Abstract
Targeting the proteasome system with bortezomib (BTZ) results in anti-tumour activity and potentiates the effects of chemotherapy/biological agents in multiple myeloma and B-cell lymphoma. Carfilzomib (CFZ) is a more selective proteasome inhibitor that is structurally distinct from BTZ. In an attempt to characterize its biological activity, we evaluated CFZ in several lymphoma pre-clinical models. Rituximab-sensitive cell lines (RSCL), rituximab-resistant cell lines (RRCL), and primary tumour cells derived from B-cell lymphoma patients were exposed to CFZ or BTZ. Cell viability and changes in cell cycle were determined. Western blots were performed to detect PARP-cleavage and/or changes in Bcl-2 (BCL2) family members. CFZ was 10 times more active than BTZ and exhibited dose- and time-dependent cytotoxicity. CFZ exposure induced apoptosis by upregulation of Bak (BAK1) and subsequent PARP cleavage in RSCL and RRCL; it was also partially caspase-dependent. CFZ induced G2/M phase cell cycle arrest in RSCL. CFZ demonstrated the ability to overcome resistance to chemotherapy in RRCL and potentiated the anti-tumour activity of chemotherapy agents. Our data suggest that CFZ is able to overcome resistance to chemotherapeutic agents, upregulate pro-apoptotic proteins to promote apoptosis, and induce G2/M cell cycle arrest in lymphoma cells. Our pre-clinical data supports future clinical evaluation of CFZ in B-cell lymphoma.
Keywords: Ubiquitin-proteasome system, carfilzomib, bortezomib, B-cell lymphoma
INTRODUCTION
The ubiquitin-proteasome system (UPS) regulates the turnover of proteins that control the cell cycle, programmed cell death, cell proliferation, survival, adhesion and differentiation (O’Connor 2005). Deregulation of these pathways has been well described in multiple malignancies including B-cell lymphoma (Adams 2004). Pharmacological inhibition of the UPS has become an attractive therapeutic strategy for certain types of malignancies and various selective and non-selective proteasome inhibitors have been developed.
Bortezomib (BTZ) was the first proteasome inhibitor approved by the Food and Drug Administration (FDA) for the treatment of multiple myeloma (MM) or relapsed/refractory mantle cell lymphoma (MCL) (Kane, et al 2007). It is a selective, reversible inhibitor of 26S proteasome chymotryptic activity. The introduction of BTZ as a single agent has demonstrated anti-tumour activity in relapsed/refractory MCL, follicular lymphoma (FL) and Hodgkin lymphoma (HL) patients (Goy, et al 2005, Kane, et al 2007, O’Connor 2005, Strauss, et al 2006, Younes, et al 2006).
Despite the observed pre-clinical and clinical activity of BTZ, a significant number of patients do not respond to BTZ-based therapies, develop acquired resistance to BTZ during therapy, or experience dose-limiting toxicities resulting in treatment discontinuation. BTZ-induced peripheral neuropathy (BIPN) was found to be the dose-limiting toxicity leading to dose-reduction or treatment discontinuation and subsequent failure to control the underlying neoplastic process (Cavaletti and Jakubowiak 2010). In MM patients, BIPN develops in 37%-44% of patients and the cumulative dose of BTZ is the strongest predictor of the severity of BIPN (Cavaletti and Jakubowiak 2010). The limited activity and the cumulative neurotoxicity observed with BTZ against certain subtypes of B-cell lymphoma stress the need to develop more potent and less toxic inhibitors of the UPS. A new generation of novel proteasome inhibitors are being developed and evaluated in pre-clinical models (e.g. Carfilzomib [CFZ]) (Dick and Fleming 2010).
CFZ is a novel irreversible proteasome inhibitor that is structurally and mechanistically different from BTZ and is now FDA-approved for treatment of relapsed/refractory MM. CFZ selectively inhibits the chymotrypsin-like activity of both the constitutive proteasome and the immunoproteasome (Parlati, et al 2009). Pre-clinical studies had shown that CFZ is more potent than BTZ in multiple myeloma cell lines when following a 1-h drug pulse (Kuhn, et al 2007). In addition, CFZ was found to be active against BTZ-resistant cell lines and primary tumour cells derived from patients with BTZ-refractory MM (Kuhn, et al 2007). A phase I clinical trial aimed at defining the maximum tolerated dose (MTD) of CFZ demonstrated that it was well tolerated and active in multiple haematological malignancies, including non-Hodgkin lymphoma (NHL), HL and MM (Alsina, et al 2012, O’Connor, et al 2009).
To better characterize CFZ’s activity, we conducted pre-clinical studies in rituximab-sensitive cell lines (RSCL) and rituximab-resistant cell lines (RRCL) representing specific subtypes of B-cell lymphoma (Burkitt lymphoma, activated B-cell [ABC] DLBCL and germinal centre B-cell [GCB] DLBCL); and primary lymphoma cells derived from patients. Our data demonstrates that CFZ has more potent anti-tumour activity compared to BTZ. CFZ has significant anti-tumour activity against various subtypes of DLBCL (ABC and GCB subtypes) and is capable of overcoming rituximab-chemotherapy resistance. Furthermore, CFZ potentiates the cytotoxic effects of paclitaxel, vincristine, gemcitabine, etoposide and carboplatin. Taken together, our data strongly suggest that CFZ is a promising proteasome inhibitor against resistant B-cell lymphoma, providing a rationale for the clinical evaluation of CFZ in combination with chemotherapy agents in rituximab-relapsed/refractory aggressive B-cell lymphoma.
MATERIALS AND METHODS
Cell Culture and Reagents
RSCL or RRCL were used for the experiments. RSCL Raji was purchased from American Type Culture Collection (ATCC, Manassas, VA). RRCL were generated by repeatedly exposing RSCL to escalating doses of rituximab (0.1-128 μg/ml) in the absence (Raji 2R) or presence (Raji 4RH) of human complement (1:1,000-1:1.875) as previously described (Czuczman, et al 2008). All the cell lines were maintained in RPMI 1640 medium (Sigma Chemical, St. Louis, MO) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Atlanta Biologicals, Norcross, GA), 5 mM HEPES, 100 u/ml penicillin and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA). CFZ was provided by Onyx Pharmaceuticals Inc. (South San Francisco, CA). In addition, BTZ, paclitaxel, and vincristine were obtained from the Pharmacy Department at Roswell Park Cancer Institute (RPCI). Cisplatin was purchased from American Pharmaceutical Partners (Schaumburg, IL) and doxorubicin obtained from Bedford Labs (Bedford, OH).
Therapeutic antibodies, rituximab (anti-CD20) and trastuzumab (anti-Her-2/neu; used as isotype control) were obtained from Genentech, Inc. (South San Francisco, CA) and, unless otherwise specified, were used at a final concentration of 10 μg/ml.
Primary mouse anti-human antibodies raised against Bak (BAK1) and actin (ACTB) were obtained from Sigma Chemical, Bik (BIK) from Santa Cruz Biotechnology (Santa Barbara, CA), PARP1 from BD Pharmigen™ (San Jose, CA), caspase 3 (CASP3; total and cleaved form) from Cell Signaling (Trask Lane Danvers, MA), Noxa (PMAIP1) from Calbiochem (San Diego, CA), p53 (TP53) and puma (BBC3) from BD Bioscience (San Jose, CA) and LC3 (MAP1LC3A) from MBL International (Woburn, MA). Alkaline phosphatase (AP) or horseradish peroxidase (HRP)-conjugated anti-mouse secondary antibodies were purchased from Jackson ImmunoResearch (West Grove, PA).
Ficoll-Hypaque was purchased from Sigma Chemical. Sodium chromate 51 (51Cr) and [3H] thymidine radioisotopes (Perkin-Elmer Life Inc., Boston MA) were utilized in functional assays assessing antibody-associated cytotoxicity and cell proliferation, respectively. Triton X-100, trypan blue and histopaque-1077 were obtained from Sigma-Aldrich Inc. (St Louis, MO). CellTiter Glo Luminescent Viability Assay reagent was purchased from Promega (Madison, WI). Three-methyladenine was obtained from Sigma Chemical; zVAD-fmk and Q-VD-OPh from MBL International.
Patient Samples
Neoplastic B-cells were isolated from biopsy tissue obtained from 28 patients with B-cell NHL or lymphocyte-predominant Hodgkin lymphoma (LPHL) treated at RPCI as previously described (Brem, et al 2011). Briefly, samples from patient biopsy specimens were procured under Institutional Review Board (IRB) RPCI protocols I42804 and I42904. Tissue specimens were placed in phosphate-buffered saline (PBS)-containing collagenase type IV (1 mg/ml; Sigma-Aldrich) and incubated for 15 min at 37°C, including manual agitation for 5 min. Next, samples were diluted with RPMI 1640 medium containing 10% FBS and the cell suspension filtered through a 100 μm cell strainer to remove large clumps. Subsequently, lymphocytes were enriched for by density centrifugation. B-cells were then isolated from enriched lymphocytes by magnetic-activated cell sorting (MACS) separation using a human B-cell Isolation Kit II (Miltenyi Biotec, Gladbach, Germany). B-cell purity was assessed by flow cytometry using antibodies against CD19 and CD20 (Becton Dickenson, San Jose, CA). Greater than 95% pure CD19+ cells were obtained from the first 8 specimens processed. Collected cells were re-suspended in RPMI media and seeded into 384-well plates at a density of 0.5 × 106/ml and treated in triplicate with various doses of CFZ or BTZ. Forty-eight hours after treatment, cell viability was determined.
In vitro effects of CFZ on the viability of NHL cells
In order to compare the anti-tumour activity between CFZ and BTZ, RRCL, RSCL, or tumour cells isolated from newly diagnosed or relapsed/refractory lymphoma patients, cells were exposed to escalating doses of CFZ, BTZ or control (dimethyl sulfoxide [DMSO]) for 24 or 48 h. Cells were plated at a cell density of 5 × 105 cells/ml. At each time period, cell viability was determined using the CellTiter Glo Luminescent Viability Assay reagent (Promega) and read out using the Thermo Fluoroscan Ascent FL scan (Thermo Fisher Scientific, Waltham, MA). Experiments were performed in triplicates.
In vitro effects of CFZ on DNA fragmentation, caspase 3/7 activity and ROS production
To further evaluate the activity of CFZ, we evaluated the degree of apoptosis induced by this novel proteasome inhibitor in B-cell lymphoma cells. In brief, after exposure to CFZ or BTZ for 24, 48 and 72 h, RSCL or RRCL cells were harvest and fixed with 70% ethanol at −20°C overnight, incubated with 100 μg/ml RNAse (Sigma-Aldrich) for 30 min and stained with PI (50μg/ml) (Sigma-Aldrich). DNA content was analysed. The percentage of apoptotic cells was measured by the number of propidium iodide (PI)-stained cells using the FACSC-Calibur (BD Biosciences) flow cytometer and analysed with the FCS express software. Hypodiploid cells accumulated at sub-G0 phase of the cell cycle were counted as apoptotic cells. Caspase 3/7 activity was detected using the luminometric Caspase-Glo 3/7 assay (Promega) following the manufacturer’s protocols. Untreated cells were used as normalized control. Reactive oxygen species (ROS) production was determined by staining cells with 5 μM dihydrorhodamine 123 (Invitrogen). We exposed lymphoma cell lines to BTZ (10 nM) or CFZ (1, 10 nM) for 24 or 48 h. Subsequently, cells were washed with PBS, re-suspended in 2.5 ml of PBS containing 5 μM dihydrorhodamine 123 and incubated at 37°C for 30 min in the dark. ROS was determined by the oxidation of dihydrorhodamine 123 to cationic rhodamine 123 that usually localizes in the mitochondria and exhibits green fluorescence. The intensity of fluorescence was read by flow cytometry.
Effect of caspase inhibition on CFZ anti-tumour activity
RSCL and RRCL were exposed to the pan-caspase inhibitors zVAD-fmk (50 μM) and Q-VD-OPh (5 μM) (MBL International, Montreal, CAN) for 1 h and subsequently treated with CFZ (5 nM) or vehicle (control). Differences in cell viability following caspase inhibition were determined using the CellTiter Glo Luminescent Viability Assay reagent.
Changes in the expression of several pro-apoptotic or anti-apoptotic proteins in RSCL or RRCL after exposure to CFZ
RSCL and RRCL cells were exposed to CFZ (1 nM) or BTZ (10 nM) for 24 h and were lysed in radioimmunoprecipitation assay (RIPA) buffer containing proteasome inhibitors (Thermo Scientific) for 30 min. Proteins were separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes (iBlot Gel transfer stacks, Invitrogen, Grand Island, NY) by iBlot Dry Blotting System (Invitrogen). Changes in key regulators of the apoptotic pathway were evaluated using specific primary and secondary monoclonal/polyclonal antibodies. Image J software (http://rsbweb.nih.gov/ij/) was used to quantify protein expression levels. Bak and Bcl-2 expression graphs were normalized to actin expression levels.
Effects of CFZ in the cell cycle of RSCL and RRCL
RSCL and RRCL were treated with CFZ or BTZ at 10 nM for 24 h. Cells were then washed with PBS and fixed with 70% ethanol at −20°C, incubated with 100 ug/ml RNase for 30 min (Sigma-Aldrich), and stained with 50 ug/ml PI (Sigma-Aldrich). DNA content was determined by flow cytometry using FACSC-Calibur (BD Biosciences) and differences in cell cycle between treated versus untreated cells were analysed using the FCS express software.
In vitro effects of CFZ on chemotherapeutic agents
After pretreatment with different doses of CFZ or BTZ for 24 h, RSCL and RRCL were plated at a cell density of 0.5 × 106 cells/ml and exposed to escalating doses of paclitaxel, gemcitabine, vincristine, gemcitabine and carboplatin for another 48 h. Cell viability was determined by the CellTiter Glo Luminescent Viability Assay reagent. Each experiment was repeated in triplicates.
51Cr release assay for the assessment of the impact that CFZ exposure has on rituximab-mediated complement-mediated cytotoxicity (CMC) and antibody-dependent cellular cytotoxicity (ADCC)
RSCL or RRCL were exposed in vitro to CFZ (1 nM), BTZ (10 nM) or DMSO (0.001%) and incubated at 37°C and 5% CO2 for 24 h. Subsequently, 2 × 106 viable cells were labelled with 51Cr at 37°C, 5% CO2 for 2 h. 51Cr-labelled-RSCL or -RRCL were then placed in 96-well plates at a cell concentration of 1 × 105 cells/well (CMC assay) or 1 × 104 cells/well (ADCC assay). Cells were then exposed to rituximab or isotype (trastuzumab) and human serum (CMC, 1:4 dilution) or peripheral blood mononuclear cells (PBMCs) (ADCC, 40:1, effector: target ratio) for 6 h at 37°C and 5% CO2, respectively. 51Cr release was measured from the supernatant by standard gamma counter and the percentage of lysis was calculated as following: % Lysis = [Test cpm – background cpm]/[Maximum cpm – background cpm]. PBMCs were obtained from healthy donors (IRB-approved protocol CIC-016). Pooled human serum was utilized as the source of complement for CMC assays.
STATISTICS
All experiments were performed in triplicate on three separate occasions. Data was plotted and analysed using the SPSS 14.0 for windows 2003 software. For in vitro studies, statistical differences between treatment groups and controls were determined by paired t-test. p values less than 0.05 were considered statistically significant.
RESULTS
CFZ is more active than BTZ against RSCL and RRCL
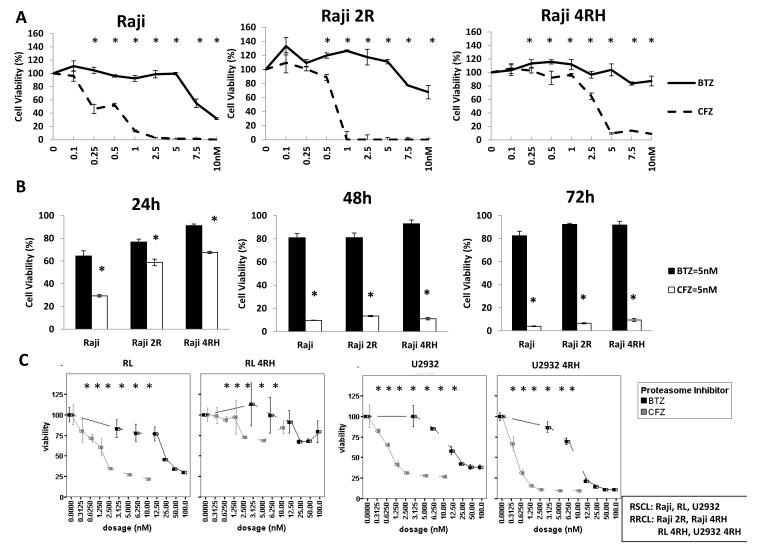
The anti-tumour activity of CFZ versus BTZ in our RSCL and RRCL was evaluated. We found that CFZ was more active than BTZ in inducing cell death in all RSCL and RRCL cell lines tested. In Raji cells and the rituximab-resistant cells (Raji-4RH and Raji 2R), CFZ induced cell death to a higher degree than BTZ at the dose-time schedule evaluated (Figure 1A, 1B). On average, the 50% inhibitory concentration (IC50) of CFZ was approximately 10-fold lower when compared to BTZ IC50 in all of the cell lines tested representing Burkitt lymphoma (Raji), GCB DLBCL (RL); or ABC DLBCL (U2932) (See Table I).
Figure 1. Carfilzomib (CFZ) induces dose- and time-dependent cell death in RRCL and RSCL.
(1A) Dose-dependent effects of CFZ and Bortezomib (BTZ) in Burkitt lymphoma cell lines. Raji and Raji therapy-resistant cell lines (i.e. Raji 2R and Raji 4RH) were exposed to CFZ or BTZ at the indicated concentrations in RPMI media in 386-well plates for 48 h and cell viability was assessed by CellTiter Glo assay. The data are presented as the mean (+ S.D.) of three independent experiments. (1B) Time-dependent effects of CFZ and BTZ in Raji family cells. Raji and Raji therapy-resistant cell lines (Raji2R and Raji4RH) were exposed to CFZ or BTZ at the indicated concentrations for 24, 48, and 72 h. (1C) Dose-dependent effects of CFZ and BTZ in germinal centre B (GCB) (i.e. RL and RL 4RH) and activated B-cell (ABC)-like (i.e. U2932 and U2932 4RH) cells. Lymphoma cells were exposed to either BTZ or CFZ at the indicated concentrations for 48 h. Each data presents the mean + S.D. of three independent experiments. Asterisks (*) represent a p-value ≤ 0.05 comparing CFZ vs. BTZ
Table I.
CFZ and BTZ IC50 values of RSCL and RRCL.
| Cell lines IC50 (nM) | BTZ | CFZ |
|---|---|---|
| Raji (Burkitt) | 8.255±0.133 | 0.575±0.028 |
| Raji 2R (Burkitt) | 10.971±0.321 | 1.985±0.489 |
| Raji 4RH (Burkitt) | 18.921±2.430 | 3.647±0.600 |
| RL (GCB) | 22.974±0.466 | 1.909±0.131 |
| RL 4RH (GCB) | 38.268±2.565 | 4.722±0.703 |
| U2932 (ABC) | 21.598±0.304 | 1.806±0.168 |
| U2932 4RH (ABC) | 10.643±1.814 | 1.406±0.005 |
Rituximab-Sensitive Cell Lines (RSCL): Raji, RL, U2932
Rituximab-Resistant Cell Lines (RRCL): Raji 2R, Raji 4RH, RL 4RH, U2932 4RH
Differences in the 50% inhibitory concentration (IC50) between Carfilzomib (CFZ) and Bortezomib (BTZ) in rituximab-sensitive cell lines (RSCL) and rituximab-resistant cell lines (RRCL) was measured as the concentration of BTZ or CFZ required reduced cell viability by 50% and assessed by CellTiter Glo. GCB, germinal centre B (GCB) cells; ABC, activated B-cells.
Carfilzomib is more potent in inducing cell death in “ABC” type versus “GCB” type of DLBCL
Clinically and pathologically, DLBCL patients are divided into two subgroups according to the cell of origin: GCB versus non-GCB (mainly ABC-like) according to the Hans (immunohistochemistry) Algorithm (Hans, et al 2004). BTZ was reported to enhance chemotherapy-associated anti-tumour activity against relapsed/refractory ABC-DLBCL (i.e. non-GCB DBCL) (Dunleavy, et al 2009). Therefore, we were interested in testing the efficacy of CFZ with ABC and GCB DLBCL cell lines, as well as rituximab-resistant cells. We exposed a panel of well characterized ABC- and GCB-DLBCL cell lines to escalating doses of either CFZ or BTZ (Fig 1C). CFZ was found to be more potent than BTZ in all cell lines tested regardless of the cell of origin (ABC or GCB). In addition, CFZ appeared to be more effective in ABC-DLBCL cell lines than in GCB-DLBCL. However, anti-tumour activity was observed in both subtypes of B-cell DLBCL. Interestingly, both BTZ and CFZ were more effective in ABC rituximab-resistant cells than rituximab-sensitive cells, but CFZ demonstrated greater potency than BTZ. This observation indicated that CFZ might have a significant role in reversing rituximab-resistance in ABC cells; further data is needed in order to validate these preliminary findings.
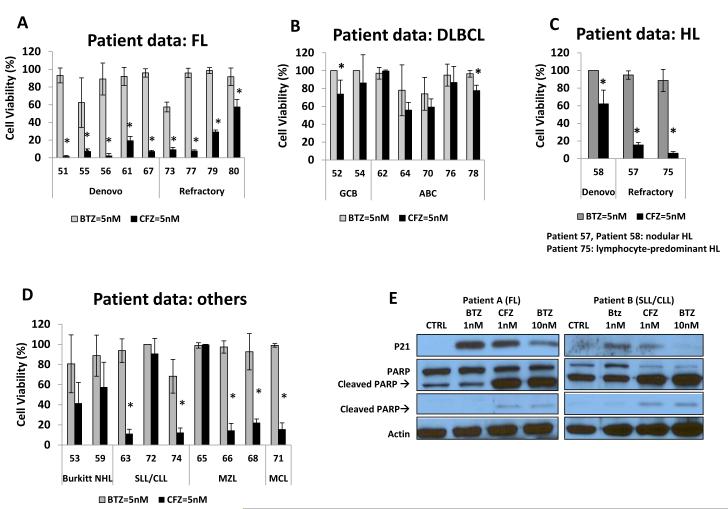
Carfilzomib has potent anti-tumour activity in tumour cells derived from lymphoma patients with FL, DLBCL, HL and other subtypes
Subsequently, we compared the anti-tumour activity of CFZ and BTZ against primary tumour cells derived from patients with various subtypes of lymphoma. A total number of 28 patient samples were obtained representing various subtypes of lymphoma, including FL (n=9), DLBCL (n=7), HL (n=3) and others (n=9) (Figure 2). Of interest, CFZ activity was observed in both de novo and relapsed/refractory lymphoma. Cell death was observed at all doses tested (1 nM and 5 nM) and was more pronounced at 48 h (data not shown). When analysing DLBCL using the Hans algorithm, decreased cell viability was observed in both GCB (n=2) and in non-GCB lymphomas (n=5) (Figure 2B). In addition, CFZ decreased cell viability in other subtypes of NHL, such as MCL, marginal zone lymphoms (MZL) and small lymphocytic leukaemia (SLL)/chronic lymphocytic leukaemia (CLL). No biological effects were noted in 2 samples obtained from patients with benign lymphoid hyperplasia (data not shown).
Figure 2. Carfilzomib (CFZ) exhibits significant antitumour activity against various subtypes of primary B-cell lymphoma patient samples.
Exposure of primary lymphoma cells derived from patients with follicular lymphoma (FL), (2A); diffuse large B-cell lymphoma (DLBCL), (2B); Hodgkin lymphoma (HL), (2C); and others (Burkitt lymphoma (Burkitt NHL), marginal zone lymphoma (MZL), mantle cell lymphoma (MCL) and small lymphocytic lymphoma / chronic lymphoma leukaemia (SLL/CLL)), (2D); to either CFZ 5 nM or BTZ 5 nM resulted in variable cell death. The percentage of cell viability was evaluated by CellTiter Glo assay after a 48-h incubation. Asterisks (*) represent a p-value ≤ 0.05 comparing CFZ vs. bortezomib (BTZ) within a given patient sample. (2E); Primary B-cells from two B-cell lymphoma patients, one with FL and the second with SLL/ CLL, were isolated for their response to either BTZ (1 nM, 10 nM) or CFZ (1 nM) at 24 h. Changes in P21 expression and apoptotic protein PARP cleavage were detected by Western Blot.
Consistent with our observations in lymphoma cell lines, CFZ also exhibited a more potent activity than BTZ in primary tumour cells derived from lymphoma patients. When compared to BTZ, CFZ induced a higher degree of cytotoxicity in de novo or relapsed/refractory FL (n=9/9), DLBCL (n=2/7) and in all HL samples analysed (n=3/3). While the activity of CFZ in tumour cells derived from HL patients is interesting (Patient 75 is lymphocyte-predominate HL, and Patients 57 and58 are nodular HL), especially because BTZ lacks significant clinical activity in HL patients; additional samples are needed to further define the anti-tumour effects of CFZ in HL (Figure 2C). In addition, CFZ decreased cell viability in 50% (2/4) of SLL/CLL, 66% (2/3) of MZL and in 1 of 1 MCL primary lymphoma samples (Figure 2D).
When sufficient tumour cells were obtained to perform downstream signalling studies, we evaluated the execution of PARP cleavage following ex vivo exposure to either BTZ or CFZ. Primary tumour cells isolated from two patients: 1) FL (Patient A) or 2) SLL/CLL, were exposed in vitro to either BTZ or CFZ. Exposure of both patients A and B to a ten-fold lower dose of CFZ induced a similar cleavage of PARP as BTZ (i.e. CTZ = 1 nM vs. BTZ = 10 nM). At the same dosage (1 nM), CFZ induced a larger cleavage of PARP than BTZ in both patients. Our data suggests that CFZ not only has potent anti-tumour activity in cell lines, but may also have better killing capacity than BTZ in more clinically relevant primary lymphoma samples.
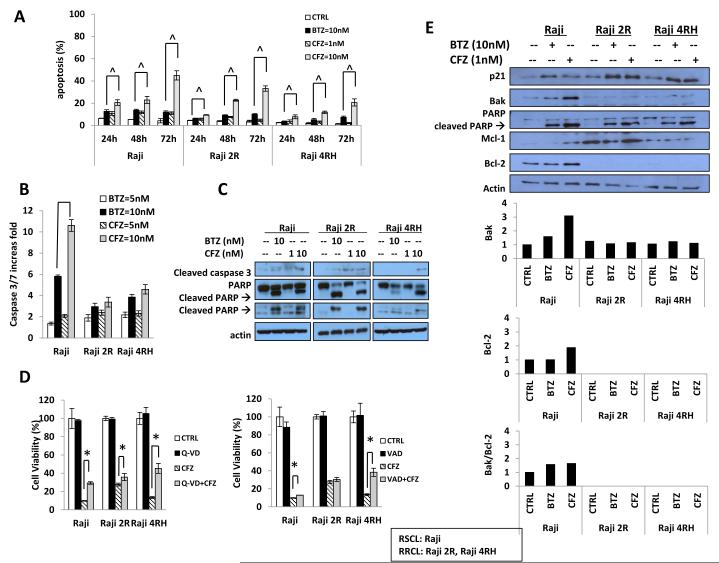
Carfilzomib induces apoptosis and up-regulates pro-apoptotic molecules in RSCL and RRCL
We previously demonstrated that BTZ has distinct and complementary mechanisms of action in RSCL and RRCL. (Olejniczak, et al 2010, Olejniczak, et al 2008). The expression of the pro-apoptotic Bcl-2 family proteins Bak, Bik and Noxa were substantially increased in our RRCL following incubation with bortezomib. Moreover, kinetic analysis of bortezomib-induced Bak, Bik and Noxa expression revealed rapid increase in the expression of these proteins following bortezomib exposure of RRCL prior to caspase-dependent PARP and Mcl-1 (MCL1) cleavage. We demonstrated that BTZ could induce cell death primarily through caspase-dependent programmed cell death (apoptosis) and secondarily by the execution of caspase-independent pathways (e.g. autophagy or senescence) (Olejniczak, et al 2010, Olejniczak, et al 2008).
In order to study if similar effects were observed in lymphoma cells exposed to CFZ, we compared the degree of apoptosis induced by either CFZ or BTZ in B-cell lymphoma cell lines. In vitro exposure of RSCL and RRCL to a 10-fold lower dose of CFZ induced a similar degree of apoptosis as BTZ (i.e. 1 nM vs. 10 nM); (Figure 3A). The degree of apoptosis was time-dependent and, as previously described in BTZ studies, the induction of apoptosis was slower in RRCL than in RSCL (48 h vs. 24 h). At equivalent doses (10 nM), CFZ induced a higher degree of apoptosis than BTZ in all the cell lines tested and at each time interval studied. The induction of apoptosis was further confirmed by caspase 3/7-activity assay, total caspase 3 reductions and formation of cleaved caspase 3. We found CFZ induced caspase 3/7 activity of a higher fold (P<0.05) than BTZ in RSCL in 24 h (Figure 3B). Moreover, CFZ induced more cleaved caspase 3 in both RSCL and RRCL (Figure 3C). To test the ability of CFZ to kill cells in the absence of caspase activity, we exposed sensitive and resistant cells to CFZ or BTZ with or without the pan-caspase inhibitors zVAD-fmk or Q-VD-OPh. We found that in RSCL, caspase inhibition repressed CFZ- and BTZ-induced cell death. In RRCL, caspase inhibition had a very limited effect on CFZ-induced cell death (Figure 3D). In vitro exposure of RSCL to CFZ at a one tenth of the BTZ dose (i.e. 1 nM vs. 10 nM) led to PARP cleavage. More importantly, in RRCL, low-dose CFZ (1 nM) induced Bak and Mcl-1 expression leading to PARP cleavage (Figure 3E). These data suggest that, in addition to triggering an apoptotic phenotype in resistant B-NHL cells, CFZ is capable of killing them in a caspase-independent manner. Our findings were similar to those we had observed with BTZ (Olejniczak, et al 2010).
Figure 3. Carfilzomib (CFZ) is more effective than bortezomib (BTZ) in inducing caspase-dependent and - independent cell death.
(3A) CFZ induced apoptosis in rituximab-sensitive (RSCL) Raji or resistant (RRCL) Raji 2R, Raji 4RH cell lines. Burkitt lymphoma cells were exposed to CFZ (1 nM, 10 nM) or BTZ (10 nM) for 24, 48, and 72 h. Subsequently, apoptosis was determined by detection of the percentage of the sub-G1 population using flow cytometry. (3B) Twenty-four hours following incubation with BTZ (10 nM) or CFZ (1, 10 nM), changes in caspase 3/7 activity were determined in RSCL and RRCL. (3C) Cleaved caspase 3 and PARP was detected by Western blot. (3D) Caspase inhibition partially reversed CFZ cytotoxic effect in both RSCL and RRCL. Raji, Raji 2R, and Raji 4RH were pretreated with the pan-inhibitors Q_VD-OPh (5 μM) or zVAD –fmk (5 μM) for 1 h, followed by 48 h exposure to CFZ. Subsequently cell viability was determined using the CellTiter Glo viability assay. (3E) Induction of pro-apoptotic proteins (BAK and PARP cleavage) after CFZ or BTZ drug exposure was demonstrated by Western blotting. Data shown represents the average of three independent experiments +/− S.D. Asterisk (*) indicate a p-value < 0.05 when comparing CFZ versus CFZ plus caspase inhibitor. In 3A ^= a significant p-value of < 0.05 when CFZ (10 nM) is compared to BTZ (10 nM). CTRL, control.
There have been several analyses showing that BTZ alone or when combined with other anti-cancer agents may induce ROS-dependent apoptosis in lymphoma cells (Bhalla, et al 2009, Perez-Galan, et al 2006, Weniger, et al 2011). We evaluated differences in the capacity of BTZ or CFZ on inducing ROS in our lymphoma cell lines. As previously described by other investigators (Bhalla, et al 2009) in vitro exposure to BTZ induced ROS in Raji cells when compared to untreated cells. However, we did not detect any induction of ROS (compared to the untreated cells) among the RRCL (Raji 2R and Raji 4RH) tested. Moreover, we did not find any ROS generation in CFZ exposed RSCL or RRCL (data not shown). We had previously demonstrated that RRCL had an abnormal mitochondrial potential that could explain the lack of ROS generation following BTZ drug exposure (Olejniczak, et al 2008). In addition, our data suggest that CFZ-induced cell apoptosis was ROS independent, and further experiments need to be done to further define if a decrease in ROS generation plays a role in rituximab resistance.
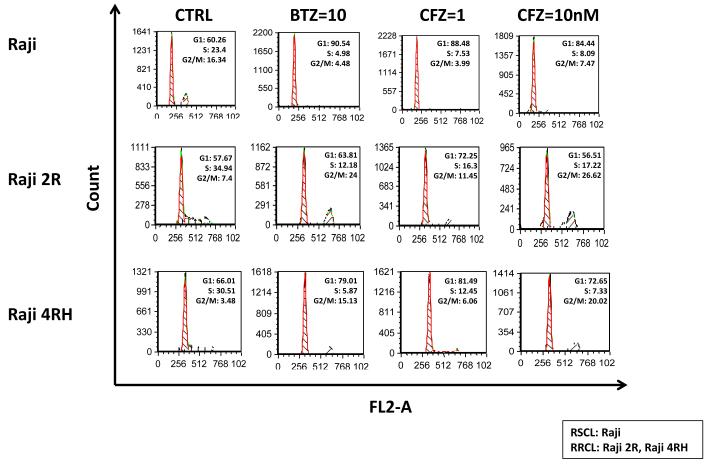
Carfilzomib induces accumulation of G2/M arrest in RRCL cells, but not in RSCL
BTZ is known to induce G2/M cell cycle arrest in pre-clinical models of solid tumour malignancies (i.e. prostate or lung cancer) (Canfield, et al 2006, Denlinger, et al 2004). In an attempt to study the caspase-independent mechanism(s) that contribute to CFZ anti-tumour activity, we examined changes in cell cycle of CFZ-(at 1 or 10 nM) or BTZ-(10 nM) exposed RSCL and RRCL.
Changes in the cell cycle pattern were different between RSCL and RRCL exposed to either CFZ or BTZ. In RSCL, exposure to either BTZ (10 nM) or CFZ (1 nM) resulted in an increase of cells at G1 phase and a decrease of cells in S or G2M phase in a dose-dependent manner (Figure 4). In contrast, in vitro exposure of RRCL to CFZ or BTZ resulted in accumulation of cells in G2M. Changes in cell cycle were dose-dependent and more evident in CFZ-than in BTZ-exposed cells.
Figure 4. Carfilzomib (CFZ) induced cell cycle arrest in G2/M phase in rituximab-resistant cells, but not in rituximab-sensitive cells.
CFZ induces a G2/M cell-cycle arrest in RRCL cells, but not parental Raji RSCL. Cell cycle analysis was performed after exposing Raji, Raji 2R and Raji 4RH to bortezomib (BTZ) 10 nM and carfilzomib (CFZ) 1 nM or 10 nM for 24 h. The experiment shown is a representative example from three different experiments. CTRL, control.
Carfilzomib overcomes chemotherapy-resistance in RRCL
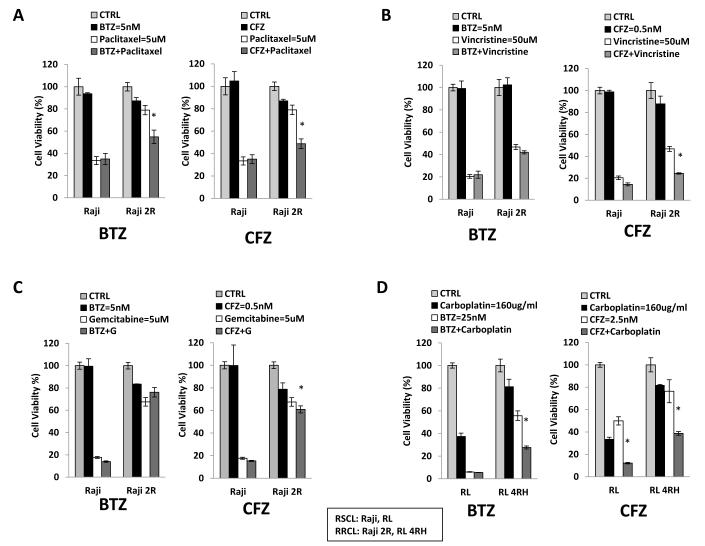
In our previous work (Olejniczak, et al 2010), we demonstrated that proteasome inhibition with BTZ lead to a decrease in the apoptotic threshold and restored chemotherapy sensitivity in RRCL. Dunleavy et al. (2009) demonstrated that bortezomib in combination with rituximab and dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin (DA-EPOCH) is active in rituximab-chemotherapy relapsed/refractory ABC-DLBCL patients. Clinically, BTZ anti-tumour activity is limited by its associated toxicities (e.g. neurotoxicity and myelosuppression) in the treatment of relapsed/refractory NHL. However, more potent proteasome inhibitors capable of achieving similar anti-tumour activity at lower concentrations may prove to be more efficacious in the clinic. To this end, we evaluated the effect of CFZ alone and in combination with several chemotherapy agents in RSCL and RRCL (Figure 5). As previously shown, we determined that the CFZ IC50 (1 nM) was 10-fold lower than BTZ (10 nM) in Raji cells.
Figure 5. Carfilzomib (CFZ) overcomes rituximab-chemotherapy resistance in combination with chemotherapy agents in both resistant cell lines and primary patient samples.
(5A, B, C) Cells were pre-treated with either bortezomib (BTZ) (5 nM) or CFZ (0.5 nM) for 24 h, and then exposed to different doses of paclitaxel, vincristine, or gemcitabine for another 48 h, after which cell viability was monitored. Values represent the means of three independent experiments on three separate occasions +/− S.D. Statistically significant differences (P<0.01) are indicated by asterisks. (5D) Raji, Raji 2R, Raji 4RH, RL, and RL 4RH cells were exposed to BTZ (25 nM) or CFZ (2.5 nM) with or without carboplatin (80 ug/ml) for an additional 48 h. There are statistical differences among Raji and RL resistant cells treated with the combination of a proteasome inhibitor and carboplatin (P<0.05). Similar results demonstrated augmented activity in RRCL to VP-16 when cells are pre-exposed to CFZ (data not shown). CTRL, control.
We utilized “subtherapeutic” doses of either BTZ (5 nM) or CFZ (0.5 nM) to evaluate its ability to augment the activity of chemotherapeutic agents against lymphoma cell lines. At 5 nM or 0.5 nM, BTZ and CFZ did not disturb the cell proliferation rate of RSCL or RRCL, respectively. On the other hand, pre-incubation of lymphoma cells with BTZ or CFZ for up to 24 - 48 h prior to exposure to chemotherapy agents (paclitaxel, vincristine, gemcitabine, etoposide, and carboplatin) resulted in a more pronounced cell death than cells exposed to chemotherapy alone. Of interest, BTZ or CFZ enhanced the cytotoxic effects of chemotherapy agents only in RRCL. CFZ effects on the cytotoxic activity of chemotherapy agents were more potent than BTZ. Similar synergistic effects were demonstrated in both ABC- and GCB-resistant cells (data not shown). Our data suggests that a much lower dose of CFZ can potentially be used to restore chemotherapy sensitivity in rituximab-chemotherapy refractory lymphoma with a better toxicity profile.
DISCUSSION
The UPS plays a pivotal role in many cellular functions by selectively degrading key regulatory proteins. The 26S ATP-dependent proteasome complex comprises a 19S regulatory subunit and a 20S multicatalytic core. Initially, proteins are attached to ubiquitin molecules recognized by the 19S proteasome regulatory complex, and destroyed by the 20S proteasome proteolytic core. Pre-clinical studies demonstrated that BTZ can increase the sensitivity of cancer cells to the cytotoxic effects of chemotherapy agents and/or radiation therapy. In addition, BTZ had been used to overcome-chemotherapy resistance in B-cell lymphoma (i.e. MCL or DLBCL) (Boccadoro, et al 2005). The combination of BTZ with multiple chemotherapy agents/regimens is widely investigated in clinical trials. de Vos et al (2009) studied the effect of adding BTZ to rituximab therapy in patients with indolent NHL. Patients with relapsed/refractory FL or MZL were randomized to receive either rituximab monotherapy or in combination with BTZ. The addition of BTZ to rituximab improved the response rate and the time to progression in relapsed/refractory indolent B-cell lymphoma patients (de Vos, et al 2009). The combination of BTZ with cytotoxic agents (i.e. doxorubicin, melphalan) or targeted agents (i.e. Bcl-2 inhibitors, kinase inhibitors, etc.) have shown promising results in B-cell lymphoma (Ning, et al 2007, San Miguel, et al 2008, Wright 2010). In addition, it has been implicated in the development of resistance to monoclonal antibodies in B-cell lymphoma (Czuczman, et al 2008, Olejniczak, et al 2008). Targeting the UPS in B-cell lymphoma is a rational and potential strategy to overcome chemotherapy-resistance and is being explored in clinical studies in patients with DLBCL and MCL.
The most common type of B-cell NHL is DLBCL, which has an aggressive clinical course. DLBCL can be divided into subgroups with distinct biological characteristics and prognosis using gene expression profiling (GEP) analysis and more recently by immunohistochemistry (IHC). There is a growing need to further characterize resistant DLBCL cells at the molecular level in an attempt to: identify biomarkers predictive of response to specific salvage therapies; better understand the mechanisms associated with acquired resistance to immunochemotherapy; and identify and develop therapeutic strategies directed against novel targets and/or pathways. DLBCL patients are primarily divided into two subgroups: GCB versus non-GCB (mainly ABC-like) DLBCL. In general, studies suggest that the ABC subtype of DLBCL is associated with an inferior clinical outcome following standard chemo-immunotherapy treatments compared to the GCB subtype. BTZ is known to inhibit nuclear factor (NF)κB (NFKB1) activity in vitro, a key regulatory pathway that plays a pivotal role in the pathogenesis of ABC-like DLBCL. This supports the hypothesis that targeting the NFκB-pathway with proteasome inhibitors can be clinically relevant in patients with ABC-like DLBCL.
Bortezomib has significant anti-tumour activity in relapsed/refractory MCL; its clinically relevant anti-tumour activity in other subtypes of B-cell lymphoma is modest at best. Grade 3-4 haematological and non-haematological toxicities that prevent the achievement of higher drug concentrations limits the activity observed with single agent BTZ in relapsed/refractory B-cell NHL. In response to the need to develop more potent and less toxic proteasome inhibitors, next generation compounds targeting the UPS are being developed, including CFZ.
Our studies suggest that CFZ, a next generation irreversible proteasome inhibitor, has more potent anti-tumour activity than BTZ against multiple subtypes of B-cell lymphoma (e.g. GCB- and ABC-DLBCL, RRCL and primary tumour cells derived from patients with de novo or relapsed/refractory B-cell lymphoma). Consistently, and across all the models utilized in our present work, we demonstrated that CFZ is essentially 10 times more potent than BTZ. The average IC50 of CFZ was 0.75 nM in contrast to 7.5 nM for BTZ. As previously described, UPS inhibition results in a decrease in the chemotherapy-induced apoptotic threshold in RSCL and RRCL. With this regard, CFZ was more effective than BTZ. The doses of CFZ necessary for overcoming chemotherapy resistance in RRCL were 10-fold lower than the necessary BTZ doses. These findings suggest that CFZ has the potential for use in the clinical setting to overcome rituximab-chemotherapy resistance in aggressive B-cell lymphomas. In addition, CFZ-associated anti-tumour activity will probably be achieved with less treatment-related toxicities than associated with BTZ.
Alternatively, because CFZ is an irreversible inhibitor, physicians and scientists are concerned about the potential of severe adverse effects associated with its being combined with chemotherapy agents. One of the most common non-haematological toxicities observed with BTZ is BIPN, which has been reported in more than 30% of patients treated with BTZ (e.g. monotherapy or in combination with chemotherapy) (Arastu-Kapur, et al 2011, Jain, et al 2011). Clinically relevant (Grade 3-4) neuropathy hinders the activity spectrum and further development of BTZ in the clinical setting, especially when it is combined with neurotoxic agents (i.e. cisplatin, vinca alkaloids, etc). Given the differences in the IC50 between proteasome inhibitors, CFZ can potentially be used in combination regimens with less non-haematological toxicity. In clinical trials, CFZ demonstrates a lower incidence of peripheral neuropathy than BTZ historical controls. The results of a phase I study aimed to define the maximum tolerated doses (MTD) of CFZ have been reported (O’Connor et al 2009). The MTD of CFZ was 15 mg/m2 and no-dose limiting toxicities (DLT) were observed. In addition, pharmacokinetics studies demonstrated that the maximum concentration of CFZ (given at a dose of 15 mg/m2) achieved was 325.9 ng/ml (452.7 nM). Of the 29 patients treated in the clinical trial, none of the patients developed grade 2 or higher peripheral neuropathy. Similar results were observed in other clinical trials (O’Connor et al 2009). Vij et al (2012) conducted a Phase II trial to evaluated the safety and tolerability of CFZ. The study enrolled 136 patients. Only 12 patients (9%) developed peripheral neuropathy, only 3 of which were grade 3 or higher. More importantly, none of the patients discontinued or required dose adjustments because of neurotoxicity (Vij, et al 2012). Most recently, Alsina et al (2012) conducted a phase I single agent of twice weekly consecutive-day dosing of CFZ in relapsed or refractory multiple myeloma or lymphoma patients. Among 37 patients with single-agent CFZ escalating doses (1.2-27 mg/m2), dosing was well tolerated and the main haematological adverse events were anaemia and thrombocytopenia (> grade III). Consistent with previous clinical trials, no grade 3 or greater peripheral neuropathy was observed in this phase I trial (Alsina, et al 2012). The data reported from these clinical trials suggests that CFZ is well tolerated and has a lower incidence of neuropathy when compared to BTZ. The reason why CFZ has less neurotoxicity compared with BTZ is yet to be fully defined. However, a recent study demonstrated that BTZ (but not CFZ) targets a mitochondrial serine protease, HtrA2/Omi, with a pivotal role in neuron survival and is associated with the induction of neuron degeneration in vitro and in vivo (Arastu-Kapur, et al 2011).
It is important to conduct pre-clinical studies using doses that can be achieved in the clinical setting. The maximum dose of CFZ used in our in vitro and ex vivo experiments was 10 nM, which is equivalent to 7.199 ng/ml, well below the maximum concentrations achieved in patients.
Similarly to BTZ, CFZ appears to have multiple mechanisms responsible for its anti-tumour effects and includes both caspase-dependent and -independent cell death pathways following drug exposure. It is unclear what triggers a neoplastic B-cell to execute one versus the other pathway. One of the potential advantages of CFZ is that the inhibition of UPS appears to be prolonged compared to BTZ, because CFZ binds irreversibly to the CT-L subunit (Groll, et al 2006).
At the molecular level, CFZ induced caspase-dependent apoptosis, increased the expression of pro-apoptotic Bcl-2 family members, and lead to cell cycle arrest at the G2/M phase. Recent studies from our laboratory and others have demonstrated that BTZ possesses several mechanisms of action that include: 1) induction of apoptosis by increasing pro-apoptotic proteins (i.e. Bax, Bak, p53 and Noxa) and decreasing inhibitors of apoptotic proteins (i.e. Bcl-2, BCL-XL [BCL2L1], bfl/A1 [BCL2A1]); 2) inhibition of the NF-kB pathway through stabilization of IκB; 3) alteration of the cell cycle regulation by the stabilization of cyclins A, B, D, E and cyclin-dependent kinase inhibitors p21 (CDKN1A) and p27 (CDKN1B); and 4) suppression of oncogenic transformation through accumulation of JUN (Barr, et al 2007, Olejniczak, et al 2010). Consistent with these observations, we found the response of RSCL and RRCL to CFZ was similar to BTZ, albeit CFZ exhibited similar effects at a much lower dose. Additional work is needed to further delineate the molecular mechanisms of action of CFZ and how they differ compared to BTZ.
In contrast to what has been previously reported in BTZ pre-clinical studies, CFZ does not appear to affect CD20 expression or rituximab activity. Bil et al (2010) reported that CD20 levels can be regulated by BTZ and therefore could influence rituximab-mediated complement-dependent cytotoxicity. Short-term (24 h) exposure of Raji cells to low-dose BTZ (10 or 20 nM) resulted in sensitizing cells to rituximab-associated CMC. On the other hand, longer BTZ exposure (48 h) or exposure of lymphoma cells to high-dose BTZ (50 nM) for 24 h resulted in a decrease in CD20 surface levels and impaired rituximab-mediated CMC (Bil, et al 2010). At the doses and schedules tested in our present work, we did not observe any (negative or positive) effects of CFZ on CD20 surface expression levels or rituximab-associated anti-tumour activity. These findings further suggest potential differences in the biological effects on lymphoma cells between reversible and irreversible proteasome inhibitors. It also stresses the need for additional studies to better characterize the mechanisms-of-action of CFZ in order to optimize its anti-tumour activity.
In summary, our results indicate that CFZ has a more favourable in vitro and ex vivo anti-tumour activity profile in B-cell lymphoma cell lines and primary lymphoma cells compared to BTZ. At much lower doses than its documented MTD, CFZ can overcome the acquired resistance to chemotherapy agents in Burkitt lymphoma and DLBCL cell lines and potentiates the anti-tumour effects of chemotherapy agents commonly used in the salvage setting (i.e. gemcitabine, etoposide, carboplatin, vinorelbine) in the clinic. The molecular mechanism(s) responsible for CFZ-associated anti-tumour activity are yet to be fully defined and probably differ from BTZ to a variable degree. Our observations provide strong pre-clinical evidence that supports the further evaluation of CFZ alone and in combination with chemotherapy agents in relapsed/refractory B-cell lymphoma patients.
ACKNOWLEGEMENTS
Grant support: This work was supported, in part, by a grant from the National Cancer Institute (Targeting the proteasome to overcome therapy resistance; Sponsor Award number 5RO1CA136907-02) and The Eugene and Connie Corasanti Lymphoma Research Fund.
Footnotes
AUTHOR CONTRIBUTIONS JG, GPK, CM, NMC performed research; FJHI, JG, MSC designed the research study; JJS contributed essential reagents or tools; JG, FJHI, MSC analysed the data; JG, FJHI, MSC wrote the paper.
CONFLICT OF INTEREST M.S.C. has served on advisory boards for Millennium Pharmaceuticals and Onyx Pharmaceuticals. The remaining authors declare no competing financial interest.
REFERENCES
- Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4:349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- Alsina M, Trudel S, Furman RR, Rosen PJ, O’Connor OA, Comenzo RL, Wong A, Kunkel LA, Molineaux CJ, Goy A. A Phase I Single-Agent Study of Twice-Weekly Consecutive-Day Dosing of the Proteasome Inhibitor Carfilzomib in Patients with Relapsed or Refractory Multiple Myeloma or Lymphoma. Clin Cancer Res. 2012;18:4830–4840. doi: 10.1158/1078-0432.CCR-11-3007. [DOI] [PubMed] [Google Scholar]
- Arastu-Kapur S, Anderl JL, Kraus M, Parlati F, Shenk KD, Lee SJ, Muchamuel T, Bennett MK, Driessen C, Ball AJ, Kirk CJ. Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: a link to clinical adverse events. Clin Cancer Res. 2011;17:2734–2743. doi: 10.1158/1078-0432.CCR-10-1950. [DOI] [PubMed] [Google Scholar]
- Barr P, Fisher R, Friedberg J. The role of bortezomib in the treatment of lymphoma. Cancer Invest. 2007;25:766–775. doi: 10.1080/07357900701579570. [DOI] [PubMed] [Google Scholar]
- Bhalla S, Balasubramanian S, David K, Sirisawad M, Buggy J, Mauro L, Prachand S, Miller R, Gordon LI, Evens AM. PCI-24781 induces caspase and reactive oxygen species-dependent apoptosis through NF-kappaB mechanisms and is synergistic with bortezomib in lymphoma cells. Clin Cancer Res. 2009;15:3354–3365. doi: 10.1158/1078-0432.CCR-08-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bil J, Winiarska M, Nowis D, Bojarczuk K, Dabrowska-Iwanicka A, Basak GW, Sulek K, Jakobisiak M, Golab J. Bortezomib modulates surface CD20 in B-cell malignancies and affects rituximab-mediated complement-dependent cytotoxicity. Blood. 2010;115:3745–3755. doi: 10.1182/blood-2009-09-244129. [DOI] [PubMed] [Google Scholar]
- Boccadoro M, Morgan G, Cavenagh J. Preclinical evaluation of the proteasome inhibitor bortezomib in cancer therapy. Cancer Cell Int. 2005;5:18. doi: 10.1186/1475-2867-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem EA, Thudium K, Khubchandani S, Tsai PC, Olejniczak SH, Bhat S, Riaz W, Gu J, Iqbal A, Campagna R, Knight J, Mavis C, Hoskin P, Deeb G, Gibbs JF, Fetterly G, Czuczman MS, Hernandez-Ilizaliturri FJ. Distinct cellular and therapeutic effects of obatoclax in rituximab-sensitive and -resistant lymphomas. Br J Haematol. 2011;153:599–611. doi: 10.1111/j.1365-2141.2011.08669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield SE, Zhu K, Williams SA, McConkey DJ. Bortezomib inhibits docetaxel-induced apoptosis via a p21-dependent mechanism in human prostate cancer cells. Mol Cancer Ther. 2006;5:2043–2050. doi: 10.1158/1535-7163.MCT-05-0437. [DOI] [PubMed] [Google Scholar]
- Cavaletti G, Jakubowiak AJ. Peripheral neuropathy during bortezomib treatment of multiple myeloma: a review of recent studies. Leuk Lymphoma. 2010;51:1178–1187. doi: 10.3109/10428194.2010.483303. [DOI] [PubMed] [Google Scholar]
- Czuczman MS, Olejniczak S, Gowda A, Kotowski A, Binder A, Kaur H, Knight J, Starostik P, Deans J, Hernandez-Ilizaliturri FJ. Acquirement of rituximab resistance in lymphoma cell lines is associated with both global CD20 gene and protein down-regulation regulated at the pretranscriptional and posttranscriptional levels. Clin Cancer Res. 2008;14:1561–1570. doi: 10.1158/1078-0432.CCR-07-1254. [DOI] [PubMed] [Google Scholar]
- de Vos S, Goy A, Dakhil SR, Saleh MN, McLaughlin P, Belt R, Flowers CR, Knapp M, Hart L, Patel-Donnelly D, Glenn M, Gregory SA, Holladay C, Zhang T, Boral AL. Multicenter randomized phase II study of weekly or twice-weekly bortezomib plus rituximab in patients with relapsed or refractory follicular or marginal-zone B-cell lymphoma. J Clin Oncol. 2009;27:5023–5030. doi: 10.1200/JCO.2008.17.7980. [DOI] [PubMed] [Google Scholar]
- Denlinger CE, Rundall BK, Keller MD, Jones DR. Proteasome inhibition sensitizes non-small-cell lung cancer to gemcitabine-induced apoptosis. Ann Thorac Surg. 2004;78:1207–1214. doi: 10.1016/j.athoracsur.2004.04.029. discussion 1207-1214. [DOI] [PubMed] [Google Scholar]
- Dick LR, Fleming PE. Building on bortezomib: second-generation proteasome inhibitors as anti-cancer therapy. Drug Discov Today. 2010;15:243–249. doi: 10.1016/j.drudis.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Dunleavy K, Pittaluga S, Czuczman MS, Dave SS, Wright G, Grant N, Shovlin M, Jaffe ES, Janik JE, Staudt LM, Wilson WH. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood. 2009;113:6069–6076. doi: 10.1182/blood-2009-01-199679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goy A, Younes A, McLaughlin P, Pro B, Romaguera JE, Hagemeister F, Fayad L, Dang NH, Samaniego F, Wang M, Broglio K, Samuels B, Gilles F, Sarris AH, Hart S, Trehu E, Schenkein D, Cabanillas F, Rodriguez AM. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:667–675. doi: 10.1200/JCO.2005.03.108. [DOI] [PubMed] [Google Scholar]
- Groll M, Huber R, Potts BC. Crystal structures of Salinosporamide A (NPI-0052) and B (NPI-0047) in complex with the 20S proteasome reveal important consequences of beta-lactone ring opening and a mechanism for irreversible binding. J Am Chem Soc. 2006;128:5136–5141. doi: 10.1021/ja058320b. [DOI] [PubMed] [Google Scholar]
- Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Muller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, Pan Z, Farinha P, Smith LM, Falini B, Banham AH, Rosenwald A, Staudt LM, Connors JM, Armitage JO, Chan WC. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- Jain S, Diefenbach C, Zain J, O’Connor OA. Emerging role of carfilzomib in treatment of relapsed and refractory lymphoid neoplasms and multiple myeloma. Core Evid. 2011;6:43–57. doi: 10.2147/CE.S13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane RC, Dagher R, Farrell A, Ko CW, Sridhara R, Justice R, Pazdur R. Bortezomib for the treatment of mantle cell lymphoma. Clin Cancer Res. 2007;13:5291–5294. doi: 10.1158/1078-0432.CCR-07-0871. [DOI] [PubMed] [Google Scholar]
- Kuhn DJ, Chen Q, Voorhees PM, Strader JS, Shenk KD, Sun CM, Demo SD, Bennett MK, van Leeuwen FW, Chanan-Khan AA, Orlowski RZ. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110:3281–3290. doi: 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning YM, He K, Dagher R, Sridhara R, Farrell AT, Justice R, Pazdur R. Liposomal doxorubicin in combination with bortezomib for relapsed or refractory multiple myeloma. Oncology (Williston Park) 2007;21:1503–1508. discussion 1511, 1513, 1516 passim. [PubMed] [Google Scholar]
- O’Connor OA. Targeting histones and proteasomes: new strategies for the treatment of lymphoma. J Clin Oncol. 2005;23:6429–6436. doi: 10.1200/JCO.2005.05.014. [DOI] [PubMed] [Google Scholar]
- O’Connor OA, Stewart AK, Vallone M, Molineaux CJ, Kunkel LA, Gerecitano JF, Orlowski RZ. A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clin Cancer Res. 2009;15:7085–7091. doi: 10.1158/1078-0432.CCR-09-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olejniczak SH, Hernandez-Ilizaliturri FJ, Clements JL, Czuczman MS. Acquired resistance to rituximab is associated with chemotherapy resistance resulting from decreased Bax and Bak expression. Clin Cancer Res. 2008;14:1550–1560. doi: 10.1158/1078-0432.CCR-07-1255. [DOI] [PubMed] [Google Scholar]
- Olejniczak SH, Blickwedehl J, Belicha-Villanueva A, Bangia N, Riaz W, Mavis C, Clements JL, Gibbs J, Hernandez-Ilizaliturri FJ, Czuczman MS. Distinct molecular mechanisms responsible for bortezomib-induced death of therapy-resistant versus -sensitive B-NHL cells. Blood. 2010;116:5605–5614. doi: 10.1182/blood-2009-12-259754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlati F, Lee SJ, Aujay M, Suzuki E, Levitsky K, Lorens JB, Micklem DR, Ruurs P, Sylvain C, Lu Y, Shenk KD, Bennett MK. Carfilzomib can induce tumor cell death through selective inhibition of the chymotrypsin-like activity of the proteasome. Blood. 2009;114:3439–3447. doi: 10.1182/blood-2009-05-223677. [DOI] [PubMed] [Google Scholar]
- Perez-Galan P, Roue G, Villamor N, Montserrat E, Campo E, Colomer D. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood. 2006;107:257–264. doi: 10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]
- San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS, Dmoszynska A, Abdulkadyrov KM, Schots R, Jiang B, Mateos MV, Anderson KC, Esseltine DL, Liu K, Cakana A, van de Velde H, Richardson PG. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- Strauss SJ, Maharaj L, Hoare S, Johnson PW, Radford JA, Vinnecombe S, Millard L, Rohatiner A, Boral A, Trehu E, Schenkein D, Balkwill F, Joel SP, Lister TA. Bortezomib therapy in patients with relapsed or refractory lymphoma: potential correlation of in vitro sensitivity and tumor necrosis factor alpha response with clinical activity. J Clin Oncol. 2006;24:2105–2112. doi: 10.1200/JCO.2005.04.6789. [DOI] [PubMed] [Google Scholar]
- Vij R, Wang M, Kaufman JL, Lonial S, Jakubowiak AJ, Stewart AK, Kukreti V, Jagannath S, McDonagh KT, Alsina M, Bahlis NJ, Reu FJ, Gabrail NY, Belch A, Matous JV, Lee P, Rosen P, Sebag M, Vesole DH, Kunkel LA, Wear SM, Wong AF, Orlowski RZ, Siegel DS. An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood. 2012;119:5661–5670. doi: 10.1182/blood-2012-03-414359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weniger MA, Rizzatti EG, Perez-Galan P, Liu D, Wang Q, Munson PJ, Raghavachari N, White T, Tweito MM, Dunleavy K, Ye Y, Wilson WH, Wiestner A. Treatment-induced oxidative stress and cellular antioxidant capacity determine response to bortezomib in mantle cell lymphoma. Clin Cancer Res. 2011;17:5101–5112. doi: 10.1158/1078-0432.CCR-10-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JJ. Combination therapy of bortezomib with novel targeted agents: an emerging treatment strategy. Clin Cancer Res. 2010;16:4094–4104. doi: 10.1158/1078-0432.CCR-09-2882. [DOI] [PubMed] [Google Scholar]
- Younes A, Pro B, Fayad L. Experience with bortezomib for the treatment of patients with relapsed classical Hodgkin lymphoma. Blood. 2006;107:1731–1732. doi: 10.1182/blood-2005-09-3731. [DOI] [PubMed] [Google Scholar]