Abstract
Background
Squamous cell carcinoma (SCC) of the oral region often metastasizes to the cervical lymph nodes. To investigate whether the risk of cervical lymph node metastasis are predictable through lymphatic vessel density (LVD) and vascular endothelial growth factor (VEGF) expression, we assessed the relationship between LVD and clinicopathological parameters, and VEGF expression in SCC of the oral region.
Methods
The subjects were 109 patients with SCC of the oral region including the lip. Clinicopathological parameters examined for the association with LVD in a peritumoral hot spot were lymph node metastasis, histological grade and disease stage. The association with VEGF expression was similarly studied. LVD was detected by immunohistochemistry using D2-40.
Results
LVD was significantly higher in lip cancer than in other oral tumors (P < 0.0001), while there were no significant differences of LVD among other cancers of the oral cavity. LVD tended to decrease with disease progression, increase of tumor size and increase of metastatic lymph node size. Eighty-four of 109 tumors were positive for VEGF-C or D. VEGF-C-positive tumor lesions were also positive for VEGF-D. Significantly higher levels of VEGF-C and D expressions were associated with large size of lymph node metastases (P = 0.02).
Conclusion
SCC of the oral region including the lip that produces VEGF-C and D is significantly more likely to cause cervical lymph node metastasis. LVD in a peritumoral hot spot does not directly indicate the risk of cervical lymph node metastasis, but instead may reflect lymphangiogenesis due to VEGF together with loss of lymphatic vessels through tumor growth and progression.
Keywords: lip, lymphatic vessel density, oral cavity, squamous cell carcinoma, vascular endothelial growth factor
The oral region has a role in various essential and complex functions to breath, masticate, swallow and vocalize, which are important for sustaining life. The oral region extends from the lips to the palatoglossal fold, classified into the lips, buccal mucosa, gingiva, floor of the mouth, tongue and palate. Although it contains various anatomically distinct tissues, squamous cell carcinoma (SCC) accounts for more than 90% of all malignant tumors originating in the oral region.1 Unlike SCC of the skin and other organs, SCC of the oral region is likely to metastasize to the cervical lymph nodes at an early period, so cervical lymph node metastasis is considered to be a prognostic factor for patients with oral cancer.2, 3
Regarding the relationship between oral SCC and cervical lymph node involvement, an association with histological grade and tumor size was suggested, 4 while association with cervical metastasis of histological grade but no primary tumor location or size 5 was suggested. Lymphatic vessels in the tongue have many connections and are linked across the midline. This anatomy of the lymphatics suggests that tumors in the oral region can easily metastasize to the opposite side from the primary tumor or can cause bilateral metastases.1
In order to establish lymph node metastasis, it is also essential for tumor lymphangiogenesis to be induced. Previously, SCC of the oral cavity, larynx/pharynx and esophagus, and squamous cancer of the lungs and gastrointestinal tract were investigated regarding the relationship between tumors and lymphangiogenesis by means of assessment of lymphatic vessel density (LVD) and expression of vascular endothelial growth factors (VEGFs). 6, 7, 8, 9, 10, 11 However, there is no detailed study of the associations of site-specific LVD in oral SCC with VEGF expression or clinicopathological findings. In the present study, we performed histopathological assessment of LVD in over 100 patients with oral SCC, and examined the association between LVD and clinicopathological findings. Our results showed that, contrary to the previous reports, LVD in peritumoral tissue “hot spots” tended to decrease with an increase of tumor size and stage progression. On the other hand, the expression of VEGFs in SCC was significantly associated with lymphatic metastasis. This suggests that growth of lymphatics may be promoted by the production of VEGFs in tumor tissue, but this is not directly reflected by LVD in peritumoral hot spots. Instead, with regard to the mechanism of cervical lymph node metastasis by oral SCC, factors other than LVD in peritumoral hot spots may be involved.
SUBJECTS AND METHODS
Subjects and clinicopathological features
The subjects were 109 patients who underwent biopsy or surgical resection of tumors in the oral region at the Division of Oral and Maxillofacial Biopathological Surgery (Tottori University Hospital, Japan) between January 1991 and 2005. They did not receive radiation therapy nor chemotherapy before biopsy or surgical resection. They included 63 men and 46 women aged between 23 and 93 years, with a mean of 65 ± 13 years.Table 1 shows their clinicopathological features. Regarding the histology of the tumor, the final diagnosis was SCC based on examination of biopsy or resected specimens in all patients. Oral SCC was classified into 6 types according to the location of the primary tumor: gingiva in 45 patients, tongue in 41 patients, buccal mucosa in 8 patients, floor of the mouth in 7 patients, palate in 5 patients and lip in 3 patients (Table 2). Clinical tumor staging according to the TNM classification was as follows: stage I in 18 patients, stage II in 28 patients, stage III in 16 patients and stage IV in 47 patients (Table 1). The subjects were also classified into 4 groups based on tumor size: T1 in 20 patients, T2 in 43 patients, T3 in 9 patients and T4 in 37 patients (Table 1). Histologically, the tumor was classified as well differentiated in 44 patients, moderately differentiated in 46 patients and poorly differentiated in 19 patients. With regard to the presence or absence of cervical lymph node metastasis, there was no cervical lymph node metastasis (N0) in 65 subjects, while 18 had a solitary lymph node metastasis measuring 3 cm or less in maximum diameter (N1) on the same side as the primary tumor, 2 had a lymph node metastasis measuring 6 cm or more in maximum diameter (N3) and 24 had a lymph node metastasis between N1 and N3 (N2). We performed histopathological assessment of LVD using the pathological specimens from each group. Furthermore, to assess the factors present at an early stage that could lead to lymphatic metastasis, we also examined the association between the above-mentioned clinicopathological parameters and LVD in stage-I and II patients. The present study was performed with approval from the ethics committee of the Tottori University Faculty of Medicine (approval number: 1499).
Table 1.
Clinicopathological characteristics of 109 patients with SCC of lip and oral cavity
| Characteristics | Patients | LVD | P-value | |
| n (%) | (mean ± SD) | |||
| Gender | Male | 63 (57.5) | 19.0 ± 11.9 | 0.345 |
| Female | 46 (42.2) | 21.3 ± 12.2 | ||
| Age (yr) | Range | 23–93 | ||
| Mean ± SD | 65 ± 13 | 20.0 ± 11.9 | 0.928 | |
| Primary tumor size | T1 | 20 (18.3) | 23.4 ± 11.0 | 0.253 |
| T2 | 43 (39.4) | 21.6 ± 13.9 | ||
| T3 | 9 (8.3) | 21.7 ± 12.3 | ||
| T4 | 37 (34.0) | 15.9 ± 8.9 | ||
| Size of regional lymph node metastasis | N0 (no metastasis) | 65 (59.6) | 21.3 ± 12.6 | 0.608 |
| N1 (≤ 3 cm) | 18 (16.5) | 20.1 ± 13.4 | ||
| N2 (between N1 and N3) | 24 (22.0) | 17.0 ± 8.6 | ||
| N3 (> 6 cm) | 2 (1.8) | 12.0 ± 2.8 | ||
| Clinical stage (UICC) | Stage I | 18 (16.5) | 22.4 ± 9.7 | 0.971 |
| Stage II | 28 (25.7) | 22.8 ± 15.5 | ||
| Stage III | 16 (14.7) | 21.9 ± 14.1 | ||
| Stage IV | 47 (43.1) | 16.7 ± 8.7 | ||
| Histological differentiation | Well differentiated | 44 (40.4) | 21.3 ± 14.1 | 0.708 |
| Moderately differentiated | 46 (42.2) | 19.0 ± 10.4 | ||
| Poorly differentiated | 19 (17.4) | 19.5 ± 10.2 | ||
LVD, lymphatic vessel density; SCC, squamous cell carcinoma; UICC, Union Internationalis Contra Cancrum.
Table 2.
LVD among the primary sites of 109 patients with SCC
| Primary site | Patient | LVD |
| n (%) | (mean ± SD) | |
| Tongue | 41 (37.6) | 20.2 ± 8.7 |
| Gingiva | 45 (41.3) | 17.8 ± 10.4 |
| Buccal mucosa | 8 (7.3) | 25.9 ± 19.4 |
| Oral floor | 7 (6.4) | 15.3 ± 8.1 |
| Palate | 5 (4.6) | 14.6 ± 7.2 |
| Lip | 3 (2.8) | 54.3 ± 6.0*** |
LVD, lymphatic vessel density; SCC, squamous cell carcinoma.
***P < 0.0001.
Histopathological examination
Serial paraffin sections measuring 4 µm in thickness were prepared from tumor tissue blocks obtained at biopsy or surgery. One of each set of serial sections was subjected to hematoxylin-eosin (HE) staining and was used to evaluate histopathological findings according to the TMN classification of the Union Internationalis Contra Cancrum. The other serial sections were subjected to immunohistochemical analysis.
Immunohistochemistry
Immunohistochemical analysis was performed by the avidin-biotinylated peroxidase complex method. Paraffin sections were deparaffinized, and then subjected to microwave heating for 10 min in 0.01 M sodium citrate buffer (pH 6.0). Next, the sections were treated in methanol containing 0.3% hydrogen peroxide solution to inhibit endogenous peroxidase, incubated with a blocking reagent to prevent nonspecific reactions, and then incubated at 4 °C overnight with various primary antibodies (Table 3) at various dilutions. The sections were subsequently washed with phosphate-buffered saline (pH 7.4) and then incubated for 30 min with the biotinylated secondary antibody at room temperature, followed by reaction for 1 h with avidin-biotinylated peroxidase complex solution at room temperature. Color was developed with 3-3'-diaminobenzidine-tetrahydrochloride, and after nuclear staining, dehydration and lucidification, specimens were served for microscopic examination. The primary antibodies used were listed in Table 3. Sections incubated in phosphate-buffered saline without any primary antibody were used as the negative control.
Table 3.
Antibodies used and dilutions
| Antibody | Clone | Dilution | Antigen retrieval | Source |
| D2-40 | D2-40 | 1:100 | Microwave heating | DAKO (Glostrup, Denmark) |
| VEGF-C | Polyclonal | 1:400 | Microwave heating | R&D Systems (Mineapolis, MN) |
| VEGF-D | 78923 | 1:100 | Microwave heating | R&D Systems (Mineapolis, MN) |
VEGF, vascular endothelial growth factor.
LVD measurement
Sections immunostained with D2-40 were used to measure the lymphatic vessel count. Clinicopathological findings were evaluated by 2 independent examiners (SW and MK) in a blinded fashion, and mean values were calculated. Lymphatic vessel measurement was performed according to a modification of Weidner’s method (1991).12 In brief, a region of the peritumoral stroma that was appropriate for determining the lymphatic vessel count was defined as a hot spot (Fig. 1), and was used to measure the number of lymphatic vessels positive for D2-40 in each of 2 arbitrary fields under an objective lens with a magnification power of 20 (field area: 0.949 mm2). Then the total number of lymphatic vessels in the 2 fields was calculated. The total lymphatic count of the 2 fields in the hot spot was compared among groups stratified for age, sex, primary tumor site, clinical stage, tumor size, grade and the presence or absence of lymphatic metastasis.
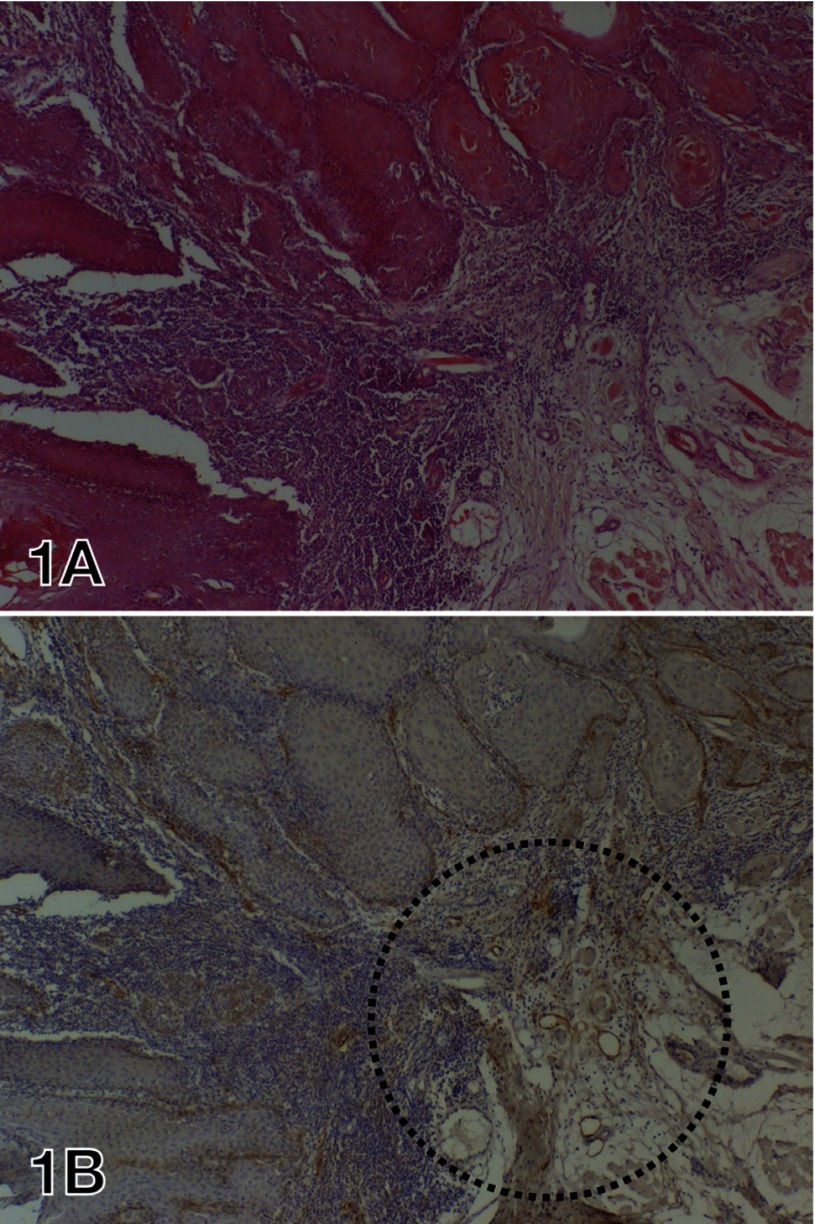
Fig. 1.
HE staining and D2-40 immunostaining of resected SCC specimens of the tongue.
A: Well differentiated SCC showing solid proliferation.
B: There are D2-40-positive lymphatic vessels in the peritumoral stroma. The cytoplasm of some tumor cells at the margin of the cancer is also positive for D2-40. The circled area was defined as the hot spot. Lymphatic vessels were counted in 2 hot spot fields and totaled.
HE, hematoxylin and eosin; SCC, squamous cell carcinoma.
Examination of VEGF-C and D expressions
Staining for VEGF-C or D was performed using serial paraffin sections. For both VEGF-C and VEGF-D, more than 50% positive tumors cells were judged as positive staining. We examined the relationship between the results of VEGF-C or D staining and clinicopathological findings or LVD.
Statistical Analysis
Relationships between clinicopathological parameters and LVD were assessed by multivariate analysis using a multiple linear regression model. Associations between the expression of VEGF-C or D and clinicopathological parameters or LVD were investigated by multivariate logistic regression analysis. For statistical analysis of the relationship between the primary site and LVD, analysis of variance was used, followed by multiple comparison with the Dunnett’s method and Scheff’s method. SPSS Statistics software version 19.0 (IBM, Tokyo, Japan) was used for all analyses, and statistical significance was accepted at P < 0.05.
RESULTS
Site-specific LVD in oral SCC
The LVD measured in the 2 hot spot fields was 20.2 ± 8.7 for tongue cancer, 17.8 ± 10.4 for gingival cancer, 25.9 ± 19.4 for cancer of the buccal mucosa, 15.3 ± 8.1 for oral floor cancer, 14.6 ± 7.2 for palatal cancer and 54.3 ± 6.0 for lip cancer (Table 2). HE- and D2-40-stained sections of representative examples of tongue, gingiva, lip, palate and oral floor cancers are shown in Figs. 2A to J. In the peritumoral stromal tissue, D2-40-poisitive lymphatic vessels of various sizes were observed (Figs. 2B, D, F, H and J). Blood vessel components apart from the lymphatic endothelium and the negative controls were negative for D2-40 staining. In addition, the margins of the tumor tissue and in infiltrating areas were D2-40 positive (Figs. 2B and F). Site-specific LVD showed a significant difference between lip cancer and cancers of the gingiva, tongue, buccal mucosa, oral floor and palate (post-hoc test, P < 0.0001). The LVD of lip cancer was significantly higher than that for cancers of the tongue, gingiva, buccal mucosa, oral floor and palate. However, no significant differences of LVD were noted among cancers of the tongue, gingiva, buccal mucosa, oral floor and palate.
Fig. 2.
HE staining and D2-40 immunostaining of SCC tissues in representative patients.
ACEGI: Well differentiated SCC showing cord-like proliferation and invasion. Bar = 100 µm.
BDFHJ: There are D2-40-positive lymphatics in the peritumoral stroma. These lymphatic vessels are clearly distinguishable from D2-40-negative blood vessels.
HE, hematoxylin and eosin; SCC, squamous cell carcinoma.
Association between clinicopathological findings and LVD
In relation to tumor size, LVD was 23.4 ± 11.0 for T1, 21.6 ± 13.9 for T2, 21.7 ± 12.3 for T3 and 15.9 ± 8.9 for T4 disease (Table 1). Regarding the association between LVD and tumor size, the lymphatic vessel count tended to decrease with an increase of tumor size, but there was no significant difference of LVD among each tumor size category. LVD was 21.3 ± 12.6 in patients without lymph node metastasis (N0 group), 20.1 ± 13.4 in the N1 group, 17.0 ± 8.6 in the N2 group and 12.0 ± 2.8 in the N3 group. There were no significant differences of LVD among these 4 groups, although LVD tended to decrease with an increase of lymph node metastasis size. According to the clinical tumor stage, LVD was 22.4 ± 9.7 in stage I, 22.8 ± 15.5 in stage II, 21.9 ± 14.1 in stage III and 16.7 ± 8.7 in stage IV. The lymphatic vessel count tended to decrease with stage progression, but multiple regression analysis revealed no significant difference of LVD between each stage. Regarding the histological grade of SCC, LVD was 21.3 ± 14.1 in well-differentiated SCC, 19.0 ± 10.4 in moderately differentiated SCC and 19.5 ± 10.2 in poorly differentiated SCC. There was no significant difference of LVD among the different grades of SCC. No significant differences of LVD were found in relation to sex and age.
Assessment of the association between LVD and clinicopathological findings in stage-I and II patients revealed no significant correlations between LVD and age (P = 0.712), sex (P = 0.873), tumor size (P = 0.772) or tumor histological grade (P = 0.057) by multiple regression analysis.
Relationship between VEGF-C or D immunostaining and LVD or clinicopathological findings
Immunostaining was positive for VEGF-C and D in the cytoplasm of tumor cells ( Figs. 3B, E and F). Tumor tissue with more than 50% positive cells is judged to be positive for VEGF staining. Based on this criterion, we determined 84 of the 109 tumors to be positive for VEGF-C (Fig. 3B and E) and 25 of them, negative (Fig. 3G). In the case of VEGF-D, we determined 84 of the 109 tumors to be positive for VEGF-D, which were also positive for VEGF-C (Figs. 3E and F). In other words, the expression patterns of VEGF-C and D were identical.
Fig. 3.
HE staining, D2-40 immunostaining, VEGF-C immunostaining and VEGF-D immunostaining of SCC.
A: Well-differentiated SCC showing cord-like proliferation and invasion.
B: The cytoplasm of tumor cells is positive for VEGF-C staining. Non-neoplastic squamous cells in the tongue mucosa are negative for VEGF-C.
C: Small cords of SCC cells proliferated and invaded the stromal tissues of the tongue.
D: Several D2-40-postivie lymphatics are observed in the stroma in the vicinity of the tumor tissues. Blood vessels are negative for D2-40.
E: The majority of the tumor cell nests are strongly positive for VEGF-C.
F: The majority of tumor cell nests are positive for VEGF-D. The VEGF-D staining pattern corresponds to that of VEGF-C.
G: A few tumor cells are positive for VEGF-C, but many tumor cells are negative.
H: Many D2-40-positive lymphatic vessels can be seen in the stroma near the tumor tissues. Tumor cells in the infiltrating lesion (left upper region) are also strongly positive for D2-40.
The same magnification was used for A to H. Bar = 100 µm. HE, hematoxylin and eosin; SCC, squamous cell carcinoma; VEGF, vascular endothelial growth factor.
When we investigated the relationship between LVD and staining for VEGF-C or D, LVD was 19.7 ± 11.9 in VEGF-C-positive patients and 21.2 ± 12.2 in VEGF-C-negative patients. Regarding the association between LVD and either positive or negative staining for VEGF-C or D, logistic regression analysis revealed no statistically significant associations. When VEGF-C/VEGF-D positivity and D2-40 staining were compared, LVD was low in some VEGF-C- and D-positive tumors (Figs. 3D–F), while LVD was high in some VEGF-C- and D-negative tumors (Figs. 3G and H).
Regarding the relationship between clinicopathological findings and VEGF staining, the VEGF-C-positive rate increased significantly with an increase in the size of lymph node metastases (logistic regression analysis, P = 0.02). Similarly, the VEGF-D-positive rate increased significantly with an increase in the size of lymph node metastases (P = 0.02). However, no significant association was observed between VEGF expression and the clinicopathological factors such as tumor size and clinical stage.
DISCUSSION
SCC of the oral region is likely to cause lymph node metastases, which influences the prognosis. For this reason, several studies have investigated the association between lymphatic vessels and clinicopathological findings in patients with oral SCC.9, 13, 14 However, these studies mainly focused on SCC of the tongue and the number of patients investigated was limited. In the present study, we investigated the relationship between histopathologically assessed LVD and clinicopathological findings in 109 patients with SCC of various parts of the oral cavity, including the lip; i.e., the gingiva, tongue, buccal mucosa, floor of the mouth and palate. No study has ever examined more than 100 patients with SCC originating in the oral region, particularly with stratification according to the site of the primary tumor.
With regard to LVD in oral SCC, the tumor site-specific LVD was 28.3 ± 12.5 for tongue cancer and 16.5 ± 5.66 for cancer at other sites.13 The peritumoral LVD of oral cancer ranged widely from 1 to 43.9 Moreover, the LVD of oral SCC was 12.89 ± 9.31.14 In the present study, we totalled the lymphatics in two 0.949-mm2 fields. When converted to a per-unit value, our LVD values were slightly lower than or similar to those reported above.
As we observed in the present investigation of site-specific LVD, lip cancer showed a significantly higher LVD level than cancer arising at other sites.15, 16 Studies on lip cancer were not many, especially with regard to LVD, and no study reported higher LVD in the peritumoral "hot spot" of lip cancer than in other types of oral cancer. This result may be related to an anatomical feature of the lip, high vascularity, which imparts the characteristic red color. Despite the high LVD observed in lip cancer at the hot spot, the prognosis of this cancer is quite good, with a low frequency of cervical lymph node metastasis. Other factor like the distance from the cervical lymph nodes might also influence the biological behavior of lip cancer. Furthermore, the lip is a prominent site where tumors are easily detected, which contributes to early diagnosis and treatment. This might also explain why the prognosis of lip cancer is better than that of cancer of other oral regions. In general, oral cancer of the tongue and floor of the mouth metastasize to the cervical lymph nodes relatively often. However, in the present investigation of site-specific LVD in peritumoral hot spots, we found no significant differences among sites other than the lip, such as the gingiva, tongue, buccal mucosa, oral floor and palate. Therefore, based on the mechanism of cervical lymph node metastasis which occurs more easily in cancer of the tongue and oral floor than in cancer of the gingiva and palate, we found that the LVD of hot spots does not directly reflect susceptibility to cervical lymph node metastasis.
Regarding the relationship between LVD and oral SCC, lymphangiogenesis was associated with metastasis-free survival in tongue cancer patients.13 In addition, the presence of lymph node metastasis was significantly associated with a higher intratumoral lymphatic density, a higher incidence of tumor relapse and shorter levels of 5-year overall survival and disease-free survival.9 Moreover, LVD was correlated with tumor grade and nodal status in oral SCC patients.17 In the present study of 109 patients with lip and oral SCC, we assessed all correlations between LVD and clinicopathological parameters including tumor size, presence or absence of lymph node metastases, histological grade and clinical tumor stage; however, multivariate analysis revealed no significant associations between LVD and clinicopathological parameters. In addition, we examined the associations between LVD and these clinicopathological parameters among stage-I and II patients without lymph node metastasis, and found no significant associations between LVD and any of these factors. Rather, our results showed lymphatic vessel count decrease along with tumor size increase, which tended to decrease with the advance of disease stage or lymph node metastasis. In an investigation of adenocarcinoma of the rectum examined on the association between LVD and clinicopathological factors,18 the prognosis was not necessarily correlated with the LVD at the peripheral zone of the tumor or inside tumor tissue, but actually with lymphangiogenesis in the regional lymph nodes. In patients with oral cancer, the primary tumor induces sentinel lymph node lymphagiogenesis prior to the onset of lymph node metastasis.19 In other words, lymphagiogenesis in the regional lymph nodes, rather than peritumoral lymphatic hyperplasia, is an important prognostic factor.
VEGF-C and D promote lymphatic vessel growth and are involved in lymphangiogenesis. 20 Both VEGFs are related to LVD, and bind to VEGF receptor-3 (VEGFR-3) on the surface of lymphatic endothelial cells to induce the proliferation and growth of new lymphatic vessels.21 Expression of both VEGFs has been reported in oral cancer.22 In the present study, immunostaining of oral SCC specimens showed a high positivity for VEGF-C and D (84/109 both; 77%). Moreover, when the staining patterns were compared, VEGF-C and D showed almost identical patterns. Significant associations between VEGF-C expression and LVD were reported.14, 17 We observed no significant association of VEGF-C and D positivity with LVD, but significantly more VEGF-C and D positivity among patients with larger lymph node metastases. It is likely that lymphangiogenesis occurs due to the production of VEGF-C by tumor cells, but conversely LVD in peritumoral hot spots tends to decrease with an increase of tumor size and stage progression. The production of VEGF-C by tumor cells also promotes lymphangiogenesis, but lymphatic vessels at the tumor periphery are damaged due to tumor progression and this may lead to a decrease of the LVD. Furthermore, there is a possibility that lymphangiogenesis also occurs in remote sentinel lymph nodes due to VEGF production. In relation to VEGF-C and D expressions in tumor tissue, it is thought that lymphagiogenesis in the regional lymph node affects the prognosis. These reports support our present finding that lymph node metastasis was more frequent in VEGF-C and D-positive patients.
In conclusion, we examined the association between clinicopathological parameters and LVD or the expression of VEGF-C or D in SCC of the oral region including the lip. Our site-specific investigation revealed a significant increase of the LVD in lip cancer, whereas no significant difference of LVD was observed in cancers arising at other sites. In addition, in terms of the relationship between clinicopathological parameters and LVD, LVD tended to decrease with increasing disease stage and size of lymph node metastasis. Furthermore, patients with larger lymph node metastases were significantly more likely to be positive for VEGF-C and D. Peritumoral LVD decreased with tumor progression, while the expression of VEGF showed a strong correlation with lymph node metastasis, suggesting that further study is needed to determine the mechanisms of cervical lymph node metastasis in patients with oral SCC.
The authors declare no conflict of interest.
REFERENCES
- 1.Johnson N, Franceschi S, Ferlay J, Ramadas K, Schmid S, MacDonald DG.Squamous cellcarcinoma In: Barnes L MacDonald DG Reichart P Sidransky D, editors. World Health Organization Classification of Tumours. Pathology & Genetics Head and Neck Tumours. Lyon: IARC Press; 2005. p. 168-175 Japanese. [Google Scholar]
- 2.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009; 45: 309-316 [DOI] [PubMed] [Google Scholar]
- 3.Kreppel M, Scheer M, Drebber U, Ritter L, Zöller JE. Impact of podoplanin expression in oral squamous cell carcinoma: clinical and histopathologic correlations. Virchows Arch. 2010; 456: 473-482 [DOI] [PubMed] [Google Scholar]
- 4.Martínez-Gimeno C, Rodríguez EM, Vila CN, Varela CL. Squamous cell carcinoma of the oral cavity: a clinicopathologic scoring system for evaluating risk of cervical lymph node metastasis. Laryngoscope. 1995; 105: 728-733 [DOI] [PubMed] [Google Scholar]
- 5.Umeda M, Yokoo S, Take Y, Omori A, Nakanishi K, Shimada K. Lymp node metastasis in squamous cell carcinoma of the oral cavity: correlation between histologic features and the prevalence of metastasis. Head Neck. 1992; 14: 263-272 [DOI] [PubMed] [Google Scholar]
- 6.Beasley NJP, Prevo R, Banerji S, Leek RD, Moore J, van Trappen P. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 2002; 62: 1315-1320 [PubMed] [Google Scholar]
- 7.Yuan P, Temam S, El-Naggar A, Zhou X, Liu DD, Lee JJ.Overexpression of podoplanin in oral cancer and its association with poor clinical outcome. Cancer. 2006; 107: 563-569 [DOI] [PubMed] [Google Scholar]
- 8.Adachi Y, Nakamura H, Kitamura Y, Taniguchi Y, Araki K, Shomori K. Lymphatic vessel density in pulmonary adenocarcinoma immunohistochemically evaluated with anti-podoplanin or anti-D2-40 antibody is correlated with lymphatic invasion or lymph node metastases. Pathol Int. 2007; 57: 171-177 [DOI] [PubMed] [Google Scholar]
- 9.Zhao D, Pan J, Li XQ, Wang XY, Tang C, Xuan M. Intratumoral lymphangiogenesis in oral squamous cell carcinoma and its clinicopathological significance. J Oral Pathol Med. 2008; 37: 616-625 [DOI] [PubMed] [Google Scholar]
- 10.Raica M, Cimpean AM, Ribatti D. The role of podoplanin in tumor progression and metastasis. Anticancer Res. 2008; 28: 2997- 3006 [PubMed] [Google Scholar]
- 11.Garcia-Carracedo D, Rodrigo JP, Astudillo A, Nieto CS, Gonzalez MV. Prognostic significance of lymphangiogenesis in pharyngolaryngeal carcinoma patients. BMC Cancer. 2010; 10: 416-425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991; 324: 1-8 [DOI] [PubMed] [Google Scholar]
- 13.Miyahara M, Tanuma J, Sugihara K, Semba I. Tumor lymphangiogenesis correlates with lymph node metastasis and clinicopathologic parameters in oral squamous cell carcinoma. Cancer. 2007; 110: 1287-1294 [DOI] [PubMed] [Google Scholar]
- 14.Oriveira-Neto HH, Gleber-Netto FO, Ferreira de Sousa S, França CM, Ferreira Aguiar MC, Silva TA.A comparative study of microvessel density in squamous cell carcinoma of the oral cavity and lip. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012; 113: 391-398 [DOI] [PubMed] [Google Scholar]
- 15.Vartanian JG, Carvalho AL, de Araújo Filho MJ, Hattori M, Jr, Magrin J, Kowalski LP. Predictive factors and distribution of lymph node metastasis in l ip cancer patients and their implications on the treatment of the neck. Oral Oncol. 2004; 40: 223-227 [DOI] [PubMed] [Google Scholar]
- 16.Batista AC, Costa NL, Oton-Leite AF, Mendonça EF, Alencar RCG, Silva TA. Distinctive clinical and microscopic features of squamous cell carcinoma of oral cavity and lip. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109: e74-e79 [DOI] [PubMed] [Google Scholar]
- 17.Sedivy R, Beck-Mannagetta J, Haverkampf C, Battistutti W, Hönigschnabl S. Expression of vascular endothelial growth factor-C correlates with the lymphatic microvessel density and the nodal status in oral squamous cell cancer. J Oral Pathol Med. 2003; 32: 455- 460 [DOI] [PubMed] [Google Scholar]
- 18.Jakob C, Aust DE, Liebscher B, Baretton GB, Datta K, Muders MH. Lymphangiogenesis in regional lymph nodes is an independent prognostic marker in rectal cancer patients after neoadjuvant treatment. PLoS One. 2011; 6: e27402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishii H, Chikamatsu K, Sakakura K, Miyata M, Furuya N, Masuyama K. Primary tumor induces sentinel lymph node lymphangiogenesis in oral squamous cell carcinoma. Oral OncoL. 2010; 46: 373-378 [DOI] [PubMed] [Google Scholar]
- 20.Clarijs R, Ruiter DJ, de Waal RMW. Lymphangiogenesis in malignant tumours: does it occur?. J Pathol. 2001; 193: 143-146 [DOI] [PubMed] [Google Scholar]
- 21.Su JL, Yen CJ, Chen PS, Chuang SE, Hong CC, Kuo IH. The role of the VEGF-C/VEGFR-3 axis in cancer progression. Br J Cancer. 2007; 96: 541-545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shintani S, Li C, Ishikawa T, Mihara M, Nakashiro K, Hamakawa H. Expression of vascular endothelial growth factor A, B, C, and D in oral squamous cell carcinoma. Oral Oncol. 2004; 40: 13-20 [DOI] [PubMed] [Google Scholar]