Abstract
Background:
The magnitude of improvement of acute heart failure achieved during treatment varies greatly among patients. We examined changes in the plasma B-type natriuretic peptide (BNP) levels of patients with acute heart failure and attempted to elucidate the clinical factors associated with amelioration of acute heart failure.
Methods and results:
The study population consisted of 208 consecutive patients admitted to our institution with acute heart failure. We measured plasma BNP levels before and after treatment of acute heart failure and evaluated these levels based on median age, body mass index (BMI), creatinine (Cr) level, and left ventricular ejection fraction (EF). Plasma BNP levels before treatment were equivalent between the younger and older age groups; however, plasma BNP levels after treatment were higher in the older age group (p<0.01). Plasma BNP levels before treatment were significantly high in the lower BMI group (p<0.05) and the higher Cr group (p<0.01). Similarly, plasma BNP levels after treatment were high in both the lower BMI and higher Cr groups (p<0.01 for both). In the low EF group, plasma BNP levels before treatment were significantly high (p<0.01), while plasma BNP levels after treatment were equivalent to those in the high EF group. A multiple linear regression analysis revealed that Cr was positively correlated and BMI and EF were negatively correlated with plasma BNP levels before treatment; however, the contributions of age, BMI, and Cr in reducing plasma BNP levels were more significant after treatment.
Conclusions:
The contributions of clinical factors working against amelioration of heart failure vary before and after treatment. Regarding plasma BNP levels, older age, very low BMI, and the presence of renal dysfunction eventually act to prevent amelioration of acute heart failure. Systolic dysfunction does not act against amelioration of acute heart failure.
Keywords: Body mass index, B-type natriuretic peptide, creatinine, diastolic dysfunction, ejection fraction
Introduction
Therapeutic advances in drug development and mechanical support have made the treatment of patients with severe acute heart failure possible. However, in some patients, acute heart failure can be improved only slightly, even with intensive treatment. Various reasons exist to explain the resistance in improvement of acute heart failure. For instance, severe deterioration of cardiac function, including systolic and diastolic dysfunction, is naturally a primary cause. Other clinical factors, including age, body mass index (BMI), and renal function, could also be involved. However, to date, the clinical factors acting against the changes in plasma BNP levels that occur with amelioration of acute heart failure have not been clarified.
B-type (brain) natriuretic peptide (BNP) is a hormone that was identified in 1988.1 Plasma BNP levels rise as heart failure becomes more severe. Plasma BNP levels have therefore been utilized for early detection of heart failure and to estimate disease severity and predict prognoses.2–14 In the present study, we attempted to elucidate the clinical factors associated with amelioration of acute heart failure by evaluating serial measurements of plasma BNP levels at admission and after treatment of acute heart failure. We used serial BNP measurements because the outcomes of patients with acute heart failure can be predicted using these measurements.3,6–10
Methods
Study patients
The study population consisted of 208 consecutive patients hospitalized due to heart failure between 1 January 2007 and 31 March 2010 at Jikei University Hospital (there were 183, 171, and 174 heart failure patients in 2007, 2008, and 2009, respectively, and there are 33 beds in the cardiology ward, including six beds in the coronary care unit).
Plasma BNP levels were measured twice: once during admission and again after intensive treatment of acute heart failure. We excluded patients with myocardial infarction because plasma BNP levels noticeably and rapidly increase during the 24 hours after its onset in a monophasic manner, then transiently decrease, possibly followed by another increase 2–3 days after onset (depending on the degree of ventricular remodelling), thus resulting in a biphasic profile.11 Patients who required emergency surgery, including coronary aorta bypass surgery and non-cardiac surgery, during the period between the collection of the first and second plasma BNP samples were also excluded. The study protocol [21–015 (5593)] was approved by the Ethics Committee of The Jikei University School of Medicine.
Diagnosis of acute heart failure
Acute heart failure was diagnosed according to the guidelines for treatment of acute heart failure published by the Japanese Circulation Society. The patients with heart failure symptoms [New York Heart Association (NYHA) functional classes II–IV] underwent several examinations (blood gas analysis, blood sampling, electrocardiogram, plain chest radiograph, echocardiogram) and decided to be admitted to our hospital.
Treatment of acute heart failure
The patients were treated with drugs, such as diuretics, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, aldosterone antagonists, beta-blockers, nitrates, carperitide (human atrial natriuretic peptide), and, if necessary, catecholamines and mechanical supports such as respirators, intra-aortic balloon pumping, or percutaneous cardiopulmonary support.
Blood sampling and measurement of plasma BNP levels
We measured plasma BNP levels immediately after admission (before intensive treatment) and at the time of improvement of acute heart failure (after intensive treatment). In each case, a second sample was collected when the clinical symptoms of acute heart failure improved and the patient’s condition stabilized. Whole blood (5 ml) was collected in tubes containing potassium EDTA (1 mg/ml blood). Plasma BNP was measured with a rapid enzyme-linked immunosorbent assay (non-extracted) using an antibody to human BNP (Shionogi, Tokyo, Japan). Serum biochemical analyses of creatinine (Cr) was performed in a central laboratory in our hospital during the study. In order to determine the effects of clinical factors on changes in plasma BNP levels, we divided the patients into two groups based on median age (72.5 years, interquartile range, IQR, 59.0–82.3 years), median BMI (23.4 kg/m2, IQR 21.2–25.6 kg/m2), median Cr level (1.20 mg/dl, IQR 0.90–1.64 mg/dl), and median left ventricular ejection fraction, EF, (39.2%, IQR 28.3–51.9%).
Echocardiographic examination and other measurements
An echocardiographic examination was performed in all patients by three expert cardiologists. EF was used as a marker of systolic left ventricular dysfunction. On the basis of height and weight at admission, BMI was calculated as the weight divided by the square of the height.
Main outcome measures
The main outcome measures were major adverse cardiovascular events (MACE), defined as cardiovascular death, myocardial infarction, stroke, heart failure, and all-cause mortality.
Statistical analysis
The continuous variables are expressed as the mean±SD or the median. The data of two groups were compared using the Wilcoxon signed-rank test or the Mann–Whitney U-test, as appropriate. Age, BMI, Cr, and EF were analysed by quintiles of plasma BNP levels, and the values of each quintile were compared with those of the lowest 20% using the Mann–Whitney U-test. Multiple regression analyses were performed when multiple values were compared. According to a multivariate analysis, natural log transformation was performed on BNP values because they were not normally distributed. The clinical factors affecting reduction of plasma BNP levels after treatment to <200 pg/ml were examined using a multiple logistic regression analysis. It has been suggested that the plasma BNP threshold level critical for predicting prognoses is approximately 180–200 pg/ml.11 It is thus generally believed that reducing BNP levels is preferable and that ensuring that plasma BNP levels are <200 pg/ml after treatment of acute heart failure is beneficial for improving prognosis.
All statistical analyses were performed using SPSS version 11.5J (SPSS Japan, Tokyo, Japan), and differences were considered to be statistically significant for p-values <0.05.
Results
Study population
The baseline characteristics of the study population are shown in Table 1. No patients died during therapy and the clinical symptoms of acute heart failure were successively improved by intensive treatment, although the remediation levels varied among the patients. This study included 44 patients (21.2% overall) in NYHA functional class II, 44 patients (21.2% overall) in class III, and 120 patients (57.7% overall) in class IV.
Table 1.
Baseline characteristics of the study population.
| Clinical characteristic | Population (n=208) |
|---|---|
| Age (years) | 69.9±15.0 |
| Body mass index (kg/m2) | 23.8±4.4 |
| BNP <200 pg/ml before treatment | AQ:Table 1: 17/208 does not equal 8.5%: please amend or clarify. Also, please delete or clarify the minus sign within the percentage brackets.17 (8.2)a |
| Creatinine (mg/dl) | 1.6±1.7 |
| Ejection fraction (%) | 40.9±15.9 |
| Male/female ( n/n, % female) | 148/60 (28.8) |
| Recovery period of BNP (days) | 42.4±56.7 |
| Underlying heart disease | |
| Ischaemic heart disease | 54 (26.0) |
| Hypertension | 50 (24.0) |
| Atrial fibrillation | 34 (16.3) |
| Dilated cardiomyopathy | 31 (14.9) |
| Valvular heart disease | 18 (8.7) |
| Other myocardiopathyb | 10 (4.8) |
| Complete atrioventricular block | 5 (2.4) |
| Congenital heart disease | 4 (1.9) |
| Constrictive pericarditis | 2 (1.0) |
| Hyperlipidaemia | 70 (33.7) |
| Type 2 diabetes mellitus | 67 (32.2) |
| Drugs | |
| ACE/ARB | 182 (87.5) |
| Beta-blocker | 115 (55.3) |
| Ca-blocker | 105 (50.5) |
| Digoxin | 39 (18.8) |
| Diuretics | 187 (89.9) |
| Statins | 50 (24.0) |
Values are n (%) or mean±SD, unless otherwise stated.
Mean±SD BNP in these patients was 142.4±52.4 pg/ml.
Other includes patients with chest pain syndrome, pre-operating checks, or others.
ACE/ARB, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker; BNP, B-type natriuretic peptide.
Changes in plasma BNP levels after treatment
Plasma BNP levels were observed to decrease after treatment in all patients (Figure 1). The mean±SD and median plasma BNP levels were 1043.8±1011.7 and 739.1 pg/ml (IQR 395.6–1334.0 pg/ml) before treatment and decreased significantly to 331.7±334.1 and 204.3 pg/ml (IQR 116.9–424.6 pg/ml) after treatment, respectively (p<0.01). The mean interval±SD between the first and second blood sample collections was 42.4±56.7 days.
Figure 1.
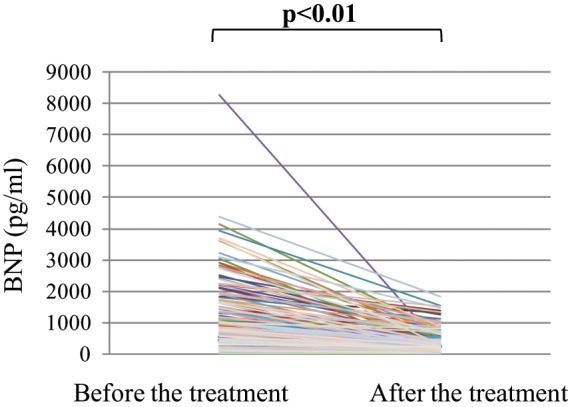
Changes in plasma B-type natriuretic peptide (BNP) levels before and after treatment in the entire study population.
Changing plasma BNP levels affected by clinical factors
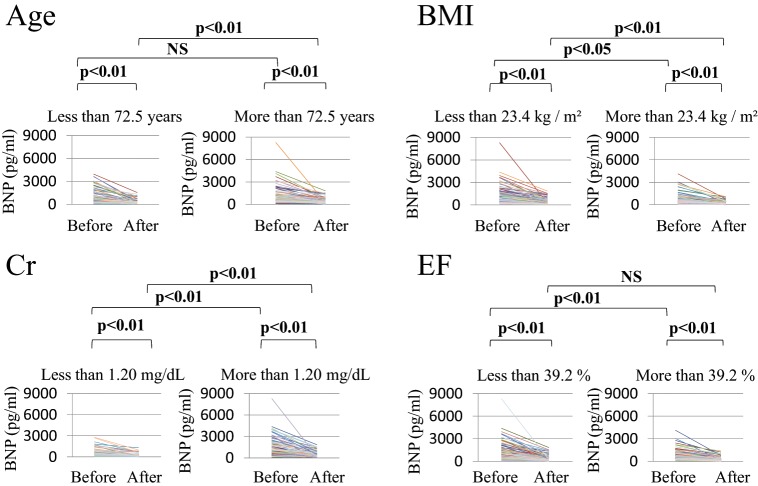
Figure 2 and Table 2 show the changes in plasma BNP levels before and after treatment according to the medians of the clinical factors. Plasma BNP levels before treatment were equivalent between the older and younger age groups (not significant); however, plasma BNP levels after treatment were significantly higher in the older age group (p<0.01). Plasma BNP levels were significantly higher in the low BMI group than in the high BMI group both before (p<0.05) and after treatment (p<0.01). Plasma BNP levels before treatment were significantly high in the low EF group compared to those in the high EF group (p<0.01); however, plasma BNP levels after treatment in both groups were equivalent (not significant). When analysed on the basis of Cr, plasma BNP levels were significantly higher in the high Cr group than in the low Cr group both before and after treatment (both p<0.01). Table 2 also shows the rate of reduction in plasma BNP levels between the first and second samples. The reduction rate was significantly high in the younger age group and the low EF group compared with that observed in the older age group and the high EF group, respectively. However, the reduction rates were equivalent in the high and low BMI and high and low Cr groups. No significant differences were observed in the values of the intervals between the first and second blood samplings.
Figure 2.
Changes in plasma BNP levels before and after treatment classified on the basis of median age, BMI, creatinine, and ejection fraction.
The leftmost graph of each pair are for below the median of each clinical factor and the rightmost for above the median.
BMI, Body mass index; BNP, B-type natriuretic peptide; Cr, creatinine; EF, ejection fraction.
Table 2.
Changing plasma B-type natriuretic peptidelevels affected by clinical factors.
| Clinical factor | n (%) | Before | After | Before vs. after | % Reduction | Interval (days) |
|---|---|---|---|---|---|---|
| Age | ||||||
| Below median | 105 (50.5) | 997.0±896.2 | 269.2±294.2 | p<0.01 | 65.2±26.1 | 35.0±35.1 |
| Above median | 103 (49.5) | 1091.6±1119.7 | 395.4±360.9 | p<0.01 | 57.8±22.0 | 50.0±71.8 |
| Below vs. above | — | NS | p<0.01 | — | p<0.01 | NS |
| Body mass index | ||||||
| Below median | 104 (50.0) | 1232.6±1198.2 | 414.2±397.0 | p<0.01 | 59.3±23.1 | 39.2±43.4 |
| Above median | 104 (50.0) | 855.1±741.2 | 249.2±230.4 | p<0.01 | 63.8±25.6 | 45.7±67.6 |
| Below vs. above | — | p<0.05 | p<0.01 | — | NS | NS |
| Creatinine | ||||||
| Below median | 103 (49.5) | 641.2±508.0 | 229.3±215.3 | p<0.01 | 59.6±24.4 | 45.8±65.8 |
| Above median | 105 (50.5) | 1438.7±1210.9 | 432.1±395.2 | p<0.01 | 63.4±24.4 | 39.2±46.3 |
| Below vs. above | — | p<0.01 | p<0.01 | — | NS | NS |
| Ejection fraction | ||||||
| Below median | 104 (50.0) | 1293.3±1190.3 | 359.6±377.7 | p<0.01 | 67.9±22.3 | 37.7±36.0 |
| Above median | 104 (50.0) | 794.4±717.4 | 303.8±283.1 | p<0.01 | 55.2±24.9 | 47.2±71.6 |
| Below vs. above | — | p<0.01 | NS | — | p<0.01 | NS |
Medians: Age, 72.5 years; BMI, 23.4 kg/m2; Cr, 1.2 mg/dl; EF, 39.2%.
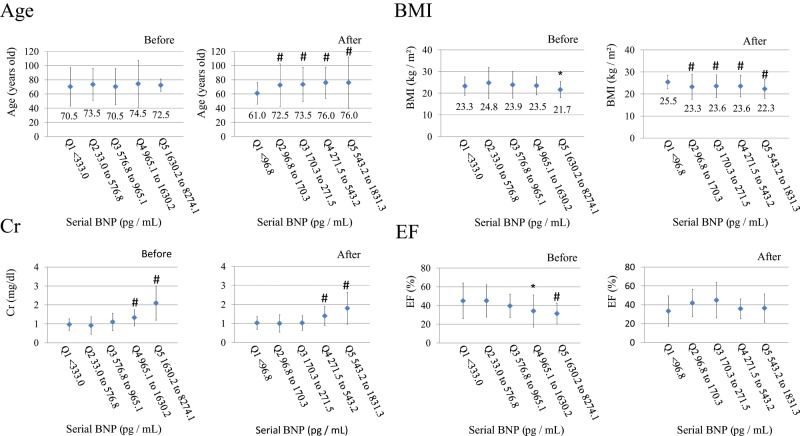
The values of BNP before and after treatment were divided into five groups according to quintiles (Q1–Q5), and age, BMI, Cr, and EF before and after treatment in group Q1 was compared to those in the other groups, from Q2 to Q5 (Figure 3). In the analysis of age, no significant differences were observed in any quintiles before treatment compared to group Q1, whereas groups Q2–Q5 showed significantly higher values after treatment. This suggests that BNP values of older patients tend to be higher after treatment than before treatment. Regarding BMI before treatment, only group Q5 showed significantly lower values than group Q1. However, BMI after treatment was significantly lower in groups Q2–Q5 compared to those observed in group Q1. Using quintiles revealed that BMI is more likely to be lower in accordance with BNP increments after treatment. Regarding the plasma Cr levels, groups Q4 and Q5 showed significantly higher levels before treatment and groups Q4 and Q5 showed significantly higher levels after treatment, which suggests that plasma BNP levels tend to be higher before and after treatment. The EF values in groups Q4 and Q5 were significantly lower than those in group Q1 before treatment. Notably, no significant differences in EF were observed in any quintiles after treatment compared to group Q1, which suggests that systolic function has no relationship with plasma BNP levels after treatment.
Figure 3.
Changes in age, BMI, creatinine, and ejection fraction before and after treatment classified on the basis of quintiles.
Significance: *p<0.05; # p<0.01.
BMI, Body mass index; BNP, B-type natriuretic peptide; Cr, creatinine; EF, ejection fraction.
Clinical factors affecting log plasma BNP levels before and after treatment
Table 3 shows the results obtained from the multiple regression analysis. BMI, Cr, and EF were significantly associated with the plasma levels of BNP before treatment (p<0.01); however, age was not significantly associated with these plasma levels. In contrast, age, BMI, and Cr were significantly associated with the plasma levels of BNP after treatment (p<0.01); however, EF did not show a statistically significant contribution after treatment.
Table 3.
Multiple regression analysis identifying clinical factors influencing the log plasma B-type natriuretic peptide levels before (R 2=0.258) and after (R 2=0.179) treatment.
| Variable | p-value | Standard β-coefficient | β-coefficient | 95% CI |
|---|---|---|---|---|
| Before treatment | ||||
| Age | NS | — | — | — |
| BMI | p<0.01 | −0.170 | −0.035 | −0.062 to −0.009 |
| Cr | p<0.01 | 0.331 | 0.176 | 0.112 to 0.239 |
| EF | p<0.01 | −0.318 | −0.018 | −0.025 to −0.011 |
| After treatment | ||||
| Age | p<0.01 | 0.282 | 0.021 | 0.010 to 0.031 |
| BMI | p<0.01 | −0.217 | −0.054 | −0.086 to −0.021 |
| Cr | p<0.01 | 0.257 | 0.163 | 0.086 to 0.240 |
| EF | NS | — | — | — |
BMI, body mass index; Cr, creatinine; EF, ejection fraction.
Clinical factors contributing to reduction in plasma BNP levels to <200 pg/ml after treatment
Table 4 shows the results of multiple logistic regression analyses performed to identify the clinical factors affecting plasma BNP levels after treatment (reducing the levels to <200 pg/ml). Plasma BNP levels were reduced to <200 pg/ml after treatment in 102 patients. A multiple logistic regression analysis showed that renal dysfunction and older age were negatively associated and high BMI were positively associated with plasma BNP levels <200 pg/ml. However, EF was not statistically significantly associated with BNP levels.
Table 4.
Multiple logistic regression analysis identifying the clinical factors contributing to reductions in plasma B-type natriuretic peptide levels to <200 pg/ml after treatment.
| Independent variable | p-value | β-Coefficient | Odds ratio | 95% CI |
|---|---|---|---|---|
| Gender | NS | — | — | — |
| Age | <0.05 | −0.026 | 0.974 | 0.950 to 0.958 |
| BMI | <0.05 | 0.085 | 1.089 | 1.006 to 1.179 |
| Cr | <0.01 | −0.763 | 0.466 | 0.290 to 0.749 |
| EF | NS | — | — | — |
BMI, body mass index; Cr, creatinine; EF, ejection fraction.
Kaplan–Meier curve for MACE and all-cause mortality
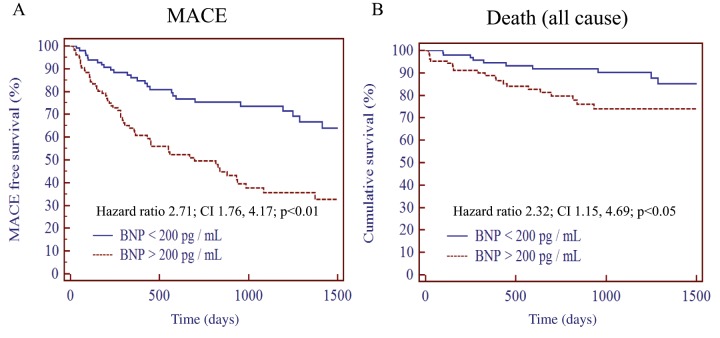
The plasma BNP values after treatment were divided by 200 pg/ml in Table 4. We investigated clinical outcomes using a Kaplan–Meier curve for MACE (Figure 4A) and all-cause mortality (Figure 4B). Patients with BNP values >200 pg/ml showed worse outcomes than those with BNP values <200 pg/ml in both the MACE-free survival curve and the cumulative survival curve.
Figure 4.
Major adverse cardiovascular event-free survival curves (A) and cumulative survival curves (B) for plasma BNP >200 and <200 pg/ml.
Patients with plasma BNP >200 pg/ml showed worse outcomes for MACE events and all-cause mortality. Eight patients died after admission and these patients are included in the survival curve.
BNP, B-type natriuretic peptide; MACE, major adverse cardiovascular event.
Discussion
In the present study, we investigated the clinical factors associated with decreases in plasma BNP levels and amelioration of acute heart failure. Improvements in acute heart failure are expected to differ depending on the specific characteristics of the patient, including degree of cardiac dysfunction, underlying heart disease, and the presence of complicating diseases. However, the present study identified standard contributing factors affecting changes in plasma BNP levels.15 Serial BNP measurements are useful in evaluating acute heart failure, and we attempted to investigate improvements in acute heart failure by assessing such measurements.9
In terms of aging, no significant differences were observed in plasma BNP levels before treatment between older and younger age groups, suggesting that the degree of acute heart failure is not related to aging in the acute phase (Figure 2). However, the reduction rate was significantly different between the two groups and plasma BNP levels were reduced only slightly in the older age group after treatment (Figure 2). Aging was negatively associated with BNP levels <200 pg/ml after treatment (Table 4). Aging was significantly associated with plasma BNP levels after treatment only. These data suggest that aging is a key factor modulating plasma BNP levels after treatment (but not before treatment) and that older age is related to poorer prognoses.16
Plasma BNP levels before treatment were significantly higher in the low BMI group compared with those observed in the high BMI group. The reduction rate was similar and plasma BNP levels remained high in the low BMI group (Figure 2). BMI was significantly associated with plasma BNP levels both before and after treatment. BMI was also significantly associated with plasma BNP levels >200 pg/ml, and this result was consistent before and after treatment, which suggests that high BMIs are positively associated with BNP reduction (Table 4). This obesity paradox has also been shown in recent studies, thus suggesting that a moderately high BMI may be preferable.17,18 However, the median BMI was only 23.4 kg/m2 in this study, which is quite low compared with that observed in Western countries.19,20 Therefore, the present study may only suggest that nutritional status is important for improving acute heart failure and reducing plasma BNP levels.
A recent study21 demonstrated that the obesity paradox does not exist in patients with diabetes mellitus (DM). In that study, all-cause mortality increased in obese patients with diabetes, which suggests that it is necessary to consider the presence of DM when investigating plasma BNP levels in patients with acute heart failure.21 We investigated the clinical outcomes in obese (above median: BMI >23.4 kg/m2) and non-obese patients (below median: BMI <23.4 kg/m2) with and without DM using a Kaplan–Meier curve for MACE and all-cause mortality. In the patients without DM, those who were non-obese showed worse outcomes than those who were obese in both the MACE-free survival curve (hazard ratio 0.32, 95% CI 0.18 to 0.56, p<0.001) and the cumulative survival curve (hazard ratio 0.21, 95% CI 0.09 to 0.51, p<0.05). In patients with DM, no significant differences were observed between non-obese and obese patients in outcomes in either the MACE-free survival curve or the cumulative survival curve (not significant, respectively, data not shown).
Plasma BNP levels before treatment were significantly high in the low EF group compared with those observed in the high EF group (Figure 2). However, the reduction rate was significantly higher in the group with an EF below median than in the group with an EF above median. No differences were observed in plasma BNP levels after treatment between these two groups (Figure 2). In addition, a multiple regression analysis showed that the contribution of EF to plasma BNP levels was reduced after treatment compared with that observed before treatment (Table 3). Given the fact that diastolic dysfunction is likely to affect plasma BNP levels after treatment, plasma BNP levels may correlate with diastolic dysfunction. To demonstrate this hypothesis, we evaluated echocardiographic diastolic function parameters [peak mitral inflow velocity and tissue Doppler velocities during early diastole (E and E′)] and plasma BNP levels after treatment in 30 patients who underwent echocardiographic examination in which echocardiographic E/E′ was evaluated. However, E/E′ was not measured in all patients and data for only 30 patients were measured, which was a limitation of this study. Plasma BNP levels after treatment were significantly correlated with E/E′ (r=0.51, p<0.05) (data not shown). These results suggest that intensive treatment during the acute phase can overcome increases in plasma BNP levels induced by systolic dysfunction and indicate that diastolic dysfunction is probably difficult to treat. EF was not significantly associated with plasma BNP levels >200 pg/ml after treatment, suggesting that EF is not a factor for determining prognosis (Table 4).
Elevated plasma BNP levels are associated with renal dysfunction.22–24 In this study, plasma BNP levels were significantly reduced in the high and low Cr groups, while the reduction rate was equivalent between the two groups (Figure 2). A multiple regression analysis showed that plasma BNP levels both before and after treatment were associated with renal dysfunction (Table 3). In addition, renal dysfunction was positively associated with levels >200 pg/ml after treatment (Table 4). These results suggest that renal dysfunction is a factor acting against amelioration of heart failure during both acute and recovery phases.
In conclusion, the contributions of clinical factors acting against amelioration of heart failure vary before and after treatment. The most important finding of this study is that plasma BNP levels are associated with systolic dysfunction in the acute phase but not after treatment. This suggests that diastolic dysfunction is hardly improved, even with intensive treatment. Regarding plasma BNP levels, an older age, very low BMI, and the presence of renal dysfunction eventually act to prevent an amelioration of acute heart failure. Systolic dysfunction does not have a negative influence on any amelioration of acute heart failure.
Acknowledgments
We thank all trial physicians and nurses in all participating hospitals for their important contributions to the study. We also thank Dr Ryuko Anzawa (Division of Cardiology, Department of Internal Medicine, The Jikei University School of Medicine) for providing suggestions regarding the survival analysis and Dr Brian Quinn (Japan Medical Communication) for their kind advice on English language.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest: No authors have any conflicts of interests to disclose.
References
- 1. Sudoh T, Kangawa K, Minamino N, et al. A new natriuretic peptide in porcine brain. Nature 1988; 332: 78–81 [DOI] [PubMed] [Google Scholar]
- 2. Mukoyama M, Nakao K, Hosoda K, et al. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest 1991; 87: 1402–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Disomma S, Magrini L, Pittoni V, et al. Usefulness of serial assessment of natriuretic peptides in the emergency department for patients with acute decompensated heart failure. Congest Heart Fail 2008; 14(4 Suppl 1): 21–24 [DOI] [PubMed] [Google Scholar]
- 4. Yasue H, Yoshimura M, Sumida H, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation 1994; 90: 195–203 [DOI] [PubMed] [Google Scholar]
- 5. Omland T, Aakvaag A, Bonarjee VV, et al. Plasma brain natriuretic peptide as an indicator of left ventricular systolic function and long-term survival after acute myocardial infarction: comparison with plasma atrial natriuretic peptide and N-terminal proatrial natriuretic peptide. Circulation 1996; 93: 1963–1969 [DOI] [PubMed] [Google Scholar]
- 6. Faggiano P, Valle R, Aspromonte N, et al. How often we need to measure brain natriuretic peptide (BNP) blood levels in patients admitted to the hospital for acute severe heart failure? Role of serial measurements to improve short-term prognostic stratification. Int J Cardiol 2010; 140: 88–94 [DOI] [PubMed] [Google Scholar]
- 7. Metra M, Nodari S, Parrinello G, et al. The role of plasma biomarkers in acute heart failure. Serial changes and independent prognostic value of NT-proBNP and cardiac troponin-T. Eur J Heart Fail 2007; 9: 776–786 [DOI] [PubMed] [Google Scholar]
- 8. Fertin M, Hennache B, Hamon M, et al. Usefulness of serial assessment of B-type natriuretic peptide, troponin I, and C-reactive protein to predict left ventricular remodeling after acute myocardial infarction (from the REVE-2 study). Am J Cardiol 2010; 106: 1410–1416 [DOI] [PubMed] [Google Scholar]
- 9. Noveanu M, Breidthardt T, Potocki M, et al. Direct comparison of serial B-type natriuretic peptide and NT-proBNP levels for prediction of short- and Long-term outcome in acute decompensated heart failure. Crit Care 2011; 15: R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xue Y, Clopton P, Peacock WF, et al. Serial changes in high-sensitive troponin I predict outcome in patients with decompensated heart failure. Eur J Heart Fail 2011; 13: 37–42 [DOI] [PubMed] [Google Scholar]
- 11. Suzuki S, Yoshimura M, Nakayama M, et al. Plasma level of B-type natriuretic peptide as a prognostic marker after acute myocardial infarction: a long-term follow-up analysis. Circulation 2004; 110: 1387–1391 [DOI] [PubMed] [Google Scholar]
- 12. Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 2004; 350: 655–663 [DOI] [PubMed] [Google Scholar]
- 13. Di Angelantonio E, Chowdhury R, Sarwar N, et al. B-type natriuretic peptides and cardiovascular risk: systematic review and meta-analysis of 40 prospective studies. Circulation 2009; 120: 2177–2187 [DOI] [PubMed] [Google Scholar]
- 14. Daniels LB, Clopton P, Jiang K, et al. Prognosis of stage A or B heart failure patients with elevated B-type natriuretic peptide levels. J Card Fail 2010; 16: 93–98 [DOI] [PubMed] [Google Scholar]
- 15. Inoue T, Kawai M, Nakane T, et al. Influence of low-grade inflammation on plasma B-type natriuretic peptide levels. Intern Med 2010; 49: 2659–2668 [DOI] [PubMed] [Google Scholar]
- 16. Ueda R, Yokouchi M, Suzuki T, et al. Prognostic value of high plasma brain natriuretic peptide concentrations in very elderly persons. Am J Med 2003; 114: 266–270 [DOI] [PubMed] [Google Scholar]
- 17. Lavie CJ, Osman AF, Milani RV, et al. Body composition and prognosis in chronic systolic heart failure: the obesity paradox. Am J Cardiol 2003; 91: 891–894 [DOI] [PubMed] [Google Scholar]
- 18. Curtis JP, Selter JG, Wang Y, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med 2005; 165: 55–61 [DOI] [PubMed] [Google Scholar]
- 19. Jain SH, Massaro JM, Hoffmann U, et al. Cross-sectional associations between abdominal and thoracic adipose tissue compartments and adiponectin and resistin in the Framingham Heart Study. Diabetes Care 2009; 32: 903–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Komukai K, Minai K, Arase S, et al. Impact of body mass index on clinical outcome in patients hospitalized with congestive heart failure. Circ J 2011; 76: 145–151 [DOI] [PubMed] [Google Scholar]
- 21. Adamopoulos C, Meyer P, Desai RV, et al. Absence of obesity paradox in patients with chronic heart failure and diabetes mellitus: a propensity-matched study. Eur J Heart Fail 2011; 13: 200–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsutamoto T, Wada A, Sakai H, et al. Relationship between renal function and plasma brain natriuretic peptide in patients with heart failure. J Am Coll Cardiol 2006; 47: 582–586 [DOI] [PubMed] [Google Scholar]
- 23. Obineche EN, Pathan JY, Fisher S, et al. Natriuretic peptide and adrenomedullin levels in chronic renal failure and effects of peritoneal dialysis. Kidney Int 2006; 69: 152–156 [DOI] [PubMed] [Google Scholar]
- 24. Tagore R, Ling LH, Yang H, et al. Natriuretic peptides in chronic kidney disease. Clin J Am Soc Nephrol 2008; 3: 1644–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]