Abstract
We demonstrate 1H amide resonance line widths <300 Hz in 1H/15N heteronuclear correlation (HETCOR) spectra of membrane proteins in aligned phospholipid bilayers. This represents a substantial improvement over typically observed line widths of ~1 kHz. Furthermore, in a proton detected local field (PDLF) version of the experiment that measures heteronuclear dipolar couplings, line widths <130 Hz are observed. This dramatic line narrowing of 1H amide resonances enables many more individual signals to be resolved and assigned from uniformly 15N labeled membrane proteins in phospholipid bilayers under physiological conditions of temperature and pH. Finding that the decrease in line widths occurs only for membrane proteins that undergo fast rotational diffusion around the bilayer normal, but not immobile molecules, such as peptide single crystals, identifies a potential new direction for pulse sequence development that includes overall molecular dynamics in their design.
Keywords: Solid-state NMR, Membrane proteins, Phospholipid bilayers, Line-narrowing
1. Introduction
It is essential to study membrane proteins in phospholipid bilayers under physiological conditions of temperature and pH, since their correct folding and function are closely linked to the chemical and physical properties of their environment. Convenient membrane mimics, such as detergent micelles and isotropic bicelles are frequently used for solution NMR experiments; however, the high concentrations of detergents in the samples have the potential to perturb the structures of these delicate and malleable proteins. Magnetically alignable bicelles with higher q values (molar ratio of long chain to short chain lipids) [1] may resemble the native environment more closely, but still contain ~30% detergent, and generally give very broad 1H amide line widths for the embedded proteins that compromise the resolution and sensitivity of the spectra. Moreover, since crystal packing effects, non-native detergents and lipid phases, and sequence mutations and modification associated with X-ray diffraction also have the potential to distort membrane protein structures, the only method currently capable of accurately determining the structures of unmodified membrane proteins in phospholipid bilayers under physiological conditions is solid-state NMR spectroscopy. Further development is necessary to improve the quality of the data, and the accuracy, precision, and speed of structure determination. This is the case for stationary, aligned samples in oriented sample (OS) solid-state NMR [2], magic angle spinning (MAS) solid-state NMR [3], and the recently developed rotational alignment (RA) NMR, which merges the two methods [4,5].
In practice, NMR spectral resolution is determined by the ratio of the line widths of individual resonances to the total frequency span covered by the relevant signals in the spectrum. For example, in solution NMR of helical membrane proteins, the 1H amide resonances are generally poorly resolved because their line widths are broadened by the relatively slow global reorientation of the protein and detergent/lipid assemblies [6], and the frequency dispersion is limited since each residue in an α-helix resides in a similar chemical and structural environment. The limited frequency dispersion of the isotropic 1H resonances also plagues MAS solid-state NMR studies. In contrast, the frequency span of signals in solid-state NMR spectra of stationary aligned or rotationally aligned samples of the same proteins are often quite large, since the frequencies are dispersed by the angular-dependence of the anisotropic chemical shift (CSA) and heteronuclear dipolar coupling (DC) nuclear spin interactions.
It is challenging to obtain narrow 1H amide line widths in solid-state NMR due to the dense network of homonuclear dipolar couplings resulting from the high abundance and high gyromagnetic ratio of hydrogens in organic molecules [7], including polypeptides. To our knowledge, the narrowest previously reported 1H line widths in the chemical shift dimension of solid-state NMR spectra of stationary aligned samples are ~1 kHz [8,9]. Even with ultrafast MAS, 1H resonance line widths of 150 Hz have only recently been achieved [10]. This is one of the main reasons that laboratory-frame proton-detected local field (PDLF) class experiments are used less frequently than the rotating-frame polarization inversion spin-exchange at the magic angle (PISEMA) class experiments [11– 15]. The design and implementation of methods that can provide additional line narrowing of 1H amide resonances from 15N labeled membrane proteins in phospholipid bilayers, while preserving the anisotropic information and large frequency spans of the spin-interactions, is an important goal for OS solid-state NMR.
In this article, we demonstrate that a pulse sequence, which provides no additional narrowing in a stationary peptide crystal, is highly effective at narrowing 1H amide line widths of membrane proteins in aligned phospholipid bilayers. It improves the resolution in the 1H chemical shift dimension of heteronuclear correlation (HETCOR) spectra, as well as in the 1H–15N dipolar coupling dimension of PDLF [16] class experiments. Notably, the line widths observed for small membrane proteins, such as the membrane-bound form of the 46-residue Pf1 coat protein [17,18], are the same as those observed for larger proteins, such as the 81-residue mercury transporter MerF [19] and the 350-residue G-protein coupled receptor CXCR1 [20]. Examples of spectra from these 46, 81, and 360 residue proteins are used to illustrate the NMR experiments.
2. Results
2.1. Narrowing of the 1H amide resonances
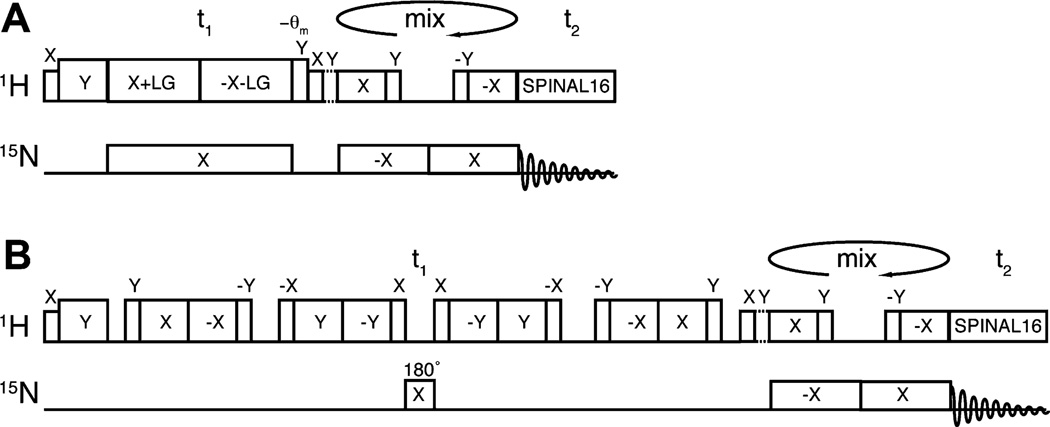
The timing diagrams for two different two-dimensional 1H/15N HETCOR pulse sequences are compared in Fig. 1; the focus of attention is the indirect 1H chemical shift dimension. In a conventional HETCOR pulse sequence (Fig. 1A), frequency-switched Lee–Goldberg (FSLG) [21,22] irradiation is applied at the 1H resonance frequency to achieve 1H/1H homonuclear decoupling, while continuous wave irradiation is applied at the 15N (and/or 13C frequencies in common variations) for heteronuclear decoupling. Results from a single crystal sample of N-acetyl leucine (NAL) (Fig. 2C) and the membrane-bound form of Pf1 coat protein in phospholipid bilayers (Fig. 2A) demonstrate that the conventional pulse sequence gives 1H amide line widths of ~700 Hz, even after extensive experimental optimization. To our knowledge, the narrowest previously reported 1H line widths in solid-state NMR HETCOR spectra are ~1 kHz [8,9]. Thus, a new generation of probes, high power irradiation, and careful optimization of conditions yields only a modest improvement in 1H line widths using the pulse sequence shown in Fig. 1A. Notably, the line widths are slightly narrower in the protein sample than in the NAL crystal sample, which can be attributed to the slightly reduced order parameter (~0.9) observed in magnetically aligned bicelles.
Fig. 1.
Timing diagrams for pulse sequences. (A) The conventional 1H/15N heteronuclear correlation HETCOR [45] pulse sequence. (B) The high-resolution variant of 1H/15N HETCOR for membrane proteins. It uses a modified magic sandwich pulse sequence (MSHOT-PI4) and a single refocusing π pulse during the 1H chemical shift evolution period. Selective magnetization transfer occurs during the “mixing” period using SAMPI4 [25].
Fig. 2.
Comparison of the application of the 1H/15N HETCOR pulse sequences to the membrane-bound form of Pf1 coat protein in magnetically aligned bilayers (top panels) and a NAL single crystal (bottom panels). (A and C) The experimental spectra were obtained using the conventional (Fig. 1A) HETCOR pulse sequence. (B and D) The experimental spectra were obtained using the new version (Fig. 1B) of the HETCOR pulse sequence. A one-dimensional spectral slice from the t1 dimension is shown on the right side of each two-dimensional spectrum, and the signal selected for each slice is labeled with an arrow on the respective two-dimensional spectra. The half brace designates the resonance peaks of I39 and V35. No apodization functions were applied in processing the t1 dimension, and Fig. 3 shows the time domain evolution of signals that contribute to spectra A and B. The resonance assignments for all peaks in spectrum B are shown in Fig. 6.
Significantly narrower line widths (<300 Hz) are observed in the 1H chemical shift dimension (Figs. 2B and 3) when the pulse sequence diagrammed in Fig. 1B is applied to the same sample of a membrane protein in phospholipid bilayers. The time domain decays and oscillations for the selected signal are shown in Fig. 3. The effects of the decreased line widths can be observed throughout the spectrum; for example, the resonances of I39 and V35 overlap in Fig. 2A, but are resolved in Fig. 2B. In contrast, the pulse sequence in Fig. 1B provides no additional narrowing of the signals from the stationary NAL crystal (Fig. 2D).
Fig. 3.
t1 Time domain evolution of the Pf1 coat protein spectra in Fig. 2 using the new (A) and conventional (B) HETCOR pulse sequence. The total t1 evolution time is approximately the same for the two spectra, however, due to their different scaling factors, the two oscillations are scaled accordingly in order to match the same frequency (shown by the dashed line).
2.2. Description of individual components of MSHOT-PI4/Pi
Since both the homonuclear and heteronuclear decoupling schemes are altered from those used previously, we investigated their effects separately. In a set of back-to-back experiments, we compared pulse sequences incorporating MSHOT-PI4 with a refocusing p pulse (top row, Fig. 4A and B), MSHOT-PI4 with CW irradiation (middle row, Fig. 4C and D), and FSLG with a refocusing π pulse (bottom row, Fig. 4E and F). The signals in Fig. 4A and B have the same line widths observed in Fig. 2, although the experiments were performed with a slightly different B1 fields and crystal orientations. While the line widths are similar in the single crystal spectra, the line widths for the membrane-bound form of the Pf1 coat protein magnetically aligned bilayers shows that the replacement of FSLG by MSHOT provides ~200 Hz of line narrowing, and the replacement of CW irradiation by a single refocusing π pulse provides ~150 Hz of line narrowing. Therefore, the changes in both the homonuclear and heteronuclear decoupling schemes contribute to the overall line narrowing provided by the pulse sequence when it is applied to a membrane protein undergoing rotational diffusion in phospholipid bilayers.
Fig. 4.
Separate evaluation of the homonuclear and heteronuclear decoupling schemes. Spectra from the membrane-bound form of Pf1 coat protein (A, C and E) and NAL single crystal (B, D and F). (A and B) MSHOT with refocusing π pulse, which is the same pulse sequence as in Fig. 1B–D. MSHOT with CW decoupling during the entire t1 interval. (E and F) FSLG with a single refocusing π pulse.
The homonuclear decoupling in the 1H chemical shift dimension results from a magic sandwich pulse sequence [23]. In an ideal case, the magic sandwich has the general pattern of τ–π/2–2π–2π– π/2 – τ, where the π/2 pulse is a δ pulse, and τ has the duration of a π pulse. In the actual NMR experiment, since the π/2 pulse cannot be a δ pulse, compensation for the finite pulse lengths has to be made. In the originally proposed MSHOT pulse sequence [24], Hohwy and Nielsen set the length of τ to be 7π/8 and kept the 2π pulse at 2π; and in a recent elaboration of the SAMPI4 pulse sequence [25], τ was set to be 3π/4 and the 2π pulse was shortened to 7π/ 4. Since in the first order approximation, the π/2 pulses retain a quarter of the homonuclear coupling evolution, both schemes average the first-order homonuclear coupling to zero. In Fig. 5, the performance of these two finite pulse length compensation schemes are compared on a single crystal sample, and found that the 3π/4 and 7π/4 combination (Fig. 5A) yields somewhat narrower line widths. Consequently, this “PI4” finite pulse compensation scheme was incorporated into the general Z-rotational decoupling of the MSHOT pulse sequence, resulting in the pulse sequence we refer to as MSHOT-PI4. Note that the 15N CW decoupling is applied only during periods without irradiation in the MSHOT pulse scheme [8,26]. On membrane protein samples, we found that application of CW decoupling during the entire t1 dimension provided superior line narrowing, though it made little difference for the single crystal sample.
Fig. 5.
Comparison of finite pulse compensation schemes for HETCOR experiment. The homonuclear decoupling during t1 is executed by MSHOT4 pulses with finite pulse compensation scheme described by either (A) Nevzorov and Opella [25] or (C) Hohwy and Nielsen [24]. The corresponding spectra acquired on a NAL single crystal sample are shown on the right side (B and D). One-dimensional slices for the left-most peak were extracted for line width comparison. Compared to the NAL single crystal spectra in Figs. 2 and 4, the slightly larger line widths observed in these spectra is most likely due to the lower 1H B1 fields used in these experiments.
2.3. Application to larger membrane proteins
All the signals from the membrane-bound form of the Pf1 coat protein in magnetically aligned bilayers can be readily assigned based on previous results [27] (Fig. 6), and significantly, we find its MSHOT-PI4/Pi HETCOR spectrum has resolution equivalent to that observed in the most recent examples of separated local field (SLF) spectra of the same sample [28,29], where except for the same two pairs of resonances that are partially overlapped in both spectra, all other peaks exhibit single-site resolution. Note that the two-dimensional SLF spectrum (1H–15N heteronuclear dipolar coupling correlated with 15N chemical shift) is the benchmark in OS solid-state NMR, in large part due to the high resolution in the 1H–15N heteronuclear dipolar coupling dimension [30]. The ability of the MSHOT-PI4/Pi pulse sequence to obtain a third high-resolution dimension expands the range of membrane proteins amenable to high resolution OS solid-state NMR without adding magnetization transfer steps, which can reduce sensitivity. The only disadvantage is that polarization inversion is not practical in the three-dimensional experiment.
Fig. 6.
Resonance assignments (residue numbers) of the signals in a two-dimensional 1H/15N HETCOR spectrum of the membrane-bound form of the Pf1 coat protein in magnetically aligned bilayers. The assignments are based on a previously recorded three-dimensional HETCOR–SLF spectrum [27].
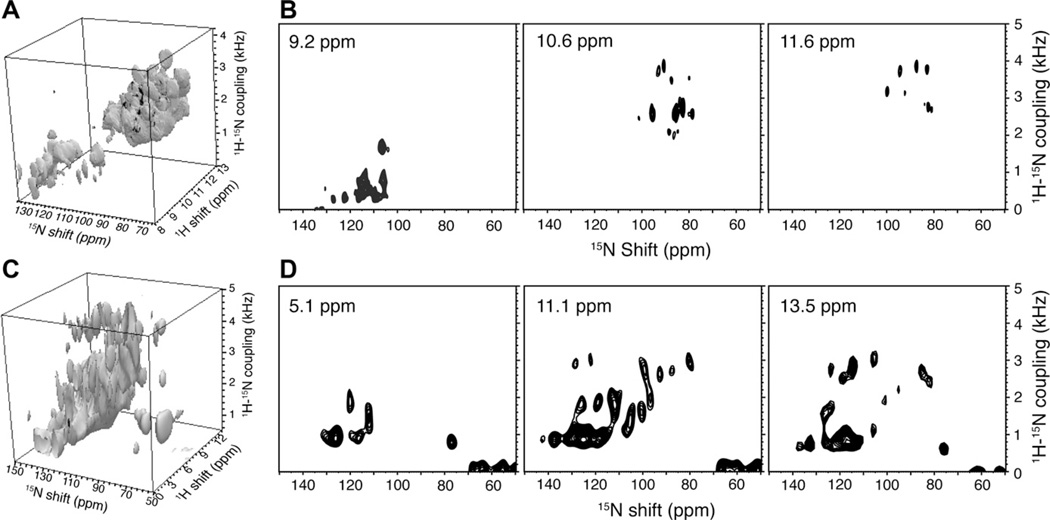
Three-dimensional HETCOR/SLF spectra were acquired from samples of two additional membrane proteins in phospholipid bilayers. Fig. 7 shows spectra acquired from a sample of uniformly 15N labeled MerFm, an 81-residue mercury transport protein with two transmembrane helices. More than 50 resonances are heavily overlapped in the helical region of a conventional two-dimensional SLF spectrum (Fig. 7A), however, almost all signals are resolved in the three-dimensional HETCOR/SLF spectrum. A cube representation of the three-dimensional spectrum is shown in Fig. 8A, and examples of HETCOR (Fig. 7B) and SLF planes (Fig. 8B) from that cube illustrate the single-site resolution. Fig. 8C and D contain data from a three-dimensional spectrum obtained from a sample of the 350-residue G-protein coupled receptor CXCR1 in phospholipid bilayers. Comparisons with the spectra of the smaller membrane proteins demonstrates that the pulse sequence in Fig. 1B is equally effective at narrowing the 1H amide resonances of both small and large membrane proteins in phospholipid bilayers.
Fig. 7.
Two-dimensional SLF spectrum (A) and selective planes from three-dimensional HETCOR/SLF spectrum (B) obtained from the uniformly 15N labeled mercury transport protein MerFm in magnetically aligned bilayers. More than 50 residues are heavily overlapped in the helical region (1H–15N dipolar couplings > 1 kHz) make it a challenging target for structure determination with two-dimensional experiments. The selected HETCOR planes from a three-dimensional experiment display resolution similar to that observed in the membrane-bound form of the Pf1 coat protein in magnetically aligned bilayers in Fig. 6.
Fig. 8.
Three-dimensional HETCOR/SLF spectra of uniformly 15N labeled membrane proteins in magnetically aligned phospholipid bilayers. (A and C) Cubes displaying the three-dimensional spectra. (A) 81-residue MerFm. (C) 350-residue CXCR1. (B) SLF planes at selected 1H frequencies from the three-dimensional spectrum of MerFm. (D) SLF planes at selected 1H frequencies from the three-dimensional spectrum of CXCR1. The two-dimensional HETCOR pulse sequence shown in Fig. 1B was used to acquire the three-dimensional HETCOR/SLF spectra with the addition of systematic incrementation of the duration of the “mixing” interval of the SAMPI4 pulse [9,25].
2.4. Line-narrowing in the heteronuclear dipolar coupling frequency dimension
Another application of this experimental approach is to enhance the resolution in the heteronuclear dipolar coupling dimension in the PDLF class of experiments. Due to the generally broader line widths of the resonances, laboratory-frame PDLF class experiments are used less frequently than the rotating-frame PISEMA class experiments [11,12]. However, PDLF experiments have the advantage that they do not truncate the values of small dipolar couplings; therefore, they yield more accurate measurements and better resolved signals from residues in loop, terminal or surface helices, which often have relatively small heteronuclear dipolar couplings in aligned samples of membrane proteins. The pulse sequences in Fig. 9 yield line widths that are similar to those (~200 Hz) achieved by either PISEMA [31] or SAMPI4 [25], as demonstrated by the spectrum of the membrane-bound form of Pf1 coat protein in Fig. 10A. Notably, the spectrum is free from dipolar coupling truncation artifacts, such as the clustering of peaks at the small dipolar coupling frequencies that are observed in many PISEMA spectra of membrane proteins. A two-dimensional PDLF spectrum was also acquired on MerFm in phospholipid bilayers, and yielded a spectrum with single-site resolution for signals in the non-helical region (Fig. 10B).
Fig. 9.
Two-dimensional PDLF pulse sequences. The pulse sequences in Fig. 1B are modified to perform PDLF class experiments by the addition of a π pulse on the 1H channel at the same position as the 15N channel refocusing π pulse. (A) Selective magnetization transfer is performed by SAMPI4 [9,25]. (B) Magnetization transfer is performed by WIM. WIM-24 follows the same phase cycle as previously described [46], and the φ pulse is cycled between Y and –Y, and in combination with the preceding Y pulse, it cancels the spin-locked component [46]. In our experience, WIM provides higher sensitivity for magnetization transfer, while SAMPI4 provides better dipolar coupling results.
Fig. 10.
The application of two-dimensional PDLF spectroscopy to non-helical residues of membrane proteins in magnetically aligned phospholipid bilayers. (A) The spectrum was acquired on the membrane-bound form of the Pf1 coat protein. The inset shows the indirect dimension time evolution of the signal from residue A46, which has a 15N chemical shift of 138 ppm and is marked in the spectrum. Its line width is noted near the resonance. (B) The spectrum was acquired on MerFm “flipped” bicelles by the additional of lanthanide ion as described previously [40,47,48]. The non-helical region of MerFm displays single-site resolution in the PDLF spectrum.
For some 1H amide signals in the PDLF spectrum, such as that from alanine 46 of the membrane-bound form of Pf1 coat protein (Fig. 10A), the time domain oscillation extends beyond 20 ms (Fig. 10A inset), which is comparable to the most extended 15N oscillations [30]. The line widths of the 1H signals would be ~40 Hz without the drawback of an unfavorable scaling factor of 0.33, which would be equivalent to the narrowest 15N amide resonance line width observed to date [30]. Considering that 15N is a dilute spin by virtue of its low gyromagnetic ratio and spatial separation in proteins, this result demonstrates the potential to achieve highly effective homonuclear and heteronuclear decoupling of amide 1H sites in membrane proteins despite their dense network of couplings and high gyromagnetic ratio.
3. Discussion
A likely reason for the enhanced line narrowing with the pulse sequences described here is their ability to avoid interference from global protein motion, since the line narrowing is only found in membrane proteins in liquid crystalline phospholipid bilayers where they undergo rapid rotational diffusion about the bilayer normal [32]. Notably, several early studies demonstrated that motions of organic compounds on a time scale similar to that of the pulse nutation frequency reduces the efficiency of pulse decoupling [33–36]. Currently, the pulse nutation frequency in most OS solid-state NMR experiments is similar to the rotational diffusion frequency of membrane proteins at approximately 105 Hz [37], and therefore interference effects may be present. These interference effects have been well characterized for CW decoupling but not for magic sandwich decoupling, and the effect has not been observed in NMR spectra of proteins. It is straightforward to understand how the replacement of CW decoupling by a single π pulse could enhance the resolution by removing the interference effect; however, it will require further theoretical and experimental studies to confirm the source of the line narrowing by the MSHOT-PI4 sequence.
It is worth noting that composite pulses, such as 9045901359045, can replace the single refocusing π pulse in the 15N channel to provide better compensation for offset and calibration errors [38]. However, the high power rf pulses used in solid-state NMR often produce transient effect, which makes the first 90° pulse being “cold”, i.e. before the RF coil is equilibrated. Consequently, the three 90° pulses are not truly equivalent, and it is in our experience a single π pulse provides better decoupling, unless elaborate steps are taken to optimize the individual components of the composite pulses.
The finding that a variation of a pulse sequence can narrow the resonances of a membrane protein by more than a factor of two illustrates the significances of global protein motions in determining the efficacy of pulse sequence performance. These effects may be generalizable to other pulse sequences applicable to both OS solid-state NMR on stationary aligned bilayer samples and RA solid-state NMR on unoriented proteoliposome samples. The pulse sequences described here narrow 1H resonances, and thereby advance the development and application of high resolution solid-state NMR spectroscopy for membrane proteins in phospholipid bilayers; together with the optimization of many other factors, for example, water-content, sample preparation, temperature, pH, dynamics, conformational heterogeneity and disorder in the membrane orientation, it improves the methods of membrane protein structure determination.
4. Experimental methods
4.1. Sample preparation and instrumentation
A full-length construct of the mercury transport protein MerF, which has its Cys residues replaced by Ser residues, is named MerFm (for modified) and its sequence, expression and purification have been described previously [39,19]. The expression and purification of Pf1 coat protein [29] and CXCR1 [30] have also been described previously. In the case of Pf1 coat protein and MerFm, magnetically aligned bicelle samples were prepared with 6-O-PC (1,2-di-O-hexyl-sn-glycero-3-phosphocholine) and 14-O-PC (1,2-di-O-tetradecyl-sn-glycero-3-phosphocholine) as previously described [29,19,40]; and in the case of CXCR1, the bicelle sample was prepared with DMPC (1,2-Dimyristoyl-sn-Glycero-3-Phosphocholine) and Triton X-100 with q = 5 and with 20% (w/v) of DMPC. In addition, 2.5 mM NaN3 and 20 mM HEPES (pH 7.3) are included in the CXCR1 sample.
The solid-state NMR spectra were obtained on 700 MHz Bruker Avance spectrometers using home-built probes. A homebuilt 1H/15N double-resonance probe with a strip-shield was used for all experiments [41] except for those on the CXCR1 sample, where a modified Alderman–Grant coil (MAGC) probe was used [42]. These probes minimize the detuning, Q lowering, and suppress sample heating associated with high frequency, high power radiofrequency irradiations on electrically lossy samples, such as protein-containing phospholipid bilayers in water.
4.2. NMR experiments
The two spectra of Pf1 coat protein in Fig. 2 were obtained back-to-back on the same sample. For both of them, the B1 field for 1H irradiation during the t1 evolution period was 71.4 kHz, and 50 kHz elsewhere in the sequence. Forty Scans were co-added for each t1 increment and 64 complex t1 point were recorded for the spectrum in Fig. 2B; consequently, 64 MSHOT-PI4 cycles gave a t1 evolution period of 10.75 ms. To match the duration of t1, 235 complex t1 points were obtained for the spectrum in Fig. 2A with 4 FSLG pulses per increment. For both spectra, 3 cycles of SAMPI4 was used to selectively transfer magnetization for signals with large dipolar couplings. For the NAL single crystal spectrum in Fig. 2C,62.5 kHz of 1H power was used during t1 evolution and 80 complex points were collected with 4 FSLG pulses per increment. The spectrum in Fig. 2D used 72.9 kHz 1H power during t1 evolution and 64 complex points were collected. In both cases, excess increments were present after the signals had decayed into the noise level. Three cycles of SAMPI4 was used in the mixing period, which selectively transferred magnetization for resonances with 1H/15N dipolar couplings >1 kHz. In all the experiments, after the first 90° pulse, a short spin-lock pulse (typically 100 µs) was incorporated in order for transient effects to dissipate.
With the same samples, the spectra in Fig. 4 were recorded back-to-back. During t1 evolution for the membrane-bound form of Pf1 coat protein sample, the B1 field was 72.9 kHz for MSHOT-PI4 pulses, 62.5 kHz for FSLG pulses, and 47.2 kHz for CW decoupling on the 15N channel. 64 complex t1 points were recorded for all the spectra in Fig. 5. Consequently, 64 MSHOT-PI4 cycles gave a t1 evolution time of 10.54 ms, and 64 FSLG cycles with 4 pulses each corresponds to 3.34 ms, where in both cases signals were allowed to decay to the noise level. A B1 field of 47.2 kHz was applied during the rest of the pulse sequence, and 3 cycles of SAMPI4 were used for magnetization transfer. The experimental conditions for the NAL crystal spectra were the same as those for the membrane-bound form of the Pf1 coat protein. The line widths were measured in NMRDraw [43] or Sparky [44].
For the spectra in Fig. 5, the B1 field was 62.5 kHz for 1H irradiation during t1 evolution, and 50 kHz elsewhere in the sequence. Sixty-four complex t1 points were collected for both spectra, giving evolution periods of 3.07 ms and 3.46 ms.
The conventional two-dimensional SLF spectrum was acquired with the SAMPI4 pulse sequence [25], and 80 real t1 points and 512 complex t2 points were co-added. For the three-dimensional HETCOR/SLF spectrum on the sample of the MerFm protein, 30 complex t1 points, 48 real t2 point, 512 complex t3 points and 40 scans were collected. The three-dimensional data set of CXCR1 consisted of 20 complex t1 points, 20 real t2 points and 256 complex t3 points.
For the PDLF spectrum on the membrane-bound form of Pf1 coat protein, 128 real t1 points and 40 transients were collected. 72.9 kHz 1H radiofrequency pulses were used during t1 evolution. Nine cycles of SAMPI4 pulses at 47.2 kHz matching B1 field were used to ensure the transfer of magnetization for residues with small dipolar couplings. For the PDLF spectrum on the MerFm sample, 76 real t1 points and 80 scans were collected. 71.4 kHz 1H radiofrequency pulses were used during t1 evolution and 3 cycles of WIM pulses at 50 kHz matching B1 field were used to ensure the transfer of magnetization for signals with small dipolar couplings.
Acknowledgments
We thank Nemil Vora for assistance with the sample preparation, and Dr. Chin Wu and Dr. Chris Grant for assistance with the instrumentation. This research was supported by grants from the National Institutes of Health, and utilized the Biotechnology Research Center for NMR Molecular Imaging of Proteins at UCSD, which is supported by Grant P41EB002031.
References
- 1.Sanders CR, Hare BJ, Howard KP, Prestegard JH. Magnetically-oriented phospholipid micelles as a tool for the study of membrane-associated molecules. Prog. Nucl. Magn. Reson. Spectrosc. 1994;26:421–444. [Google Scholar]
- 2.Cross TA, Opella SJ. Protein structure by solid state NMR. J. Am. Chem. Soc. 1983;105:306–308. [Google Scholar]
- 3.Opella SJ, Frey MH, Cross TA. Detection of individual carbon resonances in solid proteins. J. Am. Chem. Soc. 1979;101:5856–5857. [Google Scholar]
- 4.Marassi FM, Das BB, Lu GJ, Nothnagel HJ, Park SH, Son WS, Tian Y, Opella SJ. Structure determination of membrane proteins in five easy pieces. Methods. 2011;55:363–369. doi: 10.1016/j.ymeth.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park SH, Das BB, De Angelis AA, Scrima M, Opella SJ. Mechanically magnetically and “rotationally aligned” membrane proteins in phospholipids bilayers give equivalent angular constraints for NMR structure determination. J. Phys. Chem. B. 2010;114:13995–14003. doi: 10.1021/jp106043w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Son WS, Park SH, Nothnagel HJ, Lu GJ, Wang Y, Zhang H, Cook GA, Howell SC, Opella SJ. ‘q-Titration’ of long-chain and short-chain lipids differentiates between structured and mobile residues of membrane proteins studied in bicelles by solution NMR spectroscopy. J. Magn. Reson. 2012;214:111–118. doi: 10.1016/j.jmr.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waugh JS, Huber LM, Haeberlen U. Approach to high-resolution NMR in solids. Phys. Rev. Lett. 1968;20:180–182. [Google Scholar]
- 8.Fu R, Gordon ED, Hibbard DJ, Cotten M. High resolution heteronuclear correlation NMR spectroscopy of an antimicrobial peptide in aligned lipid bilayers: peptide-water interactions at the water-bilayer interface. J. Am. Chem. Soc. 2009;131:10830–10831. doi: 10.1021/ja903999g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nevzorov A, Park S, Opella S. Three-dimensional experiment for solid-state NMR of aligned protein samples in high field magnets. J. Biomol. NMR. 2007;37:113–116. doi: 10.1007/s10858-006-9121-y. [DOI] [PubMed] [Google Scholar]
- 10.Salager E, Dumez J-N, Stein RS, Steuernagel S, Lesage A, Elena-Herrmann B, Emsley L. Homonuclear dipolar decoupling with very large scaling factors for high-resolution ultrafast magic angle spinning 1H solid-state NMR spectroscopy. Chem. Phys. Lett. 2010;498:214–220. [Google Scholar]
- 11.Wu CH, Das BB, Opella SJ. 1H-13C hetero-nuclear dipole-dipole couplings of methyl groups in stationary and magic angle spinning solid-state NMR experiments of peptides and proteins. J. Magn. Reson. 2010;202:127–134. doi: 10.1016/j.jmr.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gan Z. Spin dynamics of polarization inversion spin exchange at the magic angle in multiple spin systems. J. Magn. Reson. 2000;143:136–143. doi: 10.1006/jmre.1999.1971. [DOI] [PubMed] [Google Scholar]
- 13.Ramamoorthy A, Wei Y, -K D. Lee, PISEMA Solid-State NMR Spectroscopy. In: Webb GA, editor. Annual Reports on NMR Spectroscopy. Academic Press; 2004. pp. 1–52. [Google Scholar]
- 14.Dvinskikh SV, Durr UHN, Yamamoto K, Ramamoorthy A. High-resolution 2D NMR spectroscopy of bicelles to measure the membrane interaction of ligands. J. Am. Chem. Soc. 2007;129:794–802. doi: 10.1021/ja065536k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dvinskikh SV, Yamamoto K, Ramamoorthy A. Heteronuclear isotropic mixing separated local field NMR spectroscopy. J. Chem. Phys. 2006;125:034507. doi: 10.1063/1.2212939. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt-Rohr K, Nanz D, Emsley L, Pines A. NMR measurement of resolved heteronuclear dipolar couplings in liquid crystals and lipids. J. Phys. Chem. 1994;98:6668–6670. [Google Scholar]
- 17.Zeri AC, Mesleh MF, Nevzorov AA, Opella SJ. Structure of the coat protein in fd filamentous bacteriophage particles determined by solid-state NMR spectroscopy. Proc. Natl. Acad. Sci. 2003;100:6458–6463. doi: 10.1073/pnas.1132059100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park SH, Marassi FM, Black D, Opella SJ. Structure and dynamics of the membrane-bound form of Pf1 coat protein: implications of structural rearrangement for virus assembly. Biophys. J. 2010;99:1465–1474. doi: 10.1016/j.bpj.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Angelis AA, Howell SC, Nevzorov AA, Opella SJ. Structure determination of a membrane protein with two trans-membrane helices in aligned phospholipid bicelles by solid-state NMR spectroscopy. J. Am. Chem. Soc. 2006;128:12256–12267. doi: 10.1021/ja063640w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park SH, Casagrande F, Chu M, Maier K, Kiefer H, Opella SJ. Optimization of purification and refolding of the human chemokine receptor CXCR1 improves the stability of proteoliposomes for structure determination. Biochim. Biophys. Acta. 2012;1818:584–591. doi: 10.1016/j.bbamem.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bielecki A, Kolbert AC, Levitt MH. Frequency-switched pulse sequences: homonuclear decoupling and dilute spin NMR in solids. Chem. Phys. Lett. 1989;155:341–346. [Google Scholar]
- 22.Lee M, I W. Goldburg, Nuclear-magnetic-resonance line narrowing by a rotating rf field. Phys. Rev. 1965;140:A1261. [Google Scholar]
- 23.Rhim WK, Pines AS, Waugh J. Time-reversal experiments in dipolar-coupled spin systems. Phys. Rev. B. 1971;3:684. [Google Scholar]
- 24.Hohwy M, Nielsen N. Elimination of high order terms in multiple pulse nuclear magnetic resonance spectroscopy: application to homonuclear decoupling in solids. J. Chem. Phys. 1997;106:7571–7586. [Google Scholar]
- 25.Nevzorov AA, Opella SJ. Selective averaging for high-resolution solid-state NMR spectroscopy of aligned samples. J. Magn. Reson. 2007;185:59–70. doi: 10.1016/j.jmr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Fu R, Truong M, Saager RJ, Cotten M, Cross TA. High-resolution heteronuclear correlation spectroscopy in solid state NMR of aligned samples. J. Magn. Reson. 2007;188:41–48. doi: 10.1016/j.jmr.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Lu GJ, Son WS, Opella SJ. A general assignment method for oriented sample (OS) solid-state NMR of proteins based on the correlation of resonances through heteronuclear dipolar couplings in samples aligned parallel and perpendicular to the magnetic field. J. Magn. Reson. 2011;209:195–206. doi: 10.1016/j.jmr.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Opella SJ, Zeri AC, Park SH. Structure, dynamics and assembly of filamentous bacteriophages by nuclear magnetic resonance spectroscopy. Annu. Rev. Phys. Chem. 2008;59:635–657. doi: 10.1146/annurev.physchem.58.032806.104640. [DOI] [PubMed] [Google Scholar]
- 29.Park SH, Marassi FM, Black D, Opella SJ. Structure and dynamics of the membrane-bound form of Pf1 coat protein: implications of structural rearrangement for virus assembly. Biophys. J. 2010;99:1465–1474. doi: 10.1016/j.bpj.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park SH, Opella SJ. Triton X-100 as the “Short-Chain Lipid” improves the magnetic alignment and stability of membrane proteins in phosphatidylcholine bilayers for oriented-sample solid-state NMR spectroscopy. J. Am. Chem. Soc. 2010;132:12552–12553. doi: 10.1021/ja1055565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu CH, Ramamoorthy A, Opella SJ. High-resolution heteronuclear dipolar solid-state NMR spectroscopy. J. Magn. Reson. A. 1994;109:270–272. [Google Scholar]
- 32.Park SH, Casagrande F, Das BB, Albrecht L, Chu M, Opella SJ. Local and global dynamics of the G-protein-coupled receptor CXCR1. Biochemistry. 2011;50:2371–2380. doi: 10.1021/bi101568j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothwell WP, Waugh JS. Transverse relaxation of dipolar coupled spin systems under rf irradiation: detecting motions in solids. J. Chem. Phys. 1981;74:2721–2732. [Google Scholar]
- 34.Long JR, Sun BQ, Bowen A, Griffin RG. Molecular dynamics and magic angle spinning NMR. J. Am. Chem. Soc. 1994;116:11950–11956. [Google Scholar]
- 35.McMillan DE, Hazendonk P, Hodgkinson P. Interference of homonuclear decoupling and exchange in the solid-state NMR of perfluorocyclohexane. J. Magn. Reson. 2003;161:234–241. doi: 10.1016/s1090-7807(03)00015-6. [DOI] [PubMed] [Google Scholar]
- 36.Reichert D. NMR studies of dynamic processes in organic solids. In: Webb GA, editor. Annual Reports on NMR Spectroscopy. Academic Press; 2005. pp. 159–203. [Google Scholar]
- 37.Park SH, Mrse AA, Nevzorov AA, De Angelis AA, Opella SJ. Rotational diffusion of membrane proteins in aligned phospholipid bilayers by solid-state NMR spectroscopy. J. Magn. Reson. 2006;178:162–165. doi: 10.1016/j.jmr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Levitt MH. Composite pulses. Prog. Nucl. Magn. Reson. Spectrosc. 1986;18:61–122. [Google Scholar]
- 39.Howell SC, Mesleh MF, Opella SJ. NMR structure determination of a membrane protein with two transmembrane helices in micelles: MerF of the bacterial mercury detoxification system. Biochemistry. 2005;44:5196–5206. doi: 10.1021/bi048095v. [DOI] [PubMed] [Google Scholar]
- 40.De Angelis AA, Opella SJ. Bicelle samples for solid-state NMR of membrane proteins. Nat. Protoc. 2007;2:2332–2338. doi: 10.1038/nprot.2007.329. [DOI] [PubMed] [Google Scholar]
- 41.Wu CH, Grant CV, Cook GA, Park SH, Opella SJ. A strip-shield improves the efficiency of a solenoid coil in probes for high-field solid-state NMR of lossy biological samples. J. Magn. Reson. 2009;200:74–80. doi: 10.1016/j.jmr.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grant CV, Yang Y, Glibowicka M, Wu CH, Park SH, Deber CM, Opella SJ. A modified Alderman-Grant coil makes possible an efficient cross-coil probe for high field solid-state NMR of lossy biological samples. J. Magn. Reson. 2009;201:87–92. doi: 10.1016/j.jmr.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 44.Goddard TDK. D.G. SPARKY 3. San Francisco: University of California; [Google Scholar]
- 45.Ramamoorthy A, Wu CH, Opella SJ. Three-dimensional solid-state NMR experiment that correlates the chemical shift and dipolar coupling frequencies of two heteronuclei. J. Magn. Reson. B. 1995;107:88–90. doi: 10.1006/jmrb.1995.1063. [DOI] [PubMed] [Google Scholar]
- 46.Caravatti P, Braunschweiler L, Ernst RR. Heteronuclear correlation spectroscopy in rotating solids. Chem. Phys. Lett. 1983;100:305–310. [Google Scholar]
- 47.De Angelis AA, Jones DH, Grant CV, Park SH, Mesleh MF, Opella SJ. NMR experiments on aligned samples of membrane proteins. Methods Enzymol. 2005;394:350–382. doi: 10.1016/S0076-6879(05)94014-7. [DOI] [PubMed] [Google Scholar]
- 48.DeAngelis A, Nevzorov A, Park S, Howell S, Mrse A, Opella S. High-resolution NMR spectroscopy of membrane proteins in aligned bicelles. J. Am. Chem. Soc. 2004;126:15340–15341. doi: 10.1021/ja045631y. [DOI] [PubMed] [Google Scholar]