Abstract
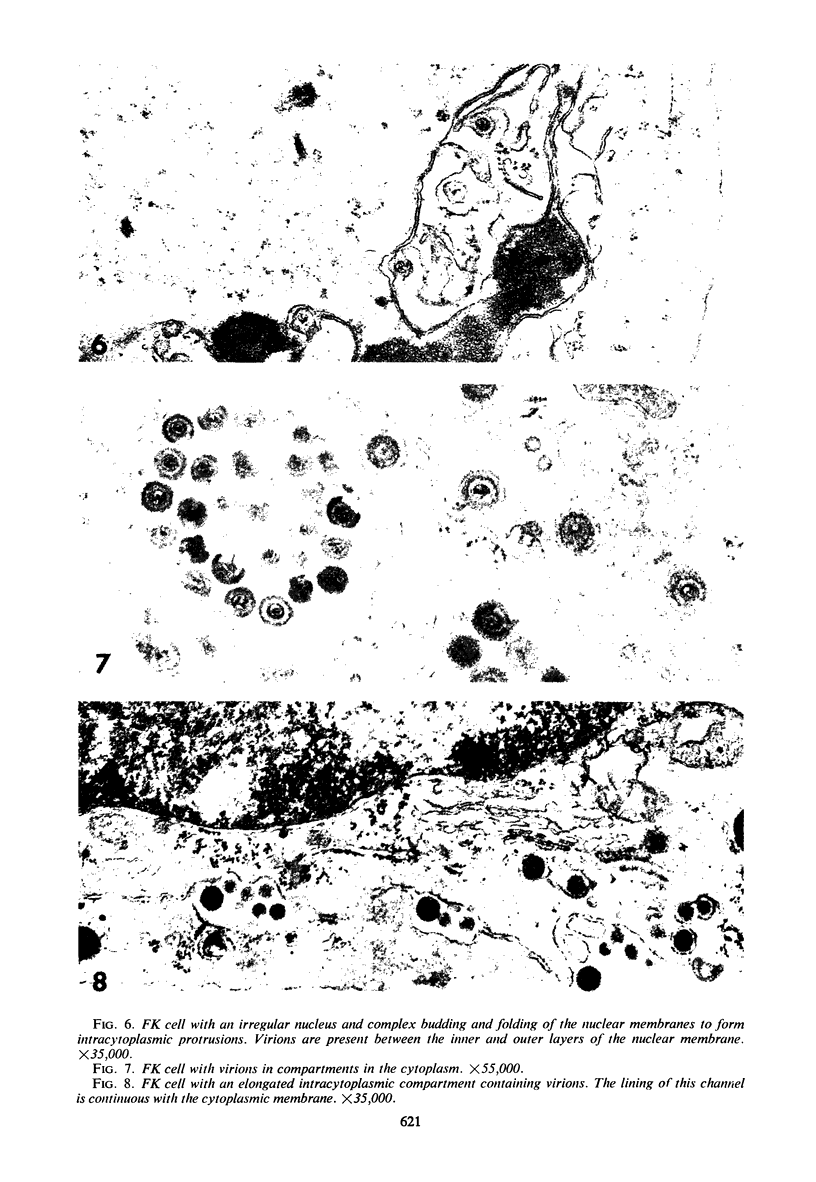
Feline herpesvirus produces characteristic morphological alterations in feline kidney cells. Nucleocapsid particles are formed in infected nuclei and are enveloped as they pass through the modified inner nuclear membrane. Aggregates of dense granular material and filamentous structures also regularly appear in infected nuclei. Infection of human embryonic lung cells by feline herpesvirus results in the appearance of intranuclear inclusion bodies, aggregates of dense granular material, and bundles of parallel filaments but no nucleocapsid particles.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHITWOOD L. A., BRACKEN E. C. REPLICATION OF HERPES SIMPLEX VIRUS IN A METABOLICALLY IMBALANCED SYSTEM. Virology. 1964 Sep;24:116–120. doi: 10.1016/0042-6822(64)90157-6. [DOI] [PubMed] [Google Scholar]
- Couch E. F., Nahmias A. J. Filamentous structures of type 2 Herpesvirus hominis infection of the chorioallantoic membrane. J Virol. 1969 Feb;3(2):228–232. doi: 10.1128/jvi.3.2.228-232.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpas A., Routledge J. K. Feline herpes virus: isolations and experimental studies. Zentralbl Veterinarmed B. 1968 Jul;15(5):599–606. doi: 10.1111/j.1439-0450.1968.tb00333.x. [DOI] [PubMed] [Google Scholar]
- MORGAN C., ROSE H. M., HOLDEN M., JONES E. P. Electron microscopic observations on the development of herpes simplex virus. J Exp Med. 1959 Oct 1;110:643–656. doi: 10.1084/jem.110.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F. A., Harrison A. K., Whitfield S. G. Intranuclear formation of filaments in herpesvirus hominis infection of mice. Arch Gesamte Virusforsch. 1967;21(3):463–468. doi: 10.1007/BF01241746. [DOI] [PubMed] [Google Scholar]
- Neff J. M., Enders J. F. Poliovirus replication and cytopathogenicity in monolayer hamster cell cultures fused with beta propiolactone-inactivated Sendai virus. Proc Soc Exp Biol Med. 1968 Jan;127(1):260–267. doi: 10.3181/00379727-127-32668. [DOI] [PubMed] [Google Scholar]
- Nii S., Rosenkranz H. S., Morgan C., Rose H. M. Electron microscopy of herpes simplex virus. 3. Effect of hydroxyurea. J Virol. 1968 Oct;2(10):1163–1171. doi: 10.1128/jvi.2.10.1163-1171.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reczko E., Böhm H. O., Straub O. C. Zur Feinstruktur des Rhinopneumonitisvirus der Pferde. Arch Gesamte Virusforsch. 1965;17(2):231–250. [PubMed] [Google Scholar]
- Siminoff P., Menefee M. G. Normal and 5-bromodeoxyuridine-inhibited development of herpes simplex virus. An electron microscope study. Exp Cell Res. 1966 Nov-Dec;44(2):241–255. doi: 10.1016/0014-4827(66)90429-0. [DOI] [PubMed] [Google Scholar]
- Spring S. B., Roizman B., Schwartz J. Herpes simplex virus products in productive and abortive infection. II. Electron microscopic and immunological evidence for failure of virus envelopment as a cause of abortive infection. J Virol. 1968 Apr;2(4):384–392. doi: 10.1128/jvi.2.4.384-392.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Enders J. F. Feline herpesvirus infection in fused cultures of naturally resistant human cells. J Virol. 1969 May;3(5):469–476. doi: 10.1128/jvi.3.5.469-476.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATRACH A. M. Intranuclear filaments associated with infectious laryngotracheitis virus. Virology. 1962 Oct;18:324–327. doi: 10.1016/0042-6822(62)90020-x. [DOI] [PubMed] [Google Scholar]