 (15) | |
| Gleason score, n (%) | 0.40 | ||||
| ≤6 | 756 (38) | 649 (33) | 326 (16) | 246 (12) | |
| 7 | 251 (39) | 191 (30) | 114 (18) | 86 (13) | |
| ≥8 | 214 (38) | 180 (32) | 83 (15) | 87 (15) | |
| D'Amico tumor risk, n (%) | <0.001 | ||||
| Low | 259 (42) | 200 (33) | 102 (17) | 52 (8) | |
| Intermediate | 527 (39) | 439 (32) | 221 (16) | 171 (13) | |
| High | 410 (37) | 354 (32) | 180 (16) | 174 (16) | |
| Unknown | 25 (27) | 27 (29) | 20 (21) | 22 (23) | |
| Primary treatment, n (%) | <0.001 | ||||
| Aggressive | 980 (41) | 753 (32) | 377 (16) | 256 (11) | |
| Surgery | 735 (46) | 499 (31) | 240 (15) | 135 (8) | |
| External-beam radiation | 245 (32) | 254 (34) | 137 (18) | 121 (16) | |
| Nonaggressive | 241 (29) | 267 (33) | 146 (18) | 163 (20) | |
| Androgen-deprivation therapy | 85 (29) | 99 (33) | 54 (18) | 60 (20) | |
| Watchful waiting | 156 (30) | 168 (32) | 92 (18) | 103 (20) |
IQR = interquartile range; PSA = prostate-specific antigen.
Percentages are reported across rows and may not add up to 100 because of rounding.
Calculated using chi-square test, except Kruskal–Wallis test used for age and PSA level.
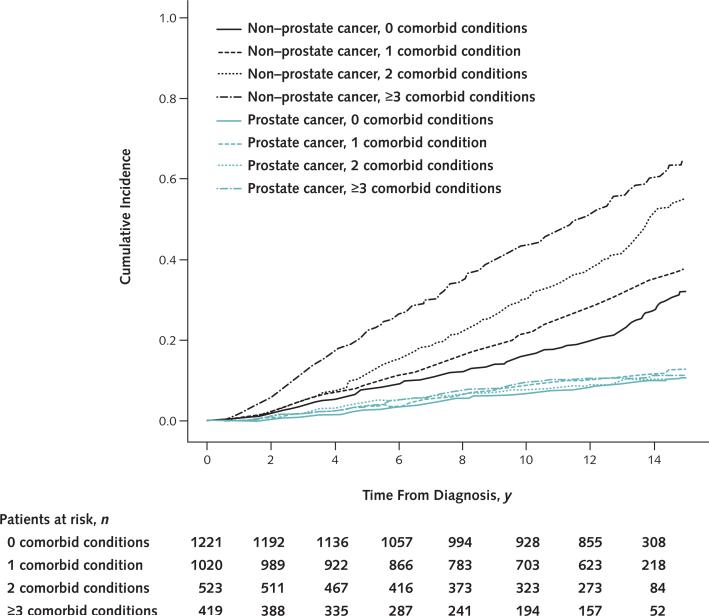
Cumulative other-cause mortality rates by number of comorbid conditions are shown in Appendix Table 2 (available at www.annals.org). At 14-year follow-up, other-cause mortality estimates were 24%, 33%, 46%, and 57% for men with 0, 1, 2, and 3 or more comorbid conditions, respectively. Cumulative incidence curves depicting other-cause and prostate cancer–specific mortality by number of comorbid conditions are shown in Figure 1.
Figure 1.
Cumulative incidence curves for other-cause and prostate cancer–specific mortality, by number of comorbid conditions.
Proportional subdistributional hazards models investigating the association between other-cause mortality and number of comorbid conditions also showed an increasing subhazard with higher numbers of comorbid conditions. When age, race, SEER site, tumor risk stratum, and treatment type were accounted for, the subhazard ratios of other-cause mortality for men with 1, 2, or 3 or more comorbid conditions were 1.2 (95% CI, 1.0 to 1.4), 1.7 (CI, 1.4 to 2.0), and 2.4 (CI, 2.0 to 2.8), respectively, compared with those with none.
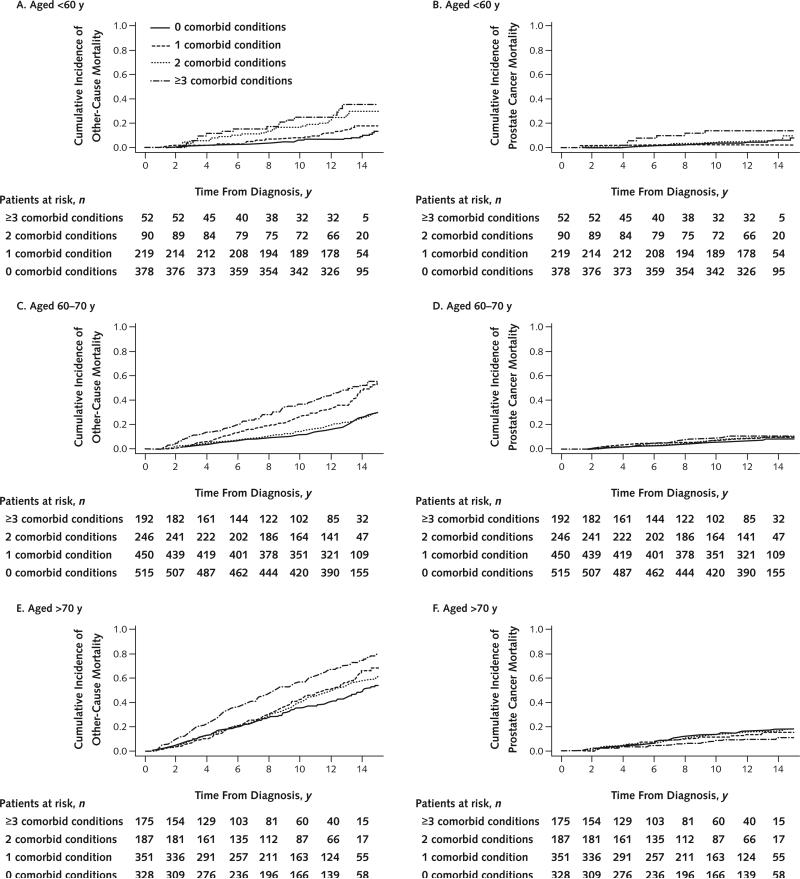
To illustrate the effect of increasing age on risk for other-cause mortality in men with the most severe comorbidity, we calculated 10-year cumulative other-cause mortality rates for men with 3 or more comorbid conditions across age strata. Among these, men younger than 60 years, aged 61 to 74 years, and older than 75 years at diagnosis had 10-year other-cause mortality rates of 26% (17 of 65), 40% (108 of 272), and 71% (58 of 82), respectively.
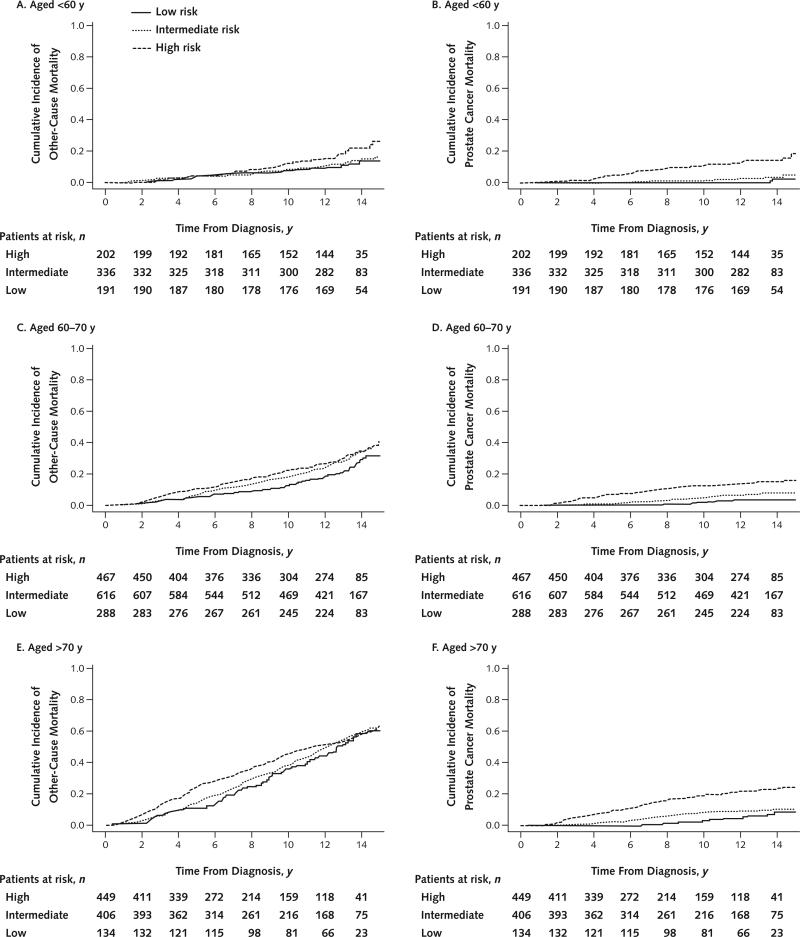
Cumulative incidence of prostate cancer–specific and other-cause mortality by D'Amico tumor risk category and number of comorbid conditions—after stratification into younger (<60 years), intermediate (60 to 70 years), and older (>70 years) groups—is shown in Figures 2 and 3. In all age groups, the risk for other-cause mortality increased with the number of comorbid conditions and the risk for prostate cancer mortality increased with higher tumor risk strata. In concordance with our multivariate models, the absolute effect of comorbidity on risk for other-cause mortality was markedly less pronounced in men younger than 60 years than in older men. Across the entire cohort, prostate cancer mortality was low in men with low-risk (3%) and intermediate-risk (7%) disease but appreciable in those with high-risk disease (18%). Proportions of men treated aggressively and nonaggressively within each subgroup of age or tumor risk are reported in Appendix Table 3 (available at www.annals.org). The risk for prostate cancer mortality associated with tumor risk was similar across age groups.
Figure 2.
Competing-risks model depicting cumulative incidence of other-cause and prostate cancer–specific mortality, by number of comorbid conditions, for men aged <60 y (A and B), 60 to 70 y (C and D), and >70 y (E and F).
Figure 3.
Competing-risks model depicting cumulative incidence of other-cause and prostate cancer–specific mortality, by D'Amico risk criteria, for men aged <60 y (A and B), 60 to 70 y (C and D), and >70 y (E and F).
A competing-risks model analyzing the subhazard of prostate cancer mortality associated with nonaggressive treatment among comorbidity groups suggested that aggressive therapy may be beneficial to men with little or no comorbid disease. However, these treatments may not be as valuable in men with more comorbid conditions. Men with 0 or 1 comorbid condition who were managed conservatively had increases of 2.4-fold (CI, 1.6 to 3.5) and 2.2-fold (CI, 1.5 to 3.3), respectively, in the subhazard of prostate cancer mortality compared with those treated aggressively. Men with 2 (hazard ratio [HR], 1.6 [CI, 1.0 to 2.7]) or 3 or more (HR, 1.5 [CI, 0.9 to 2.5]) comorbid conditions who were managed conservatively did not have a statistically significant increase in prostate cancer mortality.
To further inform clinical decision making in nonmetastatic prostate cancer, we recapitulated our analysis and included men who chose androgen deprivation therapy as primary treatment in the aggressive management group; by doing so, we effectively explored the decision of any therapy versus none. The results were virtually identical, except for those from the competing-risks model analyzing the subhazard of prostate cancer mortality associated with no treatment. Although men with no comorbid conditions still had an increased risk (HR, 2.0 [CI, 1.3 to 3.0]), men with 1 (HR, 1.1 [CI, 0.7 to 1.9]), 2 (HR, 1.2 [CI, 0.7 to 2.2]), or 3 or more (HR, 1.1 [CI, 0.6 to 2.0]) were not at increased risk.
Discussion
These results illustrate the contemporary long-term risks for other-cause and prostate cancer mortality associated with age at diagnosis, comorbidity, and tumor risk in a population-based cohort of men diagnosed with clinically localized prostate cancer. These data provide more accurate estimates of long-term prognosis for men with newly diagnosed prostate cancer because they are not limited by the selection bias incurred by using single-institution (6–9) or method-specific (10) data. In fact, several studies have shown that the risk for other-cause mortality associated with a given comorbidity score increases in a stepwise manner when men treated with radical prostatectomy, radiation therapy, and watchful waiting are compared (6, 21). Because men who have surgery are generally younger, have better functional status, and have less severe manifestations of disease than those who choose radiation therapy and, especially, those who choose watchful waiting, other-cause mortality rates among men who have surgery may be lower for a given comorbidity score than among those who choose radiation or watchful waiting. Using a population-based sample of men who are balanced in age, tumor characteristics, and primary treatment will provide a more accurate estimate of mortality risk associated with comorbidity. We believe that a population-based cohort best mimics the relevant clinical scenario: counseling a man newly diagnosed with clinically localized prostate cancer who has not yet made a treatment decision.
Our data also verify the strong prognostic utility of comorbidity in predicting risk for other-cause mortality and show that its prognostic strength persists even when its assessment is reduced to a simple count of 12 major comorbid conditions. Whether more complicated weighted systems of comorbidity assessment, such as the Charlson comorbidity index (12), provide better risk stratification than a mere count of comorbid conditions is unknown, but our data suggest that a count may be sufficient for assessing prognosis in men at the highest risk. Such a simplification of comorbidity assessment may be essential for widespread application of these findings to the clinical setting.
These data provide benchmarks for evaluating risk for prostate cancer–specific and other-cause mortality among men with different baseline comorbidity, age, and tumor features. The decision of whether to pursue aggressive treatment is preference-sensitive and is affected by one's knowledge of and attitudes about potential benefits and harms; therefore, the optimum risk ratio of prostate cancer mortality to other-cause mortality may differ among individuals. Regardless, our data provide a framework to which all patients can apply their attitudes and assess their likelihood of treatment-related benefit on the basis of their age, comorbidity, and disease characteristics. However, from a public health perspective, the widely recognized 2008 U.S. Preventive Services Task Force recommendations on cessation of screening and treatment for men older than 75 years (22) established that men with a life expectancy less than 10 years (that is, >50% other-cause mortality at 10 years) garner little or no benefit from aggressive treatment. When a similar cutoff is applied for comorbidity and age, our data show that men aged 60 years or older with 3 or more comorbid conditions approach 50% mortality at 10 years after diagnosis. Therefore, given the low likelihood of short-term prostate cancer mortality and the high likelihood of other-cause mortality, older men with more than 3 major comorbid conditions who are choosing primary treatment should strongly weigh the risk for death from other causes before realizing any potential survival benefit from aggressive therapy.
A corollary analysis investigating whether nonaggressive treatment is associated with an increased risk for prostate cancer mortality among comorbidity groups suggested that men with 2 or more comorbid conditions may derive little survival benefit from aggressive treatment. This group did not have a statistically significant increase in risk for prostate cancer mortality with nonaggressive treatment compared with men treated aggressively, although we had limited power to discern differences. Men with fewer than 2 comorbid conditions, however, did have a significant increase in risk. This contrasts with a previous claims-based study comparing overall survival in men older than 65 years who were treated aggressively versus those treated conservatively, which suggested a survival benefit with aggressive treatment that persisted after correction for comorbidity (23). That study also showed a benefit in prostate cancer–specific survival with aggressive treatment after correction for propensity scoring alone, but propensity scores were not balanced by number of comorbid conditions. Although thought-provoking, our results should be treated as exploratory given that the analysis had limited power, there was probably a selection bias between groups treated aggressively and those treated nonaggressively, and there did seem to be some benefit in other studies.
Several methodological issues limit our study. First, the comorbidity assessment relied on self-reporting by participants and was not confirmed by medical records or physicians. However, many studies have shown that patient reporting of comorbid conditions, particularly common ones like those assessed here, is fairly reliable (24). Second, because self-reporting of conditions does not allow for a detailed assessment of comorbidity severity, a broad spectrum of disease is subsumed into each of the 12 major categories of comorbid conditions. Men with mild disease manifestations may have lower rates of other-cause mortality than those estimated by our raw comorbidity assessments; the opposite may be true of men with severe disease manifestations. Furthermore, information about the presence or absence of other types of cancer at the time of diagnosis was not collected at enrollment in PCOS, so it was not included in our count of comorbid conditions. Third, because the intake questionnaire was completed within 6 months of diagnosis, some comorbid conditions may not have been present before treatment. However, most of our 12 designated conditions are chronic in nature and should have been apparent at the time of diagnosis. Fourth, a small proportion of men (n = 32) were too sick or lacked the capacity to complete the intake questionnaire and were excluded from PCOS; this may have resulted in underestimation of other-cause mortality in the sickest men. Fifth, because most of the cohort was treated aggressively for prostate cancer, rates of disease-specific mortality may have been lower than if these men had been managed conservatively, especially at more distant time points and for men with high-risk tumors. However, on the basis of survival differences observed between aggressively and non-aggressively treated men in a randomized, controlled trial (1), our study underestimated prostate cancer–specific mortality by less than 10% at 10 years for men with lowand intermediate-risk tumors. Sixth, we did not collect information on time from diagnosis to the baseline survey and, therefore, could not conduct sensitivity analyses on the assumption that no temporal bias related to comorbidity reporting was introduced into the study. Finally, although current data from randomized, controlled trials suggest that there is no significant survival benefit with aggressive treatment of early-stage prostate cancer until 8 to 10 years after local therapy (1), further data on the efficacy of aggressive treatment—especially for men with intermediate-risk disease—will help to define appropriate cut points for triage of care.
When considering these data, the reader may be inclined to inappropriately apply these findings to the decision of whether to screen for prostate cancer, particularly given the recent U.S. Preventive Services Task Force assignment of a D grade to prostate cancer screening (25). Unfortunately, these data do not inform this decision and should not be used to decide whether to screen. Although the harms from overtreatment (which are driven primarily by the morbidity associated with aggressive therapy) are cited as one of the risks of screening that led the panel to its conclusion, whether men who have been diagnosed with prostate cancer should receive treatment was not stated in the recommendation. We strongly believe that the decisions to screen or treat prostate cancer are separate entities that should always be considered individually.
In summary, because the potential for morbidity is high, men with a new diagnosis of prostate cancer should understand the likely benefit and potential harm associated with aggressive treatment. These data provide a basis on which to counsel men about their risk for prostate cancer–specific and other-cause mortality and are based on simple variables commonly available to the clinician at the time of treatment decision: age, number of major comorbid conditions at diagnosis, and tumor risk. Older men with multiple major comorbid conditions should be informed of their higher probability of death from other causes before deriving a survival benefit from surgery or radiation therapy for low- and intermediate-risk disease. The information provided herein aims to make the competing risks for mortality clearer to patients and their physicians.
Reproducible Research Statement.
Study protocol: Detailed in the Methods section and available at the discretion of Dr. Penson. Statistical code: Available at the discretion of Dr. Penson. Data set: Restricted to members of the PCOS team and their designates.
Context
Knowing a man's risk for disease-specific and other-cause mortality can help to inform decisions about whether to pursue aggressive treatment for localized prostate cancer.
Contribution
In this population-based study, the risk for other-cause mortality increased with the number of major comorbid conditions, particularly in older men. Prostate cancer mortality varied according to disease risk but not the number of comorbid conditions.
Caution
Confident risk estimates could not be made consistently according to the chosen treatment approach.
Implication
The risk estimates may assist physicians and patients who are considering preferences about prostate cancer management.
—The Editors
Acknowledgment
The authors thank the men who participated in PCOS who, by their participation, have contributed to a better understanding of the effects of prostate cancer on men's lives; the physicians in the 6 SEER areas who assisted in the collection of data from their patients and from medical records; all of the study managers and chart abstractors for their outstanding efforts in data collection; and all of the staff in the 6 cancer registries for their help with the study.
Grant Support: By grant R01CA114524 from the National Cancer Institute of the National Institutes of Health. Dr. Daskivich is supported by grants from the Robert Wood Johnson Clinical Scholars Foundation, American Cancer Society, and American Urological Association.
Appendix
Appendix Figure. Study flow diagram.
PCOS = Prostate Cancer Outcomes Study.
Appendix Table 1.
Distribution of Comorbid Conditions
| Comorbid Condition | Patients, n (%) |
||
|---|---|---|---|
| 1 Comorbid Condition | 2 Comorbid Conditions | ≥3 Comorbid Conditions | |
| Diabetes | 93 (9) | 176 (17) | 179 (11) |
| Bleeding gastrointestinal ulcer | 48 (5) | 38 (4) | 61 (4) |
| Chronic lung disease | 45 (4) | 35 (3) | 61 (4) |
| Congestive heart failure | 12 (1) | 31 (3) | 133 (8) |
| Stroke | 22 (2) | 45 (4) | 91 (6) |
| Myocardial infarction | 21 (2) | 46 (4) | 166 (11) |
| Angina | 25 (2) | 48 (5) | 168 (11) |
| Cirrhosis/liver disease | 16 (2) | 15 (1) | 26 (2) |
| Inflammatory bowel disease | 35 (3) | 32 (3) | 59 (4) |
| Arthritis/rheumatism | 207 (20) | 176 (17) | 207 (13) |
| Hypertension | 446 (44) | 341 (33) | 316 (20) |
| Depression | 50 (5) | 63 (6) | 105 (7) |
Appendix Table 2.
Cumulative Other-Cause Mortality
| Comorbid Conditions, n | Patients, n | Other-Cause Mortality, n (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Year 2 | Year 4 | Year 6 | Year 8 | Year 10 | Year 12 | Year 14 | End of Follow-up | ||
| 0 | 1221 | 22 (2) | 63 (5) | 113 (9) | 150 (12) | 197 (16) | 239 (20) | 295 (24) | 311 (25) |
| 1 | 1020 | 26 (3) | 73 (7) | 114(11) | 167 (16) | 223 (22) | 285 (28) | 332 (33) | 340 (33) |
| 2 | 523 | 9 (2) | 39 (7) | 79 (15) | 114 (22) | 158 (30) | 198 (38) | 240 (46) | 245 (47) |
| ≥3 | 419 | 25 (6) | 72 (17) | 110 (26) | 146 (35) | 183 (44) | 214 (51) | 239 (57) | 245 (58) |
Appendix Table 3.
Type of Treatment Received, by Age and Tumor Risk Stratum
| Age | Risk | Treatment, n (%) |
|
|---|---|---|---|
| Aggressive | Nonaggressive | ||
| <60 y | Low | 185 (27) | 6 (10) |
| Intermediate | 316 (46) | 20 (34) | |
| High | 172 (25) | 30 (51) | |
| Unknown | 7 (1) | 3 (5) | |
| 60–70 y | Low | 246 (22) | 42 (16) |
| Intermediate | 537 (47) | 79 (30) | |
| High | 346 (30) | 121 (46) | |
| Unknown | 10 (1) | 22 (8) | |
| >70 y | Low | 83 (15) | 51 (10) |
| Intermediate | 252 (46) | 154 (31) | |
| High | 201 (37) | 248 (50) | |
| Unknown | 11 (2) | 41 (8) | |
Footnotes
Author Contributions: Conception and design: T.J. Daskivich, P.C. Albertsen, R.M. Hoffman, J.L. Stanford, A.M. Stroup, M.S. Litwin, D.F. Penson.
Analysis and interpretation of the data: T.J. Daskivich, K.H. Fan, T. Koyama, P.C. Albertsen, J.L. Stanford, A.M. Stroup, M.S. Litwin, D.F. Penson.
Drafting of the article: T.J. Daskivich, T. Koyama, A.M. Stroup, D.F. Penson.
Critical revision of the article for important intellectual content: T.J. Daskivich, T. Koyama, P.C. Albertsen, M. Goodman, A.S. Hamilton, R.M. Hoffman, J.L. Stanford, M.S. Litwin, D.F. Penson.
Final approval of the article: T.J. Daskivich, P.C. Albertsen, M. Goodman, A.S. Hamilton, R.M. Hoffman, J.L. Stanford, A.M. Stroup, M.S. Litwin, D.F. Penson.
Provision of study materials or patients: A.S. Hamilton, J.L. Stanford, A.M. Stroup.
Statistical expertise: K.H. Fan, T. Koyama, M.S. Litwin.
Obtaining of funding: P.C. Albertsen, J.L. Stanford, M.S. Litwin, D.F. Penson.
Administrative, technical, or logistic support: M. Goodman, J.L. Stanford, A.M. Stroup, M.S. Litwin, D.F. Penson.
Collection and assembly of data: P.C. Albertsen, M. Goodman, A.S. Hamilton, R.M. Hoffman, J.L. Stanford, A.M. Stroup, D.F. Penson.
Potential Conflicts of Interest: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M12-1703.
Current author addresses and author contributions are available at www.annals.org.
References
- 1.Bill-Axelson A, Holmberg L, Ruutu M, Garmo H, Stark JR, Busch C, et al. SPCG-4 Investigators. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364:1708–17. doi: 10.1056/NEJMoa1011967. [PMID: 21542742] [DOI] [PubMed] [Google Scholar]
- 2.Mohler J, Bahnson RR, Boston B, Busby JE, D'Amico A, Eastham JA, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010;8:162–200. doi: 10.6004/jnccn.2010.0012. [PMID: 20141676] [DOI] [PubMed] [Google Scholar]
- 3.Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cook-son MS, et al. AUA Prostate Cancer Clinical Guideline Update Panel. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–31. doi: 10.1016/j.juro.2007.03.003. [PMID: 17509297] [DOI] [PubMed] [Google Scholar]
- 4.Walz J, Suardi N, Shariat SF, Jeldres C, Perrotte P, Graefen M, et al. Accuracy of life tables in predicting overall survival in patients after radical pros-tatectomy. BJU Int. 2008;102:33–8. doi: 10.1111/j.1464-410X.2008.07614.x. [PMID: 18384631] [DOI] [PubMed] [Google Scholar]
- 5.Daskivich TJ, Chamie K, Kwan L, Labo J, Palvolgyi R, Dash A, et al. Overtreatment of men with low-risk prostate cancer and significant comorbidity. Cancer. 2011;117:2058–66. doi: 10.1002/cncr.25751. [PMID: 21523717] [DOI] [PubMed] [Google Scholar]
- 6.Tewari A, Johnson CC, Divine G, Crawford ED, Gamito EJ, Demers R, et al. Long-term survival probability in men with clinically localized prostate cancer: a case-control, propensity modeling study stratified by race, age, treatment and comorbidities. J Urol. 2004;171:1513–9. doi: 10.1097/01.ju.0000117975.40782.95. [PMID: 15017210] [DOI] [PubMed] [Google Scholar]
- 7.Walz J, Gallina A, Saad F, Montorsi F, Perrotte P, Shariat SF, et al. A nomogram predicting 10-year life expectancy in candidates for radical prostatectomy or radiotherapy for prostate cancer. J Clin Oncol. 2007;25:3576–81. doi: 10.1200/JCO.2006.10.3820. [PMID: 17704404] [DOI] [PubMed] [Google Scholar]
- 8.Cowen ME, Halasyamani LK, Kattan MW. Predicting life expectancy in men with clinically localized prostate cancer. J Urol. 2006;175:99–103. doi: 10.1016/S0022-5347(05)00018-2. [PMID: 16406881] [DOI] [PubMed] [Google Scholar]
- 9.Daskivich TJ, Chamie K, Kwan L, Labo J, Dash A, Greenfield S, et al. Comorbidity and competing risks for mortality in men with prostate cancer. Cancer. 2011;117:4642–50. doi: 10.1002/cncr.26104. [PMID: 21480201] [DOI] [PubMed] [Google Scholar]
- 10.Albertsen PC, Moore DF, Shih W, Lin Y, Li H, Lu-Yao GL. Impact of comorbidity on survival among men with localized prostate cancer. J Clin Oncol. 2011;29:1335–41. doi: 10.1200/JCO.2010.31.2330. [PMID: 21357791] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potosky AL, Harlan LC, Stanford JL, Gilliland FD, Hamilton AS, Albertsen PC, et al. Prostate cancer practice patterns and quality of life: the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 1999;91:1719–24. doi: 10.1093/jnci/91.20.1719. [PMID: 10528021] [DOI] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [PMID: 3558716] [DOI] [PubMed] [Google Scholar]
- 13.D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74. doi: 10.1001/jama.280.11.969. [PMID: 9749478] [DOI] [PubMed] [Google Scholar]
- 14.Boorjian SA, Karnes RJ, Rangel LJ, Bergstralh EJ, Blute ML. Mayo Clinic validation of the D'amico risk group classification for predicting survival following radical prostatectomy. J Urol. 2008;179:1354–60. doi: 10.1016/j.juro.2007.11.061. [PMID: 18289596] [DOI] [PubMed] [Google Scholar]
- 15.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 16.Dignam JJ, Zhang Q, Kocherginsky M. The use and interprtion of competing risks regression models. Clin Cancer Res. 2012;18:2301–8. doi: 10.1158/1078-0432.CCR-11-2097. [PMID: 22282466] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26. [Google Scholar]
- 18.Grambauer N, Schumacher M, Beyersmann J. Proportional subdistribution hazards modeling offers a summary analysis, even if misspecified. Stat Med. 2010;29:875–84. doi: 10.1002/sim.3786. [PMID: 20213713] [DOI] [PubMed] [Google Scholar]
- 19.R Development Core Team. R Foundation for Statistical Computing; Vienna, Austria: 2011. [18 March 2013]. R: A language and environment for statistical computing. at www.R-project.org. [Google Scholar]
- 20.Gray RJ. cmprsk: Subdistribution Analysis of Competing Risks. R package version 2.2-2. 2011 [Google Scholar]
- 21.Fowler JE, Jr, Terrell FL, Renfroe DL. Co-morbidities and survival of men with localized prostate cancer treated with surgery or radiation therapy. J Urol. 1996;156:1714–8. [PMID: 8863577] [PubMed] [Google Scholar]
- 22.U.S. Preventive Services Task Force Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:185–91. doi: 10.7326/0003-4819-149-3-200808050-00008. [PMID: 18678845] [DOI] [PubMed] [Google Scholar]
- 23.Wong YN, Mitra N, Hudes G, Localio R, Schwartz JS, Wan F, et al. Survival associated with treatment vs observation of localized prostate cancer in elderly men. JAMA. 2006;296:2683–93. doi: 10.1001/jama.296.22.2683. [PMID: 17164454] [DOI] [PubMed] [Google Scholar]
- 24.Klabunde CN, Reeve BB, Harlan LC, Davis WW, Potosky AL. Do patients consistently report comorbid conditions over time?: results from the prostate cancer outcomes study. Med Care. 2005;43:391–400. doi: 10.1097/01.mlr.0000156851.80900.d1. [PMID: 15778642] [DOI] [PubMed] [Google Scholar]
- 25.Moyer VA, U.S. Preventive Services Task Force Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–34. doi: 10.7326/0003-4819-157-2-201207170-00459. [PMID: 22801674] [DOI] [PubMed] [Google Scholar]