Abstract
Objectives:
This study sought to determine whether 80-lead body surface potential mapping (BSPM) would improve detection of acute myocardial infarction (AMI) and occluded culprit artery in patients presenting with ST-segment depression (STD) only on 12-lead ECG.
Background:
In patients with acute coronary syndromes (ACS), the standard 12-lead ECG has limited sensitivity (50–60%) for AMI.
Methods:
Consecutive patients presenting pre- and in-hospital between 2000 and 2006 with acute ischaemic-type chest pain and an initial 12-lead ECG with STD only of ≥ 0.05 mV in two or more contiguous leads were analysed. Flow in the culprit artery at angiography was graded using the TIMI flow grade (TFG) criteria.
Results:
Enrolled were 410 patients: of these, 240 (59%) had an occluded culprit artery (TFG 0/1) with AMI, 80 (19%) had a patent culprit artery (TFG 2/3) with AMI, 67 (16%) had TFG 2/3 with cardiac troponin T (cTnT) <0.03 µg/l, and 23 (6%) had TFG 0/1 with cTnT < 0.03 µg/l. BSPM ST-segment elevation (STE) occurred in 267 (65%) patients. For the diagnosis of TFG 0/1 in the culprit artery and AMI, BSPM STE had sensitivity 91% and specificity 72% with STE occurring most commonly in the posterior territory (60%). Patients with TFG 0/1 and AMI were significantly more likely to suffer death or nonfatal MI at 30 days than those with TFG 2/3 and cTnT < 0.03 µg/l (adjusted hazard ratio 4.12, 95% CI 1.67–8.56, p = 0.003).
Conclusion:
Among 410 ACS patients presenting with only STD, BSPM identifies STE beyond the territory of the 12-lead ECG with sensitivity 91% and specificity 72% for diagnosis of occluded culprit artery with AMI.
Keywords: Acute myocardial infarction, body surface potential mapping, non-ST-elevation myocardial infarction, occluded coronary artery, ST-segment depression
Introduction
Prompt diagnosis of acute coronary occlusion and early reperfusion therapy are associated with a reduction in morbidity and mortality in ACS patients.1–4 Current clinical practice relies upon initial electrocardiographic changes for the diagnosis of ST-segment elevation myocardial infarction (STEMI). However, the initial 12-lead electrocardiogram (ECG) has limited sensitivity (50–60%) for ST-segment elevation detection, i.e. STEMI diagnosis.5 This may be due, in part, to the 12-lead ECG sampling a relatively small area of the thorax. Undiagnosed STEMI, i.e. missed acute coronary occlusion, is associated with high morbidity and mortality,6–8 with patients presenting as non-ST-elevation myocardial infarction (NSTEMI) on initial 12-lead ECG representing a high-risk patient group: at least 12.2% suffer death or non-fatal myocardial infarction at 6 months.5,8 ST-segment depression on initial 12-lead ECG is a common finding in these patients (40–50%).9
Acute transmural ischaemia caused by occlusion of a major coronary artery produces an epicardial injury current that can be detected as a deviation of the ST-segment toward the involved myocardial region.10 Acute occlusion of the left anterior descending (LAD) or right coronary artery (RCA) typically cause ST-segment elevation in leads V1–V4 or leads II, aVF, and III respectively of the 12-lead ECG and are therefore termed STEMI. However, acute occlusion of the non-dominant left circumflex (LCx) artery typically produces only ST-segment depression on the 12-lead ECG.10 This ST-segment depression in the standard leads would theoretically appear as ST-segment elevation in the negative counterparts of these leads.10
One method to improve detection of this ST-elevation and thus the diagnostic capability of the 12-lead ECG is to sample over a larger thoracic surface area. One previous approach has focused on the placement of a limited number of additional leads to cover the right ventricle (V3R–V5R) and the posterior LV wall (V7–V9). Sampling a larger and more comprehensive volume of the thorax with multiple leads – a technique known as body surface potential mapping (BSPM) – has been previously shown to improve the sensitivity of acute myocardial infarction (AMI) diagnosis over that of the 12-lead ECG.5,11–13
The primary aim of this study was to demonstrate that BSPM analysis would improve the detection of AMI associated with an occluded culprit artery in patients presenting with only ST-segment depression on initial 12-lead ECG. The secondary aims were to determine whether these patients with AMI identified only by BSPM have adverse clinical outcomes, and whether the BSPM-based approach had diagnostic advantage over the simpler method of recording additional RV and posterior leads.
Methods
Study population
Between January 2000 and December 2006, we studied retrospectively all patients admitted to our coronary care unit either using the emergency department or mobile coronary care unit. Those who fulfilled the following inclusion criteria were entered into the study:
Typical ischaemic-type chest discomfort of ≥ 20 min duration, occurring at rest and presentation within 12 h of symptom onset
ST-segment depression of ≥ 0.05 mV in two or more contiguous leads on initial 12-lead ECG
Informed consent to BSPM performed at first medical contact
Blood sampled for cardiac troponin T (cTnT) ≥ 12 h post-symptom onset
Coronary angiography during index hospitalization.
Patients were excluded from analysis if they had: ST-segment elevation ≥ 0.1 mV in any lead of the initial 12-lead ECG, or any condition precluding assessment of the ST-segment, i.e. left bundle branch block, right bundle branch block, left ventricular hypertrophy, concomitant digitalis therapy, or ventricular pacing; received fibrinolysis, nitrates, or glycoprotein IIb/IIIa inhibitor prior to initial ECG or BSPM; or BSPM recorded > 15 mins after initial 12-lead ECG (Figure 1).
Figure 1.
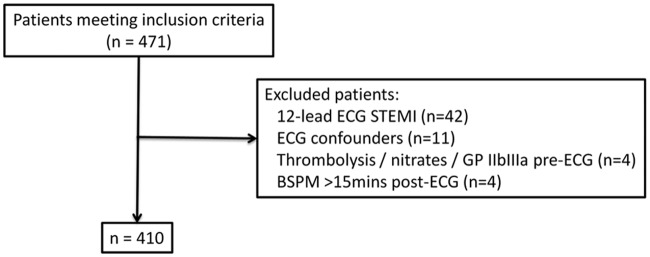
Patient flow diagram.
Demographic data and risk factors for coronary artery disease were documented (Table 1).
Table 1.
Baseline characteristics.
| cTnT +ve |
cTnT −ve |
p-value | |||
|---|---|---|---|---|---|
| TFG 0/1 (n = 240) | TFG 2/3 (n = 80) | TFG 0/1 (n = 23) | TFG 2/3 (n = 67) | ||
| Age (years) | 61 ± 12 | 63 ± 13 | 60 ± 11 | 61 ± 12 | 0.607 |
| Male gender | 178 (74) | 55 (69) | 15 (65) | 47 (70) | 0.085 |
| BMI (kg/m2) | 27 ± 3 | 26 ± 4 | 27 ± 4 | 27 ± 4 | 0.681 |
| Risk factors | |||||
| Hypertension | 132 (55) | 53 (66) | 14 (61) | 43 (64) | 0.034 |
| Hyperlipidaemia | 113 (47) | 44 (55) | 11 (48) | 36 (54) | 0.048 |
| Current smoker | 72 (30) | 26 (33) | 7 (30) | 21 (31) | 0.467 |
| Diabetes mellitus | 38 (16) | 14 (18) | 4 (17) | 10 (15) | 0.340 |
| Family history of CAD | 46 (19) | 18 (23) | 5 (22) | 14 (21) | 0.422 |
| Past medical history | |||||
| Prior MI | 26 (11) | 10 (13) | 3 (13) | 7 (10) | 0.253 |
| Prior angina | 55 (23) | 20 (25) | 5 (22) | 10 (15) | 0.083 |
| Prior PCI | 19 (8) | 8 (10) | 3 (13) | 6 (9) | 0.091 |
| Prior CABG | 8 (3) | 5 (6) | 2 (9) | 3 (5) | 0.068 |
| Multivessel disease | 29 (12) | 9 (11) | 3 (13) | 7 (10) | 0.402 |
| GFR (ml/min1) | 50 ± 10 | 46 ± 12 | 46 ± 12 | 47 ± 13 | 0.588 |
| GP IIb/IIIa use | 110 (46) | 35 (44) | 5 (22) | 17 (25) | <0.001 |
| Time to treatment | |||||
| Symptom onset to first medical contact (h) | 1.1 (0.9–1.8) | 1.6 (1.0–2.1) | 1.2 (0.9–1.7) | 1.4 (1.0–1.9) | 0.611 |
| First medical contact to 12-lead ECG (mins) | 7 (5–10) | 10 (6–13) | 9 (5–12) | 8 (5–10) | 0.424 |
| 12-lead ECG to angiography (h) | 23 (20–34) | 21 (19–32) | 26 (21–36) | 23 (19–30) | 0.372 |
Values are n (%), mean±SD, or median (IQR). BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; cTnT, cardiac troponin T; GFR, glomerular filtration rate; GP, glycoprotein; MI, myocardial infarction; PCI, percutaneous coronary intervention; TFG, TIMI flow grade.
Twelve-lead ECG analysis
A 12-lead ECG was recorded at first medical contact (25 mm/s and 10 mm/mV). ST segment shifts were measured 80 ms after the J-point for ST-segment depression using the preceding TP segment as a baseline. ST-segment depression (STD) score was defined as the total STD in all 12-lead ECG leads (mm).
BSPM analysis
The BSPM was recorded with a flexible plastic anterior and posterior electrode harness and a portable recording unit (Heartscape Technologies). The anterior harness contains 64 electrodes, including three proximal bipolar limb leads (Mason-Likar position) and a posterior harness with 16 electrodes. During the interpretation process, the electrodes are defined to represent anterior, lateral, inferior, high right anterior, right ventricular, and posterior epicardial regions.11,12
The BSPMs were uploaded and displayed on an IBM-compatible computer running PRIME analysis software. Printouts were obtained from the processed BSPM of the 80-lead ECG and a colour-contour map displaying the amount of ST-segment elevation at the J-point (ST0 isopotential map) (Figure 2). Using the 80-lead BSPM and colour-contour map, a single cardiologist familiar with BSPM interpretation and blinded to the clinical details and 12-lead ECG coded the BSPM diagnosis as AMI or non-AMI and defined the infarct location. ST-segment elevation was measured at the ST0 point and defined by the following thresholds: anterior ≥ 0.2 mV elevation; lateral/inferior/high right anterior/right ventricular ≥ 0.1 mV elevation; posterior ≥ 0.05 mV elevation; with infarct-location described by the ST0 isopotential colour-contour map.
Figure 2.
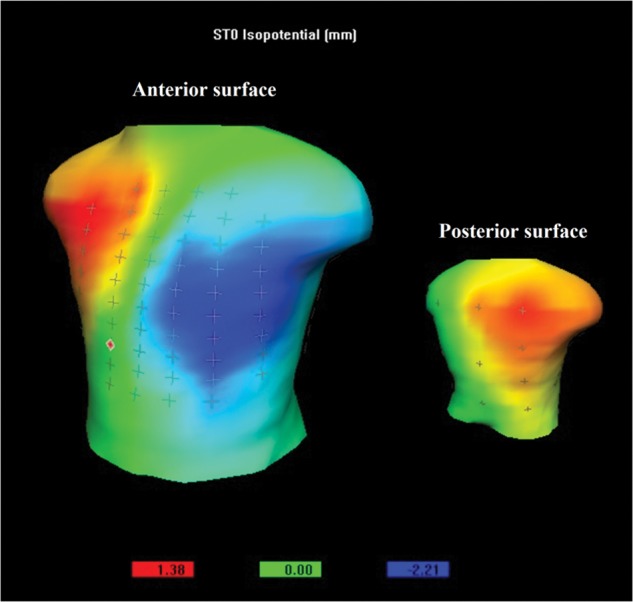
ST0 (J-point) isopotential body surface potential map showing anterior minima (blue; –2.21 mm) and high right anterior/posterior maxima (red, 1.38 mm) (high right anterior and posterior myocardial infarction).
All BSPMs were retrospectively analysed to assess ST-segment elevation in the extended 12-lead ECG leads, i.e. V3R–V5R (BSPM leads 5, 74, and 69 respectively) and V7–V9 (BSPM leads 63, 65, and 66 respectively). ST-segment elevation, measured at the J-point (ST0 isopotential map), was considered significant if ≥ 0.05 mV in any of the RV chest leads (V3R–V5R) and ≥ 0.05 mV in any of the posterior chest leads (V7–V9).
Acute myocardial infarction
Diagnosis of AMI was made when cTnT ≥ 0.03 µg/l (cTnT +ve) (Roche Diagnostics, Switzerland).
Coronary angiography
All patients underwent coronary angiography during index admission. Flow in the culprit artery was graded according to the TIMI flow grade (TFG) criteria.1 Patients were grouped into four categories based on initial TFG in the culprit artery and cTnT: (1) TFG 0/1 and cTnT +ve; (2) TFG 2/3 and cTnT +ve; (3) TFG 2/3 and cTnT <0.03 µg/l (cTnT −ve); and (4) TFG 0/1 and cTnT −ve.
Statistical analysis
Data are presented as number (%), mean±standard deviation, or median (interquartile range). Group comparisons were tested using the unpaired t-test and χ2 test. Continuous clinical variables were tested by analysis of variance. ROC analysis was used to compare STD score and BSPM ST-segment elevation in the diagnosis of TFG 0/1 in the culprit artery and cTnT +ve, with area under the curve (c-statistic) > 0.75 taken as a good performance. Hazard ratios (HR) were compared by means of Cox regression with covariates statistically significant on univariate analysis and/or those clinically relevant used as variables in the adjusted multivariate logistic regression models. Statistical analysis was performed using SPSS version 17.0 for Windows (SPSS, Chicago, Illinois). A p-value <0.05 was considered statistically significant. Ethical approval for BSPM was granted by the Local Ethics Committee.
Results
Baseline characteristics
During the study period, 410 patients met the study criteria (Figure 1). Patients with a patent culprit artery at angiography had a higher incidence of both hypertension and hyperlipidaemia than patients with an occluded culprit artery at the time of angiography (Table 1). In addition, cTnT +ve patients were more likely to receive glycoprotein IIb/IIIa antagonists than cTnT −ve patients. Otherwise, the baseline characteristics between groups were similar.
Angiographic outcomes
Of the 410 patients, 240 (59%) had an occluded culprit artery (TFG 0/1) at the time of angiography and were cTnT +ve, 80 (19%) had a patent culprit artery (TFG 2/3) and were cTnT +ve, 67 (16%) had TFG 2/3 and were cTnT −ve, and 23 (6%) had TFG 0/1 and were cTnT −ve. Among the 263 patients with an occluded culprit artery, the occluded artery was most often LCx (n = 124, 47%), followed by RCA (n = 99, 38%), LAD (n = 29, 11%) and left main coronary artery (n = 11, 4%; Figure 3).
Figure 3.
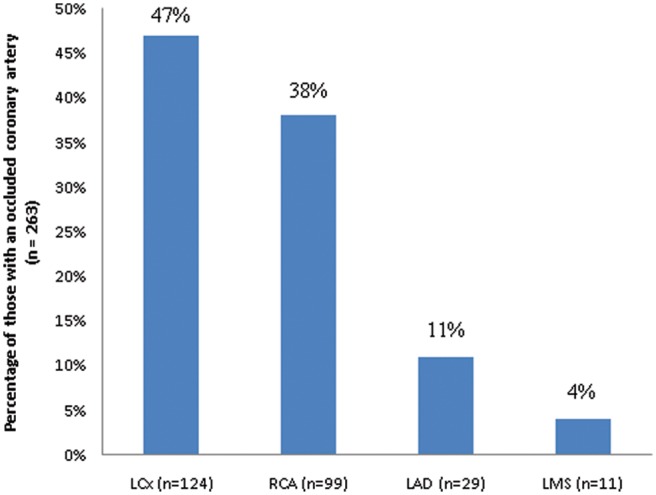
Culprit artery locations among patients with an occluded artery (n = 263). LAD, left anterior descending; LCx, left circumflex; LMS, left main stem; RCA, right coronary artery.
BSPM diagnosis
ST-segment elevation was detected on BSPM in 267 (65%) patients. Of those with TFG 0/1 in the culprit artery and cTnT +ve (n = 240), BSPM ST-segment elevation occurred in 219 patients (sensitivity 91%; specificity 72%, positive predictive value 82%, negative predictive value 85%; Table 2). Infarct locations as defined by BSPM ST-segment elevation territory are summarized in Table 3. The most commonly affected territory was the posterior region in 144/240 (60%) patients. Of these, right ventricular involvement was detected in 140 (97%). Furthermore, in patients with TFG 0/1 and cTnT +ve (n = 240), LCx was identified as the culprit artery at angiography in 114 (48%). Of these, 100 (88%) patients had ST-segment elevation on BSPM in the posterior and right ventricular territories not identified by initial 12-lead ECG.
Table 2.
Univariate analysis of body surface potential map (BSPM) and extended ECG leads in the diagnosis of AMI and TIMI 0/1 flow in the culprit artery.
| ST-segment elevation | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| BSPMa | 91 | 72 | 82 | 85 |
| RV ECG lead: ≥ 0.05 mV in V4R | 23 | 92 | 80 | 46 |
| Posterior ECG lead: ≥ 0.05 mV in V8 | 10 | 98 | 89 | 44 |
| ≥ 0.05 mV in lead V4R and/or ≥ 0.05 mV in lead V8 | 33 | 90 | 82 | 49 |
≥ 0.2 mV ST-segment elevation in anterior territory, ≥ 0.1 mV ST-segment elevation in the lateral/inferior/high right anterior/right ventricular territories, or ≥ 0.05 mV ST-segment elevation in the posterior territory.
Table 3.
Body surface potential map (BSPM) ST-segment elevation by territory in patients with an occluded culprit artery and AMI.
| TFG 0/1, cTnT +ve (n = 240) | |
|---|---|
| BSPM STE territory: | |
| High right anterior | 7 |
| Posterior | 144 |
| Right ventricular | 140 |
| Posterior and right ventricular | 140 |
| Anterior and lateral | 16 |
| Inferior and lateral | 29 |
| Inferior | 44 |
BSPM, body surface potential map; cTnT, cardiac troponin T; STE, ST-segment elevation; TFG, TIMI flow grade.
Retrospective BSPM analysis showed ST-segment elevation ≥ 0.05 mV in 42 (10%) patients in lead V3R, 69 (17%) patients in lead V4R, and 24 (6%) patients in lead V5R. All patients with ST-segment elevation in leads V3R or V5R had ST-segment elevation in lead V4R; thus, addition of either leads V3R or V5R to lead V4R did not increase the diagnostic yield of 69 patients (17%). Of those with TFG 0/1 in the culprit artery and cTnT +ve (n = 240), ST-segment elevation ≥ 0.05 mV in lead V4R occurred in 55 patients (sensitivity 23%, specificity 92%, positive predictive value 80%, negative predictive value 46%; Table 2). Of these, the culprit artery was identified as LCx in 32/55 (58%) patients and RCA in 23/55 (42%) patients.
Retrospective BSPM analysis showed ST-segment elevation ≥ 0.05 mV in 14 (3%) patients in lead V7, 28 (7%) patients in lead V8, and 20 (5%) patients in lead V9. All patients with ST-segment elevation in leads V7 or V9 had ST-segment elevation in lead V8. Of those with TFG 0/1 in the culprit artery and cTnT +ve, ST-segment elevation ≥ 0.05 mV in lead V8 occurred in 25 patients (sensitivity 10%, specificity 98%, positive predictive value 89%, negative predictive value 44%; Table 2). Of these, the culprit artery was identified as LCx in 20/25 (80%) patients and RCA in 5/25 (20%) patients.
Of those with TFG 0/1 in the culprit artery and cTnT +ve, ST-segment elevation ≥ 0.05 mV in lead V4R and/or ≥ 0.05 mV in lead V8 occurred in 80 (33%) patients and thus had sensitivity 33%, specificity 90%, positive predictive value 82%, and negative predictive value 49% for diagnosis of AMI (Table 2).
ROC analysis
ROC curves were used to compare STD score and BSPM ST-segment elevation in predicting TFG 0/1 in the culprit artery and cTnT +ve. STD score had c-statistic 0.639 (95% CI 0.521–0.771; p = 0.058) with STD score > 8 mm identified as optimal during analysis (sensitivity 54%, specificity 93%, positive predictive value 77%, negative predictive value 54%). Using BSPM ST-segment elevation, the c-statistic for TFG 0/1 in the culprit artery and cTnT +ve was 0.906 (95% CI 0.838–0.983; p < 0.001).
Clinical outcomes
The 30-day incidence of the composite of death or nonfatal MI was significantly higher among patients with TFG 0/1 and cTnT +ve than among those with TFG 0/1 and cTnT −ve and those with TFG 2/3 and either cTnT +ve or cTnT −ve (27/240, 11.3%; 1/23, 4.3%; 6/80, 7.5%; and 2/67, 3.0%, respectively; p = 0.011; Figure 4). Patients with TFG 0/1 and cTnT +ve were significantly more likely to suffer death or nonfatal MI than those with TFG 2/3 and cTnT −ve after adjustment for differences in baseline characteristics (adjusted hazard ratio, HRADJ, 4.12, 95% CI 1.67–8.56, p = 0.003). In addition, patients with cTnT +ve tended to be more likely to suffer death or nonfatal MI than those with cTnT −ve, irrespective of TFG (HRADJ 1.53, 95% CI 0.87–2.45, p = 0.071). Moreover, patients with TFG 2/3 and cTnT +ve were more likely to suffer death or nonfatal MI than those with TFG 2/3 and cTnT −ve (HRADJ 1.94, 95% CI 1.05–4.86, p = 0.052).
Figure 4.
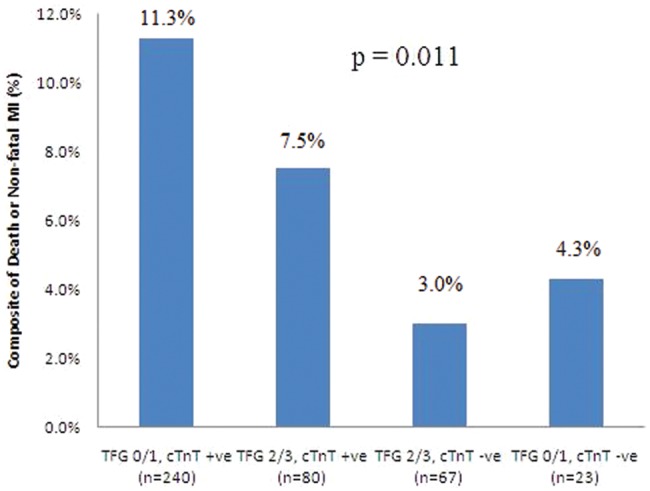
Short-term clinical outcome: composite of death or nonfatal myocardial infarction at 30 days. cTnT, cardiac troponin T; TFG, TIMI flow grade.
Discussion
In this study, among ACS patients with only ST-segment depression on initial 12-lead ECG and elevated cTnT, 240/320 (75%) patients had an occluded culprit artery. These patients had adverse short-term clinical outcomes, in keeping with previous studies.7,8 In addition, very few patients with ST-segment depression on initial 12-lead ECG and an occluded culprit artery were recognized by clinicians as necessitating urgent angiography as demonstrated by the significant median delay from initial 12-lead ECG to coronary angiography (Table 1).
Current American College of Cardiology/American Heart Association guidelines recommend a door-to-balloon time of ≤ 90 mins for STEMI patients undergoing primary PCI.7,14 Multiple studies have demonstrated increasing morbidity and mortality with treatment delay in STEMI patients.7 In addition, each 30-min delay from symptom onset to primary PCI has been estimated to increase the relative risk of 1-year mortality by 7.5%.7,15 Therefore, patients with acute coronary occlusion, i.e. STEMI, not detected by 12-lead ECG are more likely to undergo a delayed revascularization strategy and are thus at increased risk of an adverse prognosis. Furthermore, the Occluded Artery Trial found no benefit of PCI for patients with an occluded infarct artery > 24 h after symptom onset.16 Patients with an occluded culprit artery more often have pathological Q waves on their ECG, which may be attributed to either late presentation or a prior history of MI.17 Since the time from symptom onset to first medical contact is similar between the occluded and non-occluded groups in our study, late presentation is less likely an explanation for the differences in short-term clinical outcomes. The presence of previous coronary disease may limit tolerance for further ischaemic insult, thus contributing to larger infarct size and worse clinical outcomes;17 however, there was no significant difference between groups with regard to prior history of coronary artery disease in our study (Table 1). Therefore, these NSTEMI patients with an occluded infarct artery represent STEMI diagnoses missed due to the limitations of the 12-lead ECG that would benefit from earlier revascularization.
The results presented in this study corroborate findings from an analysis of NSTEMI patients enrolled in both the PARAGON-B (Platelet IIb/IIIa Antagonism for the Reduction of Acute Coronary Syndrome Events in a Global Organization Network) trial and the TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel – Thrombolysis In Myocardial Infarction 38) substudy.7,17 In the PARAGON-B trial, 27% patients with NSTEMI had an occluded culprit artery at the time of angiography, identified as the LCx in 25.1% patients.17 Despite similar in-hospital treatment, patients with an occluded culprit artery in the PARAGON-B trial had larger infarcts, as defined by peak cardiac biomarker titre, and higher risk-adjusted 6-month mortality (HR 1.72, 95% CI 1.07–2.79). Furthermore, a substudy of the TRITON-TIMI 38 trial revealed that 26.2% of patients with isolated anterior ST-segment depression had an occluded culprit artery at angiography.7 Of these, acute LCx artery occlusion was identified in 48.4%.7 In this substudy, patients with an occluded culprit artery and elevated biomarkers were significantly more likely to suffer death or nonfatal MI at 30days than those with a patent artery and negative biomarkers (HR 3.06, 95% CI 1.33–7.03, p = 0.008).
BSPM has been shown to have sensitivity 76%, specificity 92%, and c-statistic 0.84 for AMI diagnosis in consecutive patients presenting with acute ischaemic-type chest pain at rest (n = 755).11 Improvement in sensitivity over the 12-lead ECG (sensitivity 68%) has been shown to be mainly due to detection of ST-elevation in the high right anterior, posterior, and right ventricular territories.11 In the OCCULT-MI trial, BSPM increased STEMI detection by 27.5% over the 12-lead ECG.13 In this trial, ST-segment elevation on BSPM was associated with an increased risk of death or MI at 30 days (odds ratio 3.4) in those without initial 12-lead ECG STEMI.13 Furthermore, Menown et al.9 have previously shown that, in a small number of consecutive patients (n = 54) with ischaemic-type chest pain and ST-segment depression only on 12-lead ECG, BSPM has sensitivity 88% and specificity 75% for AMI diagnosis. In addition, Martin et al.10 have shown that 12-lead ECG ST-segment elevation has only 50% sensitivity for AMI using contrast-enhanced cardiac magnetic resonance imaging as the diagnostic gold-standard. However, sensitivity increased significantly to 84% (p < 0.0001) with the inclusion of ST-segment depression criteria: ≥ 0.1 mV ST-segment depression in two or more anatomically contiguous leads or in one lead that was anatomically contiguous to a lead with ST-segment elevation criteria.10
In our study, BSPM at presentation identified ST-segment elevation ‘missed’ by the 12-lead ECG in 91% of those with an occluded culprit artery and elevated cTnT. The addition of RV and posterior leads to the standard 12-lead ECG lead set identified only 33% of those with AMI and acute coronary occlusion presenting with isolated ST-segment depression on the 12-lead ECG. Clearly this is an improvement from the 12-lead dataset but still represents a substantial underdetection of myocardial infarction.
Schmitt et al.18 have shown the sensitivity of the conventional ECG to be only 50% in patients with chest pain and AMI, with addition of ST-segment elevation ≥ 0.1 mV in the posterior leads (V7-V9) improving the diagnostic sensitivity by only 11%. Isolated ST-segment elevation in the RV (V3R-V6R) or posterior leads was detected in only 4–8% patients in this study.18 Furthermore, in a study of 345 patients with AMI by Zalenski et al.,19 only 29 (8%) had ST-segment elevation ≥ 0.1 mV in at least two nonstandard leads (V7–V9 and V4R–V6R) when the 12-lead ECG was negative for ST-segment elevation, increasing diagnostic sensitivity by 8.4% (p = 0.03). In our study, BSPM superiority over the 18-lead ECG in the detection of RV or posterior infarction was likely to be due to the BSPM sampling a greater area of the patients’ right-side and permitting ST-segment elevation detection in the lower posterior and right posterolateral regions through additional posterior chest lead placement.
It is well known that the 12-lead ECG is particularly insensitive for LCx artery occlusion due to the absence of lateral precordial leads and the late depolarization of the lateral wall.17 Classical ST-segment elevation in leads I, aVL, V5, and V6 is seen in only 48% of patients with LCx artery occlusion.17,20 In this study of patients with only ST-segment depression on initial 12-lead ECG, 47% of those with an occluded coronary artery had LCx occlusion (Figure 3). Of those with LCx occlusion and AMI, 100/114 (88%) were successfully identified by ST-segment elevation on BSPM. Unfortunately, BSPM did not influence clinical decision making in our study and was part of a research protocol only; as such, clinical outcomes are comparable with previous work on acute coronary occlusion in NSTEMI patients.7,17 In summary, early BSPM has the potential to identify those patients with a non-diagnostic 12-lead ECG at presentation who have the potential to benefit from emergent revascularization. Future randomized studies are required to assess whether early BSPM in these patients improves prognosis.
Limitations
This study is a nonrandomized retrospective analysis and as such it is possible that both identified and unidentified confounders may have influenced the clinical outcomes. Consecutive patients admitted to the coronary care unit with ST-segment depression of ≥ 0.05 mV in two or more contiguous leads on initial 12-lead ECG were studied. This represents a selected potentially high-risk patient group and is not representative of all patients presenting to the emergency department with ischaemic-type chest discomfort. In addition, patients had to survive long enough to undergo angiography and the distribution of patent and occluded arteries among patients who died prior to angiography is not known. Thus, there is a component of survival bias such that patients with an occluded artery and elevated cTnT survived to angiography, a median of 23 h later. In addition, 12-lead ECG, BSPM, and angiography were not performed simultaneously; therefore, a patent artery at the time of ECG may have occluded in the time from ECG/BSPM to angiography and vice versa. Diagnosis of acute myocardial infarction required cTnT ≥ 0.03 µg/l ≥ 12 h postsymptom onset and did not require dynamic changes in cTnT elevation.
Footnotes
Funding: This research received funding from the not-for-profit sector. MJD is supported by the Heart Trust Fund (Royal Victoria Hospital).
Conflict of interest
None.
References
- 1. TIMI Study Group The Thrombolysis in Myocardial Infarction (TIMI) trial. N Engl J Med 1985; 312: 932–936 [DOI] [PubMed] [Google Scholar]
- 2. The GUSTO Angiographic Investigators The effects of tissue plasminogen activator, streptokinase, or both on coronary-artery patency, ventricular function, and survival after acute myocardial infarction. N Engl J Med 1993; 329: 1615–1622 [DOI] [PubMed] [Google Scholar]
- 3. Vogt A, von Essen R, Tebbe U, et al. Impact of early perfusion status of the infarct-related artery on short-term mortality after thrombolysis for acute myocardial infarction: retrospective analysis of four German multicenter studies. J Am Coll Cardiol 1993; 21: 1391–1395 [DOI] [PubMed] [Google Scholar]
- 4. Mathew TP, Menown IB, McCarty D, et al. Impact of pre-hospital care in patients with acute myocardial infarction compared with those first managed in-hospital. Eur Heart J 2003; 24: 161–171 [DOI] [PubMed] [Google Scholar]
- 5. Owens CG, McClelland AJJ, Walsh SJ, et al. Pre-hospital 80-lead Mapping: Does it add significantly to the diagnosis of acute coronary syndromes? J Electrocardiol 2004; 37 suppl: 223–232 [DOI] [PubMed] [Google Scholar]
- 6. Mills NL, Churchhouse AMD, Lee KK, et al. Implementation of a sensitive troponin I assay and risk of recurrent myocardial infarction and death in patients with suspected acute coronary syndrome. JAMA 2011; 305: 1210–1216 [DOI] [PubMed] [Google Scholar]
- 7. Pride YB, Tung P, Mohanavelu S, et al. ; for the TIMI Study Group. Angiographic and clinical outcomes among patients with acute coronary syndromes presenting with isolated anterior ST-segment depression. J Am Coll Cardiol Intv 2010; 3: 806–811 [DOI] [PubMed] [Google Scholar]
- 8. Collinson J, Flather MD, Fox KAA, et al. ; for the PRAIS-UK Investigators. Clinical outcomes, risk stratification and practice patterns of unstable angina and myocardial infarction without ST elevation: Prospective Registry of Acute Ischaemic Syndromes in the UK (PRAIS-UK). Eur Heart J 2000; 21: 1450–1457 [DOI] [PubMed] [Google Scholar]
- 9. Menown IB, Allen J, Anderson JMcC, et al. ST depression only on the initial 12-lead ECG: Early diagnosis of acute myocardial infarction. Eur Heart J 2001; 22: 218–227 [DOI] [PubMed] [Google Scholar]
- 10. Martin TN, Groenning BA, Murray HM, et al. ST-segment deviation analysis of the admission 12-lead electrocardiogram as an aid to early diagnosis of acute myocardial infarction with a cardiac magnetic resonance imaging gold standard. J Am Coll Cardiol 2007; 50: 1021–1028 [DOI] [PubMed] [Google Scholar]
- 11. Owens C, McClelland A, Walsh S, et al. Comparison of value of leads from body surface maps to 12-lead electrocardiogram for diagnosis of acute myocardial infarction. Am J Cardiol 2008; 102: 257–265 [DOI] [PubMed] [Google Scholar]
- 12. McClelland AJJ, Owens CG, Menown IBA, et al. Comparison of the 80-lead body surface map to physician and to 12-lead electrocardiogram in detection of acute myocardial infarction. Am J Cardiol 2003; 92: 252–257 [DOI] [PubMed] [Google Scholar]
- 13. Hoekstra JW, O’Neill BJ, Pride YB, et al. Acute detection of ST-elevation myocardial infarction missed on standard 12-lead ECG with a novel 80-lead real-time digital body surface map: primary results from the multicenter OCCULT MI trial. Ann Emerg Med 2009; 54: 779–788 [DOI] [PubMed] [Google Scholar]
- 14. Antman EM, Hand M, Armstrong PW, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Canadian Cardiovascular Society; American Academy of Family Physicians; American College of Cardiology; American Heart Association. J Am Coll Cardiol 2008; 51: 210–247 [DOI] [PubMed] [Google Scholar]
- 15. De Luca G, Suryapranata H, Ottervanger JP, et al. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation 2004; 109: 1223–1225 [DOI] [PubMed] [Google Scholar]
- 16. Hochman JS, Lamas GA, Buller CE, et al. ; for the Occluded Artery Trial Investigators. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med 2006; 355: 2395–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang TY, Zhang M, Fu Y, et al. Incidence, distribution and prognostic impact of occluded culprit arteries among patients with non-ST-elevation acute coronary syndromes undergoing diagnostic angiography. Am Heart J 2009; 157: 716–723 [DOI] [PubMed] [Google Scholar]
- 18. Schmitt C, Lehmann G, Schmieder S, et al. Diagnosis of acute myocardial infarction in angiographically documented occluded infarct vessel: limitations of ST-segment elevation in standard and extended ECG leads. Chest 2001; 120: 1540–1546 [DOI] [PubMed] [Google Scholar]
- 19. Zalenski RJ, Rydman RJ, Sloan EP, et al. Value of posterior and right ventricular leads in comparison to the standard 12-lead electrocardiogram in evaluation of ST-segment elevation in suspected acute myocardial infarction. Am J Cardiol 1997; 79: 1579–1585 [DOI] [PubMed] [Google Scholar]
- 20. Huey BL, Beller GA, Kaiser DL, et al. A comprehensive analysis of myocardial infarction due to left circumflex artery occlusion: comparison with infarction due to right coronary artery and left anterior descending artery occlusion. J Am Coll Cardiol 1988; 12: 1156–1166 [DOI] [PubMed] [Google Scholar]


