Abstract
Background
Brain white matter abnormalities have been hypothesized to play an important role in the neurobiology of bipolar disorder. The nature of these abnormalities is not well characterized, however, and it is unknown whether they occur following disease onset or represent potential markers of genetic risk.
Methods
We examined white matter integrity (assessed via fractional anisotropy [FA]) using diffusion tensor imaging in patients with bipolar disorder (n = 26), unaffected siblings of patients with bipolar disorder (n = 15) and healthy volunteers (n = 27) to identify white matter biomarkers of genetic risk.
Results
FA differed significantly (p< .05; corrected) among the three groups within the right temporal white matter. Unaffected siblings had FA values that were intermediate to and significantly different from those of healthy volunteers and patients with bipolar disorder (healthy controls > unaffected siblings > bipolar disorder). Moreover, FA values in this region correlated negatively and significantly with trait impulsivity in unaffected siblings. Probabilistic tractography indicated that the regional abnormality lies along the inferior frontal occipital fasciculus, a large intrahemispheric association pathway.
Conclusions
Our results suggest that lower white matter integrity in the right temporal lobe may be a biomarker for genetic risk of bipolar disorder. It is conceivable that the attenuated nature of these white matter abnormalities present in unaffected siblings allows for some preservation of adaptive emotional regulation, whereas more pronounced alterations observed in patients is related to the marked emotional dysregulation characteristic of bipolar disorder.
Keywords: Bipolar, DTI, endophenotype, siblings, fractional anisotropy, tractography
Introduction
Brain abnormalities in regions involved in emotion regulation and behavioral control have been consistently implicated in the pathophysiology of bipolar disorder (1, 2). A critical unanswered question concerns whether these structural alterations occur following disease onset or if they represent potential markers of genetic risk. Twin studies suggest that genetic factors play a key role in risk for bipolar disorder, with concordance rates in monozygotic twins ranging from 50–90% versus concordance rates in dizygotic twins of approximately 10% (3). The investigation of unaffected siblings, in addition to patients with bipolar disorder and healthy volunteers, may therefore be an optimal design for identifying biomarkers of genetic risk for developing this disorder. Specifically, although unaffected siblings share a proportion of genes with their ill probands, they do not share the typical confounds that limit data interpretation in patient studies such as illness duration, treatment or clinical state.
Because white matter morphology has been demonstrated to be highly heritable in bipolar disorder (4) it is possible that it may represent an endophenotype for understanding mechanisms of core illness features. There is some evidence that white matter morphology may fulfill the four major criteria for potential endophenotypes (5,6): (a) association with the illness (2); (b) independence from clinical state (7); (c) evidence of genetic influence (4); and (d) co-segregation within families (8). Data are particularly limited, however, regarding potential white matter abnormalities in family members. Genetic liability to developing bipolar disorder was associated with less white matter volume within the left frontal and temporal lobes (8) within a sample of patients, unaffected relatives, and healthy controls. Similarly, unaffected co-twins of patients with bipolar disorder were found to have less left hemispheric white matter volume relative to controls (9). In a study of patients, unaffected relatives, and healthy controls, relatives were found to have less right medial frontal white matter compared to controls, although notably patients and controls did not differ in this region (10). Two of the most prominent white matter findings in patients with bipolar disorder, a reduction in corpus callosum volume (11, 12) and an increase in white matter hyperintensities (e.g. 12), have been examined in unaffected first degree relatives. No differences in callosal volume (13) or number of white matter hyperintensities were apparent between relatives and controls (14, 15), suggesting that potential white matter abnormalities in relatives may be distinct from or more subtle than those present in patients.
Diffusion tensor imaging (DTI) allows for a potentially more sensitive assessment of white matter abnormalities than assessment of white matter volume (16). Recent DTI studies have demonstrated alterations in fractional anisotropy (FA), a putative measure of white matter fiber integrity, in unaffected relatives of patients with bipolar disorder. Children with a first-degree relative with bipolar disorder demonstrated lower FA in bilateral superior longitudinal fasiculi compared to healthy control children; however, FA in these tracts did not differ between patients and relatives (17). Healthy children with at least one parent with bipolar disorder demonstrated negative associations between age and FA in corpus callosum and right temporal lobe WM whereas control children demonstrated positive associations (18). Chaddock and colleagues (19) reported an association between increased genetic liability to developing bipolar disorder and lower FA throughout the brain, although no differences were identified in relatives when they were directly compared with patients and controls. More recently, Sprooten and colleagues (20) examined young adult unaffected relatives of patients with bipolar disorder and healthy controls and found that relatives demonstrated lower FA throughout the brain white matter.
A potential limitation of several prior studies investigating FA abnormalities in unaffected relatives may be the lack of a patient group in the analysis (e.g., 18, 20). It is important to ensure that specific differences identified between unaffected relatives and healthy controls are also present in patients to clarify the endophenotypic nature of FA abnormalities in bipolar disorder. Abnormalities identified in the same regions among unaffected siblings compared to their ill probands in attenuated form provides the strongest evidence that these alterations may potentially serve as endophenotypes given that they are directly implicated in the disease process. A second potential limitation of previous studies may be the inclusion of mixed relatives, such as unaffected parents and children in addition to siblings. The inclusion of unaffected parents raises the possibility that some parent participants would not contribute heredity risk factors for developing bipolar disorder and could potentially weaken findings in samples of unaffected relatives in which they are included. Moreover, the inclusion of child and young adult relatives who are not yet past the age of risk for developing bipolar disorder may also be problematic. Prior work examining the age-at-onset in a sample of several hundred patients with bipolar disorder demonstrated that the majority of patients had developed the disorder by the age of 25 (21), and that over half of the participants had an age of onset in the early twenties. Moreover, this same study assessed affected siblings of patients and found that the age of onset was quite similar within sibling pairs. Siblings who are themselves past the age of 25 and who are older than their affected siblings were at their age of onset may represent an excellent subject group to investigate FA abnormalities in unaffected relatives.
To our knowledge, the present study is the first to directly compare patients, adult unaffected siblings, and healthy controls using a conservative tract-invariant voxelwise analysis of FA data (i.e. tract based spatial statistics [22]) to examine the possibility that FA abnormalities may serve as an endophenotype in bipolar disorder. We also examined the relationship between FA and trait impulsivity among unaffected siblings. We hypothesized that siblings would demonstrate FA values that were intermediate to those of patients and controls in regions implicated in emotion regulation, and that lower FA would be associated with greater impulsivity.
Methods and Materials
Sample
Twenty-six patients diagnosed with either Bipolar I Disorder (n=20) or Bipolar II Disorder (n=6) as determined using the Structured Clinical Interview for DSM-IV Disorders (SCID) (23) participated in this study. None of the bipolar patients were related to one another and all patients were being treated on an outpatient basis at the time of the scan. Nineteen patients were receiving antipsychotic medication with the majority (n=18) of patients taking second generation antipsychotics; one patient was being treated with a first-generation antipsychotic. Sixteen patients were taking mood stabilizers at the time of the scan. Eleven patients were taking both an antipsychotic medication and a mood stabilizer and two patients were not taking any psychotropic medications at the time of the scan. Fifteen siblings of patients with a diagnosis of bipolar disorder and 27 healthy volunteers also participated in this study and were free from current Axis I major mood or psychotic disorders as determined from the SCID (non-patient version) (24). Siblings were at least 25 years of age and were past the age of onset in their affected sibling. One sibling met criteria for a single post-partum depressive episode that remitted without treatment eight years prior to the scan. Two other siblings met criteria for prior substance use disorders, and one of these participants also met criteria for Anxiety Disorder NOS. All other siblings and all healthy volunteers were free from any current or lifetime Axis I diagnoses. One sibling was being treated with an SSRI for anxiety; all other siblings and healthy controls were free from exposure to psychotropic medication. All participants denied substance abuse or dependence in the three months prior to the scan. Exclusion criteria for all participants included: (1) MRI contraindications; (2) significant medical illness (3) prior psychosurgery; (4) DSM-IV mental retardation; and (5) pregnancy. All procedures were approved by the local IRB and written informed consent was obtained from all participants.
Impulsivity Measures
Trait impulsivity was assessed in all siblings using the Barratt Impulsivity Scale, version 11 (BIS-11) (25). The BIS-11 is a self-report measure of trait impulsivity that assesses three empirically derived factors: (a) an Attention subscale; (b) a Motor subscale and (c) a Non-Planning subscale.
Image Acquisition and Processing
All subjects received a diffusion tensor imaging (DTI) exam at the North Shore University Medical Center on a GE Signa HDx 3.0 T system. The DTI sequence included volumes with diffusion gradients applied along 31 non-parallel directions (b = 1000 s/mm2) and five volumes without diffusion weighting (TR = 14 s, TE = min, matrix = 128 × 128, FOV = 240 mm). Each volume consisted of 51 contiguous 2.5-mm axial slices acquired parallel to the anterior-posterior (AC-PC) commissural line using a ramp sampled, spin-echo, single shot echo-planar imaging (EPI) method. Image processing was conducted using TBSS (22) within the Functional Magnetic Resonance Imaging of the Brain Library (FSL; Oxford, United Kingdom). Each subject’s DTI data was aligned to the first volume, which was acquired without diffusion weighting, to eliminate eddy- current distortion; the b-vector table for each participant was then adjusted to account for this eddy-current correction. Fractional anisotropy (FA) was calculated in each voxel of the brain for each participant using DTIFIT, part of FSL’s Diffusion Toolbox. Each image was then nonlinearly transformed using FSL’s Nonlinear Image Registration Tool (FNIRT) into standard MNI-152 space for cross subject comparisons at the voxel level.
Statistical Analysis
A nonparametric voxelwise ANCOVA with subject-type (patients, siblings, and controls) as the between-subjects factor and age as a covariate was carried out using permutation statistics (number of permutations = 5000) via the Randomise tool in FSL with family-wise error correction. We also employed threshold-free cluster enhancement in FSL (26) to eliminate the need for arbitrary smoothing and cluster threshold criteria. Mean FA within each significant cluster for all participants was exported to SPSS for direct pair-wise comparisons and examined in relationship to impulsivity using Pearson Product Moment correlations (p < .05; two-tailed).
Probabilistic Tractography
We utilized probabilistic tractography in FSL (27) to identify the white matter tract(s) that comprised clusters that differed significantly among groups. Using the Bedpostx tool, the local (i.e., within-voxel) probability density functions of the principal diffusion direction were estimated using Markov Chain Monte Carlo sampling; a spatial probability density function across voxels was then estimated based on these local probability density functions using the Probtrackx tool. The resulting spatial probability density function allows for the visualization of a “connectivity distribution” between any given voxel in the brain and every other voxel (27) and serves as an estimate of the anatomical white matter tracts that may course between two or more points in the brain. Clusters identified as significantly different among groups in the TBSS analysis were used as seed regions for the tractography analysis. Seed regions were masked in MNI space and linearly registered to each subject’s native DTI space. Probtrackx parameters were as follows: number of samples = 5000, curvature threshold = 0.2, step length = 0.5mm, and number of steps per sample = 2000. Tractography was conducted in native DTI space; resultant pathways were normalized at a threshold of .01 in each participant, linearly registered into MNI space, and averaged across all participants for visualization of the “average” pathway(s) passing through the seed region(s). Although the choice of a normalization threshold is inherently arbitrary in probabilistic tractography, we chose .01 as it allowed for the propagation of plausible probability density functions within each participant while also excluding highly implausible voxels.
Results
Nine complete sibling pairs participated in the study. Six additional unaffected siblings participated in the study but the corresponding patients were unable to participate due to inability to tolerate the scanning procedure or lack of interest. All six of these non-participating patients underwent the SCID and had confirmed diagnoses of bipolar disorder. Seventeen patients with bipolar disorder participated without a corresponding sibling participant.
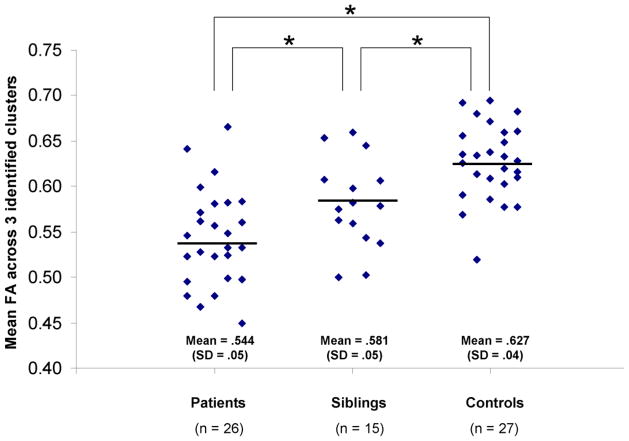
The three groups did not differ significantly in distributions of age, race, sex, laterality quotient, or years of education (Table 1). The TBSS voxelwise analysis revealed three clusters in close proximity to one another within the right temporal lobe where FA was significantly different among the three groups (Figure 1). Peak coordinates of the clusters in MNI space and their size was as follows: (1) x = 34, y = −17, z = −4 (10 voxels); (2) x =37, y = −24, z = −3 (14 voxels); and (3) x = 39, y = −30, z = −3 (14 voxels). FA within each cluster was extracted from the TBSS analysis and averaged given their close proximity, high correlations with one another, and to limit Type-I error in subsequent analyses. Univariate analysis of variance indicated that mean FA across all three clusters was significantly different among groups (F(2,68) = 19.99, p < .01, effect size (partial eta squared) = .38). Posthoc analyses indicated that patients had significantly lower FA in the average of these clusters compared to controls (t(51) = 6.36, p < .05) and that unaffected siblings had FA that was intermediate to and significantly different from healthy volunteers (t(40) = 3.23, p <.05) and patients (t(39) = 2.19, p < .05) (see Figure 2). This same pattern of results was still evident when each of the three clusters was examined individually.
Table 1.
Sample characteristics
| Bipolar Patients (n = 26) | Unaffected Siblings (n = 15) | Controls (n = 27) | df | Statistic | p-value | |
|---|---|---|---|---|---|---|
| Age (years) | 40.6 (12.4) | 42.0 (11.7) | 40.8 (12.5) | 2, 67 | F = .067 | .94 |
| Sex (M/F) | 11/15 | 6/9 | 15/12 | 2 | χ2 = 1.32 | .52 |
| Laterality Quotienta | 0.88 (.34) | 0.75 (.41) | 0.76 (.58) | 2, 67 | F = .52 | .60 |
| Raceb | 5,8,13 | 3,5,7 | 4,15,8 | 4 | χ2 = 3.95 | .41 |
| Education (years) | 13.9 (1.7) | 14.1 (2.6) | 14.6 (2.7) | 2, 67 | F = .76 | .47 |
Note. Data are presented as mean +/− standard deviation unless otherwise indicated.
Handedness for all participants was assessed using a modified, 20-item version of the Edinburgh Inventory. A laterality quotient was computed for each participant according to the following formula: (Total R−Total L)/(Total R + Total L), where Total R and Total L refer to the total number of right- and left-hand items scored, respectively. Laterality quotients derived from this formula ranged from + 1.00 (totally dextral) to −1.00 (totally nondextral).
Race was coded as African American, Caucasian, Hispanic, Asian, and other. Because more than 40% of the categories for race had expected frequencies of less than 5 we combined the latter three groups (i.e. Hispanic, Asian, and other) into a single group for analysis.
Figure 1.
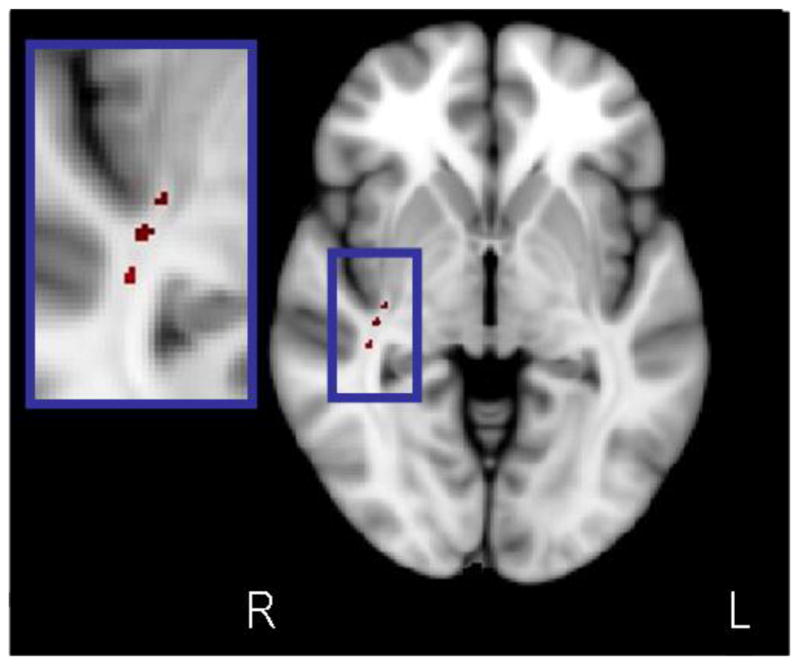
Voxelwise ANCOVA in TBSS revealed three clusters in close proximity to one another in which FA was significantly different among the three groups. Figure is in radiologic convention (R = right, L = Left).
Figure 2.
Mean FA within the three clusters is significantly different among the three groups.
Asterisk indicates groups differed at p <.05. The horizontal line represents the mean for each group.
Ancillary analyses investigated the effects of psychotropic medication, sibling diagnoses, diagnoses of Bipolar II disorder, and sex on the observed findings. The overall pattern of findings remained similar when we excluded patients taking antipsychotic medication (F(2,49) = 10.77, p < .05) and those taking mood stabilizers (F(2, 52) = 17.44, p < .05); the findings also remained after we excluded the three siblings with a DSM-IV-TR diagnosis (F(2,24)= 21.73, p < .01). Similarly, excluding the six patients with a diagnosis of Bipolar II disorder did not affect the main findings (F(2,60) = 18.01, p < .01). In addition, there was neither a significant main effect of sex nor a significant group-by-sex interaction for the FA measures. We also performed a post-hoc analysis in which we excluded unrelated siblings and patients and included only the controls (n = 27) and the related patients (n = 9) and siblings (n = 9). The main findings remained significant (F(2,44) = 9.60, p < .05); patients as well as siblings demonstrated significantly lower right temporal FA compared to controls. Findings were similar among the unrelated patients (n = 17) and siblings (n = 6) when compared to controls (F(2,49) = 19.81, p < .05).
We examined the correlation between abnormal FA within these clusters and impulsivity in the group of unaffected siblings. Results indicated that mean FA across the three clusters was significantly negatively correlated with the Attention subscale of the BIS-11 (r = −.55, p < .05). There were no other significant correlations between mean FA in the identified clusters and other subscales of the BIS-11.
The three clusters that differed significantly among groups were used as seed regions for tractography. We expected that the results would approximate one of several major intrahemispheric tracts known to course through this region including the inferior-frontal occipital fasciculus, inferior longitudinal fasciculus, and/or superior longitudinal fasciculus (e.g. 28). Probabilistic connectivity distributions were generated in each participant’s native DTI space and then linearly registered into MNI space. For display purposes, the tracts were averaged across the entire sample (see Figure 3). Based on visual inspection, reference to the Johns Hopkins University White Matter Tractography Atlas available in FSL, and post-mortem work (29, 30) the clusters in which patients and siblings demonstrated lower FA compared to controls were located along the fronto-occipital fasciculus.
Figure 3.
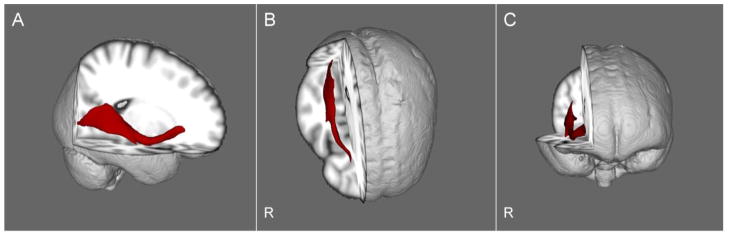
Visualization of the group averaged pathway passing through the three clusters wherein the groups differed significantly. The three clusters were used as seed regions for probabilistic tractography. Results are shown in sagittal (panel A), axial (panel B), and coronal (panel C) views. The resultant pathway approximates the inferior fronto-occipital fasciculus. R indicates the right side of the brain.
Discussion
Our data provide evidence that white matter integrity, as inferred by diffusion tensor imaging, may serve as an intermediate endophenotype in genetic studies of bipolar disorder. Specifically, as predicted apriori, our findings are consistent with a linear effect in that FA within the right temporal lobe white matter of unaffected siblings was intermediate to and significantly different from patients with bipolar disorder and healthy volunteers. Moreover, this finding was identified using a rigorous tract-invariant approach for image analysis and survived strict family-wise error correction. Although several prior studies reported abnormal white matter integrity in unaffected relatives of patients with bipolar disorder, these studies were conducted in samples comprised of unaffected parents and children in addition to siblings, which could potentially reduce statistical power and obscure study findings. Moreover, several prior studies reported white matter abnormalities among unaffected relatives compared to healthy volunteers, but did not include a patient group. These previous studies have provided valuable evidence that relatives of patients with bipolar disorder exhibit deficits in the white matter; identifying such abnormalities among unaffected siblings in the same regions as patients, but in attenuated form, provides evidence that these deficits are implicated in the neurobiology of the disorder and thus, might successfully serve as an intermediate endophenotype.
Few diffusion tensor imaging studies in unaffected relatives of patients with bipolar disorder have been conducted to date. In general, our results are broadly consistent with findings of widespread lower FA in relatives (17, 20) as well as an association between genetic liability to developing bipolar disorder and lower FA (19). Our data, however, further point to the right temporal lobe white matter as a potentially critical region for further investigation. The findings reported herein are consistent with a report of a negative correlation between age and FA in right temporal white matter in healthy children with at least one parent with bipolar disorder (18). Our study taken together with this prior work suggests that significantly lower right temporal FA in individuals at increased genetic risk for bipolar disorder may be the result of aberrant neurodevelopment in childhood and adolescence.
Investigation of the functional correlates of abnormal white matter integrity indicated that lower FA was significantly associated with impulsivity in the unaffected sibling group, suggesting that the region of abnormal FA identified in the present study is related to brain mechanisms that underlie behavioral and temperamental aspects of this disorder. In this regard, it is noteworthy that impulsivity has been demonstrated to be elevated in unaffected relatives of patients with bipolar disorder (31). In particular, the attention subscale of the BIS-11 reflects a tendency toward racing thoughts and difficulty concentrating (32) and has been found to be associated with a more severe course of illness in patients with bipolar disorder (33). Our findings thus suggest that aspects of bipolar phenomenology, including impulsivity, may be mediated by structural alterations within the right temporal lobe white matter.
The use of probabilistic tractography indicated that the region of abnormal white matter in unaffected siblings lies along the inferior frontal occipital fasciculus and is consistent with prior reports of FA abnormalities in this region in bipolar disorder (19, 34–36). This long association tract is comprised of fibers that course along the inferior portion of the brain between the occipital and frontal lobes (37). A portion of the inferior frontal occipital fasciculus consists of bidirectional connections between the posterior occipital and orbital frontal cortices that are postulated to be involved in higher-order top-down processing of highly refined visual information (38) as well as in visual-spatial processing (39). In addition to occipital-frontal connections, the inferior frontal occipital fasciculus also consists of a subpopulation of fibers terminating in the frontal operculum (e.g. 40). Recent post-mortem anatomical work has demonstrated that other subpopulations of fibers with the inferior frontal occipital fasciculus terminate within the inferior temporal gyrus (41). The inferior frontal occipital fasciculus therefore connects not only occipital and frontal cortex, but temporal and frontal cortex as well. Given that our findings were restricted to regions within the temporal portion of the inferior frontal occipital fasciculus an abnormality in this location may be indicative of disconnectivity between the orbital frontal and temporal cortices. This possibility is supported by a wealth of clinical, functional, and structural data suggesting that frontal-temporal connectivity abnormalities are strongly implicated in bipolar disorder (e.g. 42) and with neurobiological models of bipolar disorder that posit abnormalities within frontal-temporal circuits that contribute to deficits in the generation and regulation of emotion (2, 42–46).
More specifically, abnormalities in connectivity between right temporal and frontal lobes could contribute to an attenuation of prefrontal inhibitory control over subcortical limbic structures, a major component of most models of emotion regulation in bipolar disorder (e.g., 42, 43). A tendency toward inattention and a relative intolerance for cognitive complexity could arise from alterations along the IFOF as it has been shown that this tract is strongly implicated in the identification of behaviorally relevant stimuli as part of the ventral attention system (47,48). In particular, decreased FA within an anterior portion of the right IFOF has been shown to be correlated with decreased response inhibition in healthy volunteers (49). It is therefore possible that our finding of lower FA in patients with bipolar disorder and their siblings compared to healthy volunteers is indicative of altered connectivity between regions known to be involved in both emotion regulation and attention, which could lead to deficits in these domains.
It may be noteworthy that our findings of lower FA in patients and unaffected siblings compared to healthy volunteers were lateralized to the right hemisphere. Neurobiological models of bipolar disorder have emphasized a disruption in cognitive reappraisal and/or suppression of emotional information that is partially mediated by deficits within the right dorsolateral prefrontal cortex (reviewed in 42). A recent meta-analysis of 10 functional MRI studies investigating emotion regulation in bipolar disorder identified several regions of decreased activiation in patients that were restricted to the right hemisphere (50). Moreover, alterations in white matter integrity in regions comprising right intrahemispheric connections have been reported in several independent studies (19, 51) as well as in a recent meta-analysis of whole brain DTI studies in bipolar disorder (7). Further work is required to examine the significance of right-sided structural and functional alterations in bipolar disorder and to clarify the specificity of these findings in unaffected relatives.
There were several limitations to the present study that should be noted. We did not assess impulsivity in patients or healthy volunteers to determine whether a similar association exists in these groups. Given the small sample size of the sibling group and number of correlations performed our analyses should be interpreted with caution. Due to these factors, these findings require replication in a larger sample consisting of both patients and siblings. As we included only one patient and/or one sibling from each family, we were unable to assess whether right temporal FA abnormalities co-segregate within families. Future studies should include multiply affected families to assess this aspect of the white matter to further clarify the endophenotypic status of the present finding. Furthermore, the sample of unaffected siblings was small relative to patients and healthy volunteers and may have limited our ability to detect additional regions of abnormal FA in this group compared to the other two groups. It should also be acknowledged that with a larger sample size additional abnormalities, including those previously reported to be abnormal in bipolar disorder, such as the corpus callosum, might have been identified as well as regions in the left hemisphere. Also, we should note that the finding of intermediate right temporal FA within the sibling group could be related to aspects of subsyndromal symptomotology that were not assessed in this study. Moreover, FA differences among the groups could also be due to other factors including an increase in orthogonal cross fibers and differences in hydration.
The present study is the first to identify a region in which siblings demonstrate FA values intermediate to those of patients and controls in a direct, three-way comparison. Although medication effects in the patients cannot be ruled out, the significant difference in FA between siblings and controls suggests that the abnormal FA identified in the present study is not due to the effect of medication. Further work is required to elucidate the genetic mechanisms underlying abnormal white matter integrity along the IFOF, as well as the functional implications of this abnormality. In addition, the identification of protective mechanisms among individuals at increased genetic risk for developing bipolar disorder may be critical in developing potential treatment strategies for this disorder.
Acknowledgments
Funding for this study was provided by the Brain and Behavior Research Foundation, National Institute of Mental Health (R01 MH076995 to PRS; and K23 MH077807 to KEB), a Center for Intervention Development and Applied Research (P50 MH080173) and a General Clinical Research Center (M01 RR018535).
Footnotes
Financial Disclosures
Dr. Malhotra reports having received research and grant support from Eli Lilly and has served as a consultant for this company. He is on the Scientific Advisory Board of Genomind and Shire, and is a member of the Speaker’s Bureau for Schering-Plough/Merck and Sunovian Pharmaceuticals, Inc. The remaining authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Heng S, Song AW, Sim K. White matter abnormalities in bipolar disorder: insights from diffusion tensor imaging studies. J Neural Transm. 2011;117:639–654. doi: 10.1007/s00702-010-0368-9. [DOI] [PubMed] [Google Scholar]
- 2.Mahon K, Burdick KE, Szeszko PR. A role for white matter abnormalities in the pathophysiology of bipolar disorder. Neurosci Biobehav Rev. 2010;34:533–554. doi: 10.1016/j.neubiorev.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kieseppä T, Partonen T, Haukka J, Kaprio J, Lönnqvist J. High concordance of bipolar I disorder in a nationwide sample of twins. Am J Psychiatry. 2004;161:1814–1821. doi: 10.1176/ajp.161.10.1814. [DOI] [PubMed] [Google Scholar]
- 4.van der Schot AC, Vonk R, Brans RG, van Haren NE, Koolschijn PC, Nuboer V, et al. Influence of genes and environment on brain volumes in twin pairs concordant and discordant for bipolar disorder. Arch Gen Psychiatry. 2009;66:142–151. doi: 10.1001/archgenpsychiatry.2008.541. [DOI] [PubMed] [Google Scholar]
- 5.Gottesman II, Shields J. Genetic theorizing and schizophrenia. Br J Psychiatry. 1973;122:15–30. doi: 10.1192/bjp.122.1.15. [DOI] [PubMed] [Google Scholar]
- 6.Glahn DC, Bearden CE, Niendam TA, Escamilla MA. The feasibility of neuropsychological endophenotypes in the search for genes associated with bipolar affective disorder. Bipolar Disord. 2004;6:171–182. doi: 10.1111/j.1399-5618.2004.00113.x. [DOI] [PubMed] [Google Scholar]
- 7.Vederine FE, Wessa M, Leboyer M, Houenou J. A meta-analysis of whole-brain diffusion tensor imaging studies in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1820–1826. doi: 10.1016/j.pnpbp.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 8.McDonald C, Bullmore ET, Sham PC, Chitnis X, Wickham H, Bramon E, et al. Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Arch Gen Psychiatry. 2004;61:974–984. doi: 10.1001/archpsyc.61.10.974. [DOI] [PubMed] [Google Scholar]
- 9.Kieseppä T, van Erp TG, Haukka J, Partonen T, Cannon TD, Poutanen VP, et al. Reduced left hemispheric white matter volume in twins with bipolar I disorder. Biol Psychiatry. 2003;54:896–905. doi: 10.1016/s0006-3223(03)00373-1. [DOI] [PubMed] [Google Scholar]
- 10.Matsuo K, Kopecek M, Nicoletti MA, Hatch JP, Watanabe Y, Nery FG, et al. New structural brain imaging endophenotype in bipolar disorder. Mol Psychiatry. 2011;2011 doi: 10.1038/mp.2011.3. e-pub ahead of print 15 Feb 2011. [DOI] [PubMed] [Google Scholar]
- 11.Arnone D, McIntosh AM, Chandra P, Ebmeier KP. Meta-analysis of magnetic resonance imaging studies of the corpus callosum in bipolar disorder. Acta Psychiatr Scand. 2008;118:357–362. doi: 10.1111/j.1600-0447.2008.01229.x. [DOI] [PubMed] [Google Scholar]
- 12.Kempton MJ, Geddes JR, Ettinger U, Williams SC, Grasby PM. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry. 2008;65:1017–1032. doi: 10.1001/archpsyc.65.9.1017. [DOI] [PubMed] [Google Scholar]
- 13.Walterfang M, Wood AG, Barton S, Velakoulis D, Chen J, Reutens DC, et al. Corpus callosum size and shape alterations in individuals with bipolar disorder and their first-degree relatives. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1050–1057. doi: 10.1016/j.pnpbp.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Gulseren S, Gurcan M, Gulseren L, Gelal F, Erol A. T2 hyperintensities in bipolar patients and their healthy siblings. Arch Med Res. 37:79–85. doi: 10.1016/j.arcmed.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Gunde E, Novak T, Kopecek M, Schmidt M, Propper L, Stopkova P, et al. White matter hyperintensities in affected and unaffected late teenage and early adulthood offspring of bipolar parents: a two-center high-risk study. J Psychiatr Res. 45:76–82. doi: 10.1016/j.jpsychires.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Fjell AM, Westlye LT, Greve DN, Fischl B, Benner T, van der Kouwe AJ, et al. The relationship between diffusion tensor imaging and volumtery as measures of white matter properties. Neuroimage. 2008;42:1654–1668. doi: 10.1016/j.neuroimage.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frazier JA, Breeze JL, Papadimitriou G, Kennedy DN, Hodge SM, Moore CM, et al. White matter abnormalities in children with and at risk for bipolar disorder. Bipolar Disord. 2007;9:799–809. doi: 10.1111/j.1399-5618.2007.00482.x. [DOI] [PubMed] [Google Scholar]
- 18.Versace A, Ladouceur CD, Romero S, Birmaher B, Axelson DA, Kupfer DJ, et al. Altered development of white matter in youth at high familial risk for bipolar disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49:1249–1259. doi: 10.1016/j.jaac.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaddock CA, Barker GJ, Marshall N, Schulze K, Hall MH, Fern A, et al. White matter microstructural impairments and genetic liability to familial bipolar I disorder. Br J Psychiatry. 2009;194:527–534. doi: 10.1192/bjp.bp.107.047498. [DOI] [PubMed] [Google Scholar]
- 20.Sprooten E, Sussmann JE, Clugston A, Peel A, McKirdy J, Moorhead TW, et al. White matter integrity in individuals at high genetic risk of bipolar disorder. Biol Psychiatry. 2011;70:350–356. doi: 10.1016/j.biopsych.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Bellivier F, Golmard JL, Rietschel M, Schulze TG, Malafosse A, Preisiq M, et al. Age at onset in bipolar I disorder: further evidence for three subgroups. Am J Psychiatry. 2003;160:999–1001. doi: 10.1176/appi.ajp.160.5.999. [DOI] [PubMed] [Google Scholar]
- 22.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 23.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM IV TR Axis I Disoders, Patient Edition (SCID-I/P) Biometrics Research Department, New York State Psychiatric Institute; New York: 1994. [Google Scholar]
- 24.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM IV TR Axis I Disoders, Non-Patient Edition (SCID-I/NP) Biometrics Research Department, New York State Psychiatric Institute; New York: 2001. [Google Scholar]
- 25.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 26.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localization in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 27.Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fiber orientations: what can we gain? Neuroimage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wakana S, Jiang H, Nagae-Poetscher LM, Van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 29.Martino J, Brogna C, Robles SG, Vergani F, Duffau H. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex. 2010;46:691–699. doi: 10.1016/j.cortex.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Martino J, De Witt Hamer PC, Vergani F, Brogna C, de Lucas EM, Vázquez-Barquero A, et al. Cortex-sparing fiber dissection: an improved method for the study of white matter anatomy in the human brain. J Anat. 2011;219:531–541. doi: 10.1111/j.1469-7580.2011.01414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quintin P, Benkelfat C, Launay JM, Arnulf I, Pointereau-Bellenger A, Barbault S, et al. Clinical and neurochemical effect of acute tryptophan depletion in unaffected relatives of patients with bipolar affective disorder. Biol Psychiatry. 2001;50:184–90. doi: 10.1016/s0006-3223(01)01140-4. [DOI] [PubMed] [Google Scholar]
- 32.Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. Fifty years of the Barratt Impulsiveness Scale: an update and review. Personality and Individual Differences. 2009;47:385–395. [Google Scholar]
- 33.Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. Increased trait-like impulsivity and course of illness in bipolar disorder. Bipolar Disord. 2009;11:280–288. doi: 10.1111/j.1399-5618.2009.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnea-Goraly N, Chang KD, Karchemskiy A, Howe ME, Reiss AL. Limbic and corpus callosum abberations in adolescents with bipolar disorder: a tract-based spatial statistics analysis. Biol Psychiatry. 2009;66:238–244. doi: 10.1016/j.biopsych.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 35.Bruno S, Cercignani M, Ron MA. White matter abnormalities in bipolar disorder: a voxel-based diffusion tensor imaging study. Bipolar Disord. 2008;10:460–468. doi: 10.1111/j.1399-5618.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 36.Zanetti M, Jackowski MP, Versace A, Almeida JRC, Hassel S, Duran FL, et al. State dependent microstructural white matter changes in bipolar I depression. Eur Arch Clin Neurosci. 2009;259:316–328. doi: 10.1007/s00406-009-0002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmahmann JD, Pandya DN. Fiber pathways of the brain. Oxford, UK: Oxford University Press; 2006. [Google Scholar]
- 38.ffytche DH, Catani M. Beyond localization: from hodology to function. Philos Trans R Soc Lond B Biol Sci. 2005;360:767–779. doi: 10.1098/rstb.2005.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rizolatti G, Matelli M. Two different streams from the dorsal visual system: anatomy and functions. Exp Brain Res. 2003;153:146–157. doi: 10.1007/s00221-003-1588-0. [DOI] [PubMed] [Google Scholar]
- 40.Catani M, Jones DK, Donato R, ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- 41.Martino J, Brogna C, Robles SG, Vergani F, Duffau H. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex. 2010;46:691–699. doi: 10.1016/j.cortex.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 42.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:829, 833–57. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strakowski SM, DelBello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- 44.Green MJ, Cahill CM, Malhi GS. The cognitive and neurophysiological basis of emotion dysregulation in bipolar disorder. J Affect Disord. 2007;103:29–42. doi: 10.1016/j.jad.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 45.Lyoo IK, Hwang J, Sim M, Dunn BJ, Renshaw PF. Advances in magnetic resonance imaging methods for the evaluation of bipolar disorder. CNS Spectr. 2006;11:269–280. doi: 10.1017/s1092852900020770. [DOI] [PubMed] [Google Scholar]
- 46.Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33:699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 48.Umarova RM, Saur D, Schnell S, Kaller CP, Vry MS, Glauche V, et al. Structural connectivity for visuospatial attention: significance of ventral pathways. Cereb Cortex. 2010;20:121–129. doi: 10.1093/cercor/bhp086. [DOI] [PubMed] [Google Scholar]
- 49.Forstmann BU, Jahfari S, Scholte HS, Wolfenstellar U, van den Wildenberg W, Ridderinkhof KR. Function and structure of the right inferior frontal cortex predict individual differences in response inhibition: a model-based approach. J Neurosci. 2008;28:9790–9796. doi: 10.1523/JNEUROSCI.1465-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Houenou J, Frommberger J, Carde S, Glasbrenner M, Diener C, Leboyer M, et al. Neuroimaging-based markers of bipolar disorder: evidence from two meta-analyses. J Affect Disord. 2011;132:344–55. doi: 10.1016/j.jad.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 51.Versace A, Almeida JC, Hassel S, Walsh ND, Novelli M, Klein CR, et al. Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Arch Gen Psychiatry. 2008;65:1041–1052. doi: 10.1001/archpsyc.65.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]