Abstract
Background:
Most patients hospitalized for acutely decompensated heart failure (ADHF) present with symptoms and signs of volume overload, which is also associated with substantially high rates of death and rehospitalization in ADHF.
Objective:
To review the recent experimental and clinical evidence on existing therapeutic algorithms and investigational drugs used for the treatment of volume overload in ADHF patients.
Methods:
A systematic search of peer-reviewed publications was performed on Medline and EMBASE from January 1990 to March 2012. The results of unpublished trials were obtained from presentations at national and international meetings.
Results:
Apart from intrinsic renal insufficiency and neurohormonal activation, volume overload through venous congestion may be the primary haemodynamic factor triggering the worsening of renal function in ADHF patients. It is well known that heart and kidneys are closely interrelated and an acute or chronic disorder in one organ may induce acute or chronic dysfunction in the other organ. Established therapeutic strategies, (e.g. loop diuretics, vasodilators, and inotropes), are sometimes associated with limited clinical success due to tolerance and the need for frequent up titration of the doses in order to achieve the desired effect. That leads to an increasing interest in novel options, such as the use of adenosine A1 receptor antagonists, vasopressin antagonists, and renal-protective dopamine. Initial clinical trials have shown quite encouraging results in some heart failure subpopulations but have failed to demonstrate a clear beneficial role of these agents. On the other hand, ultrafiltration appears to be a more promising therapeutic procedure that will improve volume regulation, while preserving renal and cardiac function.
Conclusion:
Further clinical studies are required in order to determine their net effect on renal function and potential cardiovascular outcomes. Until then, management of volume overload in ADHF patients remains a challenge for the clinicians.
Keywords: Decompensated heart failure, pathophysiology, renal failure, salt retention, therapeutic options, volume overload
Introduction
Despite significant advances in the pharmacological and device therapy of heart failure (HF), acutely decompensated heart failure (ADHF) remains the most common reason for hospitalization among patients over the age of 65 years and a major economic burden for the healthcare system worldwide.1 It may result from new onset of ventricular dysfunction or, more often, exacerbation of chronic heart failure (CHF) symptoms.2 ADHF is a heterogenous syndrome with a complex pathophysiology and increased morbidity and mortality worldwide. Furthermore, this condition is frequently complicated with worsening of renal function, which has been associated with further increase in rehospitalization and mortality rates.3–5
The majority of patients admitted in hospital with ADHF have dyspnoea and signs of fluid overload. Their treatment is focused on reversing pulmonary and/or peripheral congestion, identifying and treating potential precipitating causes, and optimizing the therapeutic approach.6 In the ADHERE (Acute Decompensated Heart Failure National Registry) population, most patients admitted in hospital had signs and symptoms of congestion; 89% presented with dyspnoea, 68% presented with rales, and 66% with peripheral edema.7 Because these volume overload states are described as ‘congestive heart failure’, heart function has been given more attention than kidney function. Nowadays, it is well accepted that the interaction between the heart and the kidney plays a crucial role in the progression of HF. Nevertheless, the adverse prognostic value of renal dysfunction on the duration of hospitalization and outcomes in HF population is frequently underestimated.8,9 Most epidemiological information of renal dysfunction in the HF population comes from large registries, since most outpatient CHF clinical trials did not include patients with significant chronic kidney disease. ADHERE database showed that 31% of the enrolled patients suffered from chronic renal insufficiency, 5% were receiving dialysis, and 20% had a serum creatinine level ≥2 mg/dl. It is also important that as renal function worsened in the ADHERE population, prognosis deteriorated.10 In addition, in the SOLVD (Studies of Left Ventricular Dysfunction) trial, patients with a glomerular filtration rate (GFR) less than 60 ml/min/1.73 m2 had a 40% higher mortality risk.11
The cross-talk between kidneys and heart is important to control blood pressure, regulate renal sodium and water excretion, and provide sufficient arterial perfusion and oxygenation of tissues. An acute or chronic disorder in one organ may induce an acute or chronic dysfunction in the other organ and vice versa.12 In the HF population, kidney dysfunction is an often comorbidity with multifactorial origin, which may play a key role in the cascade involving salt and fluid retention and subsequent decompensation. It is important to understand the interaction between these two organs, so as to tailor therapy for these patients.10,12
Pathophysiology of renal insufficiency and volume overload in ADHF
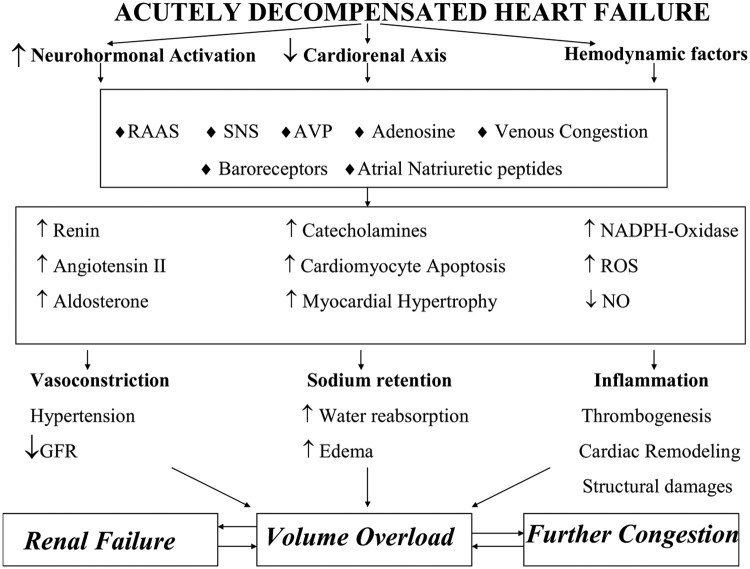
Optimal volume management in ADHF patients requires a deep knowledge of the underlying pathophysiological mechanisms that lead to worsening of renal function (WRF), as well as salt and water retention despite hypervolaemia. Although the main topic of this review is to present the therapeutic options for ADHF with volume overload, we make a brief reference to the pathophysiology of WRF and fluid overload, so as to provide a more comprehensive approach to the therapeutic strategies. Figure 1 summarizes the underlying pathophysiological mechanisms of volume overload in congestive heart failure.
Figure 1.
The underlying pathophysiological mechanisms of volume overload in acutely decompensated heart failure
AVP, arginine vasopressin; GFR, glomerular filtration rate; NO, nitric oxide; RAAS, renin–angiotensin–aldosterone system; ROS, reactive oxygen species; SNS, sympathetic nervous system.
Worsening renal function
WRF is defined as an absolute increase in serum creatinine levels ≥0.3 mg/dl during hospitalization and by ≥25% increase relative to baseline. It complicates about one-third of HF admissions and it has been associated with increased duration of hospitalization, increased readmission rate, and increased short-and long-term mortality.13
WRF pathophysiology is multifactorial. In many cases, decreased renal perfusion causes deterioration of renal function. This may be caused by hypovolaemia (decreased preload), neurohormonally mediated excessive vasoconstriction (increased after-load), and hypotension with low-output syndrome (severe pump failure, cardiogenic shock). It can also be provoked by diuretic resistance and nephrotoxicity induced by certain classes of coadministered drugs, e.g. nonsteroidal anti-inflammatory drugs (NSAIDs), cyclosporine, angiotensin-converting enzyme inhibitors (ACE inhibitors), angiotensin II receptor I blockers (ARBs), and contrast agents.10 Venous congestion is another precipitating factor; for the patient with HF and fluid overload, the combination of high central venous pressure with low systemic pressure may lead to a severe compromise of the net renal perfusion pressure and, thus, result in significant deterioration of the renal blood flow and urine output.14 Mullens et al. showed that venous congestion is the most important haemodynamic factor enhancing WRF in ADHF patients.15
In daily clinical practice, it is of great importance to distinguish between real congestion and hypovolaemia, since there is a very narrow window of optimal hydration for these patients. Overhydration or inappropriate dehydration might prove devastating for heart and kidney function, respectively. The careful clinical evaluation of the patient, the echocardiogram, and the use of Swan-Ganz catheter can guide the physician to take the best therapeutic decisions for the patient.
Salt and water retention
Maintenance of total body salt and fluid within normal range is under the control of the atrial–renal reflexes, the renin–angiotensin–aldosterone system (RAAS), and the sympathetic nervous system (SNS).12,16,17
In a normal heart, any increase in left atrial pressure suppresses the release of the antidiuretic hormone, decreases the tone of the SNS in the kidneys, and, finally, it enhances the release of the atrial natriuretic peptide. The latter promotes sodium and water excretion at the distal nephron, improves GFR, causes vasodilatation, decreases the release of the antidiuretic hormone, and activates both the SNS and the RAAS. In CHF, the former actions are blunted due to renal vasoconstriction, reduced sensitivity of its receptors, and reduced sodium delivery to the distal nephron. Therefore sodium and water retention occur despite elevated atrial pressures.12,18
Under normal circumstances, activation of the RAAS by low renal perfusion pressure or low blood flow works as a defence mechanism that protects vital organs from underperfusion. In HF states, this response can be devastating; retention of salt and water due to the haemodynamic and reabsorptive actions of angiotensin causes further congestion. Therefore the RAAS has a central role in the initiation and maintenance of edema in HF.19 More specifically, renin release from the kidneys leads to stimulation of angiotensin II, which in turn activates receptors on the proximal tubule and causes constriction of the glomerular efferent arterioles, leading to increased sodium reabsorption. Besides its direct tubular and vascular effects in the kidney, angiotensin II stimulates central neural centres associated with increased thirst, causes systemic vasoconstriction, stimulates the SNS, promotes aldosterone secretion,20 and activates NADPH oxidase, which results in the formation of reactive oxygen species.21 The increased oxidative stress enhances negative inotropic effects and induces cardiac remodelling. Therefore, a vicious cycle sets in promoting structural and functional damage to both kidneys and heart.22 Aldosterone release, in turn, causes continuous renal sodium reabsorption and increases the myocardial fibrosis of the failing heart.23
The SNS contributes to a long-term regulation of the intravascular volume and blood pressure. In CHF, it is initially activated by the reflex to provide inotropic support and preserve cardiac output. But excessive SNS activity, caused partially by the attenuated receptor sensitivity met in CHF, can increase cardiomyocyte apoptosis and focal myocardial necrosis, while the direct actions of catecholamines can lead to myocardial hypertrophy.24 Moreover, the aggressive use of diuretics may cause further neurohormonal activation and provoke systemic and renal vasoconstriction, leading to additional WRF. The consequent decline in blood flow and filtration contribute actively to the appearance of diuretic resistance.13
The antidiuretic hormone is released in response to arterial underfilling and increased osmolality. It has detrimental effects on cardiorenal performance by fluid retention, enhancement of angiotensin II and noradrenaline actions, and stimulation of myocardial hypertrophy.25
Adenosine and the related tubuloglomerular feedback is a newly identified contributing factor. Adenosine binds to A1-receptors on the afferent arterioles, induces vasoconstriction, and consequently decreases renal blood flow and GFR. Activation of these receptors promotes sodium reabsorption in the tubules, leading to further water and sodium retention. Treatment with diuretics in ADHF acutely delivers sodium to the distal tubules, which in turn stimulates further adenosine release and further reduction in GFR.26
Treatment of patients with ADHF and volume overload
Trying to manage ADHF patients with volume overload is challenging due to the complex underlying pathophysiology of the disease, the unique medical history, coexisting risk factors and comorbidities of each patient, the effort to avoid renal impairment, electrolyte abnormalities, and hypotension, and, finally, the frequent development of resistance to many standard therapies such as diuretics and vasodilators. That leads to a growing concern about developing novel treatment options. Unfortunately, we have no evidence from clinical HF trials on which to base our therapeutic decisions for patients with significant renal dysfunction, since most studies predominantly enrolled patients with relatively preserved renal function.27
Another point to highlight is that treating ADHF patients with volume overload often involves taking therapeutic decisions that are mutually contradictory. The aggressive use of diuretics and volume depletion in order to treat congestion and volume overload directly deteriorates renal function. On the other hand, replacing intravascular volume and salt in order to preserve renal function leads directly to further cardiac congestion. Not surprisingly, many patients are discharged either still volume-loaded or deteriorated in terms of renal function.14 In ADHERE, 21% of patients admitted for ADHF were discharged without weight loss or even with a gain in body weight, which led to a high readmission rate for these patients.9,28
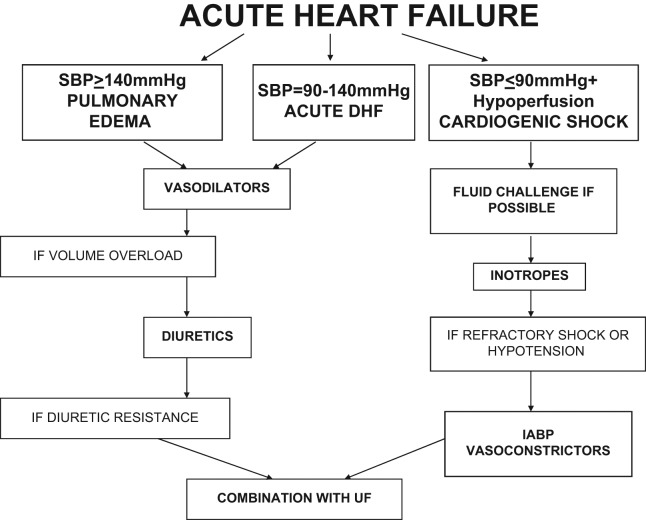
Treatment of patients with ADHF and symptomatic congestion aims at the rapid removal of fluid and symptomatic relief. Despite the arising difficulties, it is important to individualize the treatment according to the patient’s clinical characteristics, volume and haemodynamic status, and severity of the underlying disease, as well as to decide on the duration of each therapeutic choice. The goal of the therapeutic efforts is to stabilize the patient haemodynamically without further myocyte damage, arrythmias, hypotension, electrolyte abnormalities, or WRF.12 The Canadian Cardiovascular Society suggest the rapid clinical assessment of the patient and the consequent categorization based on the clinical perfusion status (warm or cold) and volume load (wet or dry). Patients who are ‘warm’ and ‘wet’ (almost 70% of those with ADHF) are candidates for combined early diuretic and vasodilator therapy.29,30 Figure 2 summarizes some practical recommendations for the management of volume overload in ADHF patients.
Figure 2.
ADHF treatment algorithm
IABP, intraaortic balloon pump; SBP, systolic blood pressure; UF, ultrafiltration.
Table 1 summarizes the current and possible future treatment options for ADHF patients, which are discussed further below.
Table 1.
Current and possible future treatment options for ADHF patients
| Current treatments | Possible future treatments |
|---|---|
| Oxygen administration and positive airway pressure to correct hypoxaemia | Ularitide |
| Opioids (e.g. morphine) to reduce anxiety and pre-load | Relaxin |
| Vasodilators (nitrovasodilators, neseritide) to reduce both pre-load and after-load | Istaroxime |
| Diuretics (i.v. loop diuretics) to effect venodilation and diuresis | Cardiac myosin activator (omecamtiv mecarbil) |
| Low-dose dopamine (≤3 µg/kg/min) | Vasopressin antagonists |
| Inotropes (dobutamine, milirinone, levosimendan) | Adenosine antagonists |
| Ultrafiltration | |
| Intra-aortic balloon pump |
Established therapeutic strategies
Diuretics
The pharmacological armamentarium for managing symptomatic volume overload (including loop diuretics, nitrates, morphine, and oxygen) has slightly changed during the last three decades. Loop diuretics are undoubtedly an essential component of current treatment for the acute symptomatic relief of patients with ADHF, despite the growing recognition of their limitations.9,12 Intravenous (i.v.) administration of loop diuretics is recommended as a first-line treatment option, because they rapidly decrease the ventricular filling pressure, reduce pulmonary congestion, and relieve from dyspnoea.31,32 Despite decades of clinical experience with these agents, prospective data to guide the use of loop diuretics are sparse and current guidelines are based primarily on experts’ opinion. Such trials are difficult to perform because diuretics are supposed to be absolutely necessary in patients with acute decompensation.33 The great extent of the use of diuretics is illustrated by data from the ADHFNR (Acute Decompensated Heart Failure National Registry), which revealed that almost 81% of patients included in the registry were on chronic diuretic therapy at the time of admission, while 88% were treated acutely with an i.v. diuretic during their admission for decompensation.34
Despite the acute symptomatic relief that diuretics provide, they are not free from adverse events, especially when used in high doses (>80 mg of i.v. furosemide).35 They activate the neurohormonal system and indirectly deteriorate the function of the left ventricle, cause significant electrolyte abnormalities (e.g. hypokalaemia), potential arrythmias, and ototoxicity (when administered in high i.v. doses), and increase systemic vascular resistance, plasma renin and aldosterone activity, and plasma levels of neurohormones. Through the above mechanisms, they result in deterioration of renal function and increase of the mortality risk.36,37
A challenging clinical problem while treating patients with ADHF and impaired renal performance is resistance to diuretics, which is also an indicator of poor prognosis. It is described as a clinical entity in which the diuretic response is reduced or lost before the therapeutic goal has been achieved. It is attributed to many different precipitating factors, such as decreased intestinal absorption of oral diuretics due to mucosal edema, impaired renal perfusion, decreased diuretic excretion into the urine (due to hypertrophy of distal tubular epithelial cells), inadequate drug dosing, excess salt intake, and finally the concomitant use of NSAIDs.28,38,39 This effect could be overdriven with a continuous infusion of furosemide, rather than bolus doses. Another approach is to use simultaneously a second diuretic agent (e.g. an i.v. thiazide diuretic), so as to cause sequential nephron blockade of sodium reabsorption. However, combination therapy requires close monitoring, as it may lead to excessive sodium and potassium loss.29 A Cochrane review examined eight trials comparing continuous infusion of a loop diuretic with bolus injections in 254 CHF patients. The urine output was significantly greater in patients who were given continuous infusion and the duration of hospitalization was significantly shortened.35 On the other hand, The Diuretic Optimization Strategies Evaluation (DOSE) trial was designed to evaluate the safety and efficacy of i.v. furosemide at ‘low’ and ‘high’ doses and in a continuous infusion vs. a bolus one every 12 hours in 308 patients with ADHF (156 patients were assigned to intermittent dosing, 74 of which received low-dose furosemide and 82 received high dose, while 152 patients were assigned to continuous dosing, 77 of which received low dose and 75 high dose). The results of this trial showed that there was no significant difference in symptom relief or change in renal function at 72 hours in the four groups, but patients in the high-dose group had significant improvements in terms of weight loss, HF biomarkers, and dyspnoea.40
Several studies on ADHF have demonstrated that aggressive diuretic therapy could promote diuretic-induced hypovolaemia, exaggerating any pre-existing renal insufficiency and increasing mortality.41 On the other hand, Testani et al. showed that aggressive decongestion, even in the face of worsened renal outcomes, may positively impact post-discharge survival. Future research is necessary to replicate these results and change current strategies.42
Mineralcorticoid antagonists
Low and high-output HF are both hyperaldosteronism states. Aldosterone increases sodium reabsorption in the collecting duct and promotes reactive perivascular and interstitial myocardial fibrosis. Thus, it might be effective to combine natriuretic doses of antagonists of the mineralcorticoid receptors (>25 mg daily of spironolactone) with loop diuretics. This combination may prevent or attenuate diuretic refractoriness and reverse the aldosterone-mediated sodium retention and fibrosis.43 The Randomized Aldactone Evaluation Study (RALES) added spironolactone to an ACE inhibitor and showed decreased mortality in HF patients with complete suppression of aldosterone secretion.44 A recent subanalysis of Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS), showed that the early administration (3–7 days) of 25–50 mg/daily of eplerenone in post-myocardial patients with left ventricular ejection fraction <40% improved outcomes and did not significantly increase the risk of hyperkalaemia.45 The onset of action of spironolactone is slower than loop diuretics and its peak effect is at 48 hours. Therefore, in acute states the diuretic of choice is a loop diuretic.12 However, these agents have not been systematically studied in patients with acute heart failure (AHF) syndromes.
ACE inhibitors and ARBs
Inhibitors of the RAAS (ACE inhibitors and ARBs) are the cornerstone in the treatment of patients with systolic dysfunction of the left ventricle.4 Although they do not have a direct effect on fluid and salt excretion in congestive states and they do not belong to the first-line treatment during acute decompensation, they have been clearly shown to improve survival and long-term outcomes in patients with HF. This emphasizes the importance of instituting or optimizing disease-modifying therapy as soon as possible. Nevertheless, in ADHF these drugs should be used cautiously whenever there is an underlying renal disease, because they might be associated with increases in serum creatinine levels.46–48 In order to reduce the possibility of renal deterioration, patients should be started on the lowest dose of an ACE inhibitor, when the patient is considered not to be dehydrated (due to the previous diuresis) and concomitant use of NSAIDs should be avoided.27,49 In addition, up titration of dosage should be done very gradually and carefully. It is important to remember that, when these agents are administered, their effect on the haemodynamic status and renal function requires close monitoring.29 An effective approach is to continue these agents during decompensation, unless renal dysfunction is steadily impaired and severe hyperkalaemia develops. However, an expert physician should evaluate extreme clinical conditions, such as cardiogenic shock or acute renal failure.
Low-dose dopamine
On daily clinical practice low renal-protective doses of dopamine are often used in combination with i.v. diuretics in an effort to improve diuresis and natriuresis. Nevertheless, available data do not clearly support favourable effects on renal function. Dopamine is an endogenous catecholamine that acts on a variety of receptors (renal, splanchnic, cardiac, vascular) according to the dose infused. When infused at low doses (≤3 µg/kg/min), it selectively stimulates receptors in the renal and splanchnic vasculature, improving blood flow in these tissues. In addition, it attenuates the effects of norepinephrine and aldosterone.50 In a prospective, double-blind, randomized, controlled study by Lauschke et al.,51 40 patients, 10 without and 30 with acute renal failure, were given i.v. dopamine (2 µg/kg/min) or placebo in alternating sequence for four subsequent periods of 60 min and renal resistance indices were determined by Doppler ultrasound. The study showed that low-dose dopamine can worsen renal perfusion in patients with acute renal failure, which adds to the trend to abandon the routine use of low-dose dopamine in critically ill patients.51 This can be explained by the fact that its beneficial effects on renal blood flow and tubular natriuresis are blunted in patients presenting with acute renal failure and oliguria.52
On the other hand, Giamouzis et al. (DAD-HF clinical trial)53 compared the effects of low-dose dopamine (5 µg/kg/min) plus low-dose furosemide (5 mg/h) vs. high-dose furosemide (20 mg/h) alone on kidney function and subjective perception of dyspnoea in 60 ADHF patients. There were no differences in urine output or in dyspnoea score, but those patients who received dopamine plus low-dose furosemide were associated with improved renal function profile and potassium homeostasis at 24 hours.53 Moreover, Aziz et al. showed that continuous infusion of furosemide in addition to low-dose dopamine in patients with ADHF is less nephrotoxic and carries a lower readmission rate at 30 days.52 Although dopamine seems to have been forgotten for a long time, it now emerges again among our therapeutic choices for ADHF in combination with furosemide infusion, since its positive effects seem to be preserved in patients without compromised renal function.
Inotropes
Traditional positive inotropic drugs include beta-adrenergic agonists (e.g. dobutamine) and phosphodiesterase inhibitors (e.g. milirinone). Their stimulation leads to increased levels of cyclic adenosine monophosphate (cAMP), resulting in increased levels of calcium ions within the cardiac myocyte and increased cardiac contractility. They are indicated in patients with ADHF and peripheral hypoperfusion (hypotension, deterioration of renal function, cutaneous signs of poor peripheral perfusion) or refractoriness to diuretics and vasodilators. Inotropes should be restricted only to circulatory collapse states, for short term and under close monitoring, as they are susceptible to malignant arrhythmogenesis and loss of myocardial cells by ischaemia or apoptosis. The latter is attributed to the increased myocardial oxygen demand in a period of myocardial energy depletion. It has been shown that in both acute and chronic HF, inotropic agents, compared with placebo and vasodilators, have been related to an increased risk of death. Until new clinical studies provide more data, inotropes should be reserved for those patients with marked haemodynamic compromise, cardiogenic shock, or evidence of end-organ hypoperfusion.54–56
Levosimendan is a promising new inotropic agent that belongs to the novel class of ‘calcium sensitizers’. It exerts its influence on the cardiovascular system by two mechanisms of action. It binds to cardiac troponine C, stabilizing the conformational alteration of troponin C through binding to calcium, therefore improving cross-bridging and contractility. It also stimulates peripheral vasodilation through activation of adenosine triphosphate (ATP)-sensitive potassium channels of vascular smooth muscle cells. As it has a neutral effect on cAMP levels, it seems to be superior to classical positive inotropes in improving central haemodynamic parameters and symptoms of congestion in patients hospitalized with low cardiac output syndrome.57 Zager et al. showed in an experimental study that in critical situations such as sepsis or AHF, levosimendan protects against ischaemic acute renal failure due to severe renal vasoconstriction.58 In addition, there is clinical evidence that it improves renal function in patients with ADHF in comparison to placebo or dobutamine, by improving more effectively cardiac output and therefore renal perfusion.59 Finally, the results obtained in clinical trials (LIDO, RUSSLAN, and CASINO) suggest that levosimendan is more effective compared to dobutamine in ADHF and has lower mortality rates compared to dobutamine or placebo.60–62 Despite the contradictory results of REVIVE II and SURVIVE studies (maybe due to different study designs), levosimendan is suggested as an excellent therapeutic option when inotropic treatment is considered necessary, especially in patients without hypotension and relative depletion of intravascular volume.63
Digoxin is an inotropic agent that increases the myocardium’s contractility through inhibition of the Na+-K+-ATPase and subsequent increase of the intracellular calcium ions. It also provokes bradycardia through stimulation of the parasympathetic nervous system. It appears to be beneficial in patients with a high heart rate, atrial fibrillation, and enlarged ventricles with systolic dysfunction. The DIG trial demonstrated that digoxin does not reduce overall mortality but it appears to reduce the hospitalization rate both overall and for worsening HF. However, the role of digoxin in the management of AHF based on the results of the DIG trial remains equivocal.64
Vasodilators and natriuretic peptide
Vasodilators such as i.v. nitroglycerin are recommended for the first-line treatment of ADHF associated with elevated systemic blood pressure at presentation. They have been shown to be much less harmful to kidney function, especially when used at low doses that do not cause hypotension and hypoperfusion. Vasoconstriction plays a central role in the pathogenesis of ADHF. Vasodilators can rapidly reduce ventricular filling pressures and central venous pressures and reduce myocardial oxygen consumption. I.v. nitroglycerine is a vasodilator used to alleviate pulmonary congestion and dyspnoea in patients with ADHF. Frequent dose titration according to systemic blood pressure is necessary to achieve the desired haemodynamic effects and symptomatic relief.13 The use of continuous i.v. administration of nitrates is associated with tolerance, up titration against blood pressure is required in order to maintain efficacy.65 The reduction in venous pressure may be beneficial in decreasing transrenal perfusion pressure. However, their use is still based on limited evidence and experts’ opinion rather than large-scale clinical trials.13
B-type natriuretic peptide (BNP) is formed in the ventricular myocardium in response to overload and wall stress. BNP causes dilation of both arteries and veins, enhances sodium renal excretion, and suppresses the RAAS. Nesiritide, a recombinant human B-type atrial natriuretic peptide, is an effective vasodilator with a mild diuretic action. It is the only vasodilator recently approved in the USA for the treatment of AHF.66 Its administration results in venous, arterial, and coronary vasodilatation and reduction of the cardiac pre-load and after-load, which consequently increases cardiac output without direct inotropic effects. These haemodynamic effects are followed by natriuresis and diuresis, which are attenuated in severe HF. Nevetheless, creatinine clearance was not improved by nesiritide, even in patients who showed satisfactory natriuresis and diuresis.67,68
The Vasodilatation in the Management of Acute Congestive Heart Failure (VMAC) trial, which assessed the impact of early nesiritide infusion on dyspnoea and pulmonary congestion in patients with ADHF, confirmed the favourable effects of neseritide. A total of 489 patients with impaired renal function were given either nesiritide or nitroglycerin. At 24 hours, 83% of the patients with renal insufficiency and 91% of patients without renal insufficiency who were treated with neseritide, were improved in terms of dyspnoea. Nesiritide might induce symptom relief in HF patients with renal dysfunction but has no effect on kidney function itself.69 A substudy of the Follow-Up Serial Infusions of Nesiritide trial (FUSION I), showed that in HF population who were at high risk for cardiorenal syndrome, infusion of nesiritide at two doses (0.005 or 0.01 µg/kg/min) was well tolerated without deterioration of kidney function.70 The serial infusion of nesiritide (FUSION II) trial was designed to examine the intermittent infusion of nesiritide in patients with severe HF. Infusions were given either once or twice weekly over 12 weeks. It was shown that there was not a significant effect on outcome or quality of life, but there was an effect on the kidney; an increasing serum creatinine level of more than 0.5 mg/dl was favourably affected by nesiritide.71 Although first data show that low doses of nesiritide are potentially renal protective, additional information on its efficacy and safety are needed before it becomes an established therapy. The Acute Study of Clinical Effectiveness of Neseritide in Subjects with Decompensated Heart Failure (ASCEND-HF) study has recently shown no superior effect of neseritide vs. placebo on either symptoms or survival.72
Novel therapeutic strategies
Mechanical methods
Mechanical fluid removal/ultrafiltration
Ultrafiltration is a very promising treatment option for patients with ADHF and volume overload refractory to diuretics. The term ‘ultrafiltration’ was first introduced in 1907 by the German physician H Bechhold. Ultrafiltration is the mechanical removal of fluid and small-molecular-weight compounds from the vasculature. Hydrostatic pressure is applied to blood across a semi-permeable membrane to separate isotonic plasma water from blood. Serum concentration of electrolytes and other solutes is not affected, which permits large amounts of fluid to be removed at the discretion of the treating physician. The equipment consists of a circuit with blood leaving the patient, a filter composed of small porous tubes which allow water and small molecules to exit, and filtered blood returning to the patient. A pump directs blood through the filter and a second one exerts negative pressure on the filter to remove ultrafiltrate. Ultrafiltration requiring central venous access is more frequently used, especially if the patient is extremely edematous. Criteria for the initiation of mechanical fluid removal are pulmonary edema with significant renal dysfunction (creatinine clearance <30 ml/min), marked volume overload in patients with ADHF, including significant dysfunction of the right ventricle, and refractoriness to i.v. diuretics.73 Ultrafiltration slightly influences the patients’ haemodynamic status. The usual volume of water removed per ultrafiltration session is 3–4 lt. The reduction in water is accompanied by decreases in right atrial pressure and wedge pressure. Cardiac output and stroke volume do not change or rise slightly. Compared to diuretic-based therapy, ultrafiltration is more efficient in removing sodium, while the neurohormonal activation is less for the same degree of volume reduction. Moreover, the fluid removal volumes and rates are adjustable and weight loss is sustained relatively to furosemide treatment. Loop diuretics should not be administered during the ultrafiltration sessions, so as to minimize electrolyte abnormalities and further neurohormonal activation.74
The efficacy of ultrafiltration has been assessed by several clinical trials. The multicentre randomized controlled study RAPID CHF (Relief for Acutely Fluid-Overloaded Patients with Decompensated Congestive Heart Failure)75 compared a single 8-hour ultrafiltration intervention to usual care of 40 patients hospitalized with ADHF. Total fluid removal at 24 hours was greater with ultrafiltration than with the usual care, with a trend towards greater weight loss at 24 hours in the ultrafiltration group.75 Ultrafiltration can be a potential treatment for patients with severe HF and WRF, decreased urine output despite escalating doses of diuretics or diuretic resistance. In the Ultrafiltration versus Intravenous Diuretics for patients Hospitalized for Acute Decompensated Heart Failure (UNLOAD) trial, patients with ADHF were randomly assigned to ultrafiltration with flows of up to 500 ml/h vs. standard i.v. diuretics. The ultrafiltration group showed a greater weight loss and water removal at 48 hours, although the changes in dyspnoea score did not differ and both groups improved. At a 3-month follow up, the rehospitalization rates and the duration of hospitalization were significantly lower in the ultrafiltration group.76
The recent ULTRADISCO Study (Effects of ULTRAfiltration vs. DIureticS on clinical, biohumoral and haemodynamic variables in patients with deCOmpensated heart failure) showed that ultrafiltration promotes a greater clinical improvement compared with diuretic infusion, by ameliorating haemodynamics without a marked increase in aldosterone and NT-proBNP levels.77 Finally, the ongoing Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) compares ultrafiltration vs. stepped pharmacological care and is expected to provide information and evidence for the management of WRF in patients with ADHF.78
In summary, compared with the administration of i.v. diuretics with or without accompanying vasoactive therapy, ultrafiltration provides a quick and predictable removal of fluid that is free of induced electrolyte abnormalities and associated consequences. On the other hand, it may be related to high daily cost, the need for large vein access and closer patient supervision.74 Table 2 summarizes potential benefits and risks of peripheral ultrafiltration application in ADHF patients.
Table 2.
The potential benefits and risks of peripheral ultrafiltration application in ADHF patients
| Benefits | Risks |
|---|---|
| Predictable reduction of fluid overload | Local complications (e.g. anticoagulation, vein access) |
| Protection from electrolyte disturbances | Local and systematic infections |
| Correction of hyponatraemia | No long-term mortality data |
| No neurohormonal activation | Close patient supervision |
| Improvement of exercise capacity | Cost |
| Less hospitalizations |
Cardiocirculatory mechanical support
Extra-corporeal membrane oxygenation (ECMO) is instituted for the management of life-threatening pulmonary or cardiac failure (or both) when no other treatment has been successful. It can be deployed in a veno-arterial configuration for the treatment of cardiogenic shock and improvement of peripheral perfusion. It is used as temporary support, usually awaiting recovery of organs, or can be used as a bridge to a more permanent device (e.g. BiVAD) or cardiac transplantation.79 There are no large-scale clinical trials about ECMO in cardiogenic shock and only case reports or small series of patients have been announced. Subjects with resistant cardiorenal failure are rarely candidates for advanced HF interventional therapies, such as cardiocirculatory mechanical support or heart transplantation, because of their high surgical risk and poor prognosis. However, there are mechanical interventions such as intra-aortic balloon pump (IABP) or other portable LV assist device implantations, which are used in low cardiac output states and contribute to the haemodynamic stabilization of these patients and, therefore, the preservation of renal function.
Pharmacological methods
Ularitide
Ularatide is a synthetic form of urodilatin, a member of the family of atrial natriuretic peptides. Urodilatin is synthesized in renal distal tubular cells and plays an important role in sodium and water excretion. Early studies in patients with HF have demonstrated favourable haemodynamic effects and possible increased diuresis and natriuresis with ularatide.80 In SIRIUS I and SIRIUS II studies, three doses of ularatide were compared to placebo among patients hospitalized for AHF. Mitrovic et al.81 showed that ularatide lowered cardiac filling pressures and improved dyspnoea without early deteriorating effects on renal performance in ADHF patients. Only 5% of patients in the active group presented hypotension. Large-scale clinical studies are required in order to evaluate ularatide’s effects on symptomatic improvement and outcomes in ADHF.81
Relaxin
Relaxin is a natural peptide that was first identified as a reproductive hormone. It has been shown to play a major role in the haemodynamic and renal adjustments that occur during pregnancy. The actions of relaxin include the production of NO, VEGF, matrix metalloproteinases, and inhibition of endothelin and angiotensin II. These actions promote systemic and renal vasodilatation and increased arterial compliance.82 The recognition of these physiological properties of relaxin has led to its evaluation as a pharmacological agent for the treatment of AHF, which is characterized by vasoconstriction and vasomotor nephropathy. The Preliminary study of RELAX in Acute Heart Failure (Pre-RELAX-AHF) assessed the efficacy and safety of relaxin in patients hospitalized with AHF, mild to moderate renal insufficiency, signs of volume overload, and elevated plasma concentrations of BNP or NT-proBNP. Administration of relaxin showed a trend towards a potential improvement in persistent dyspnoea and prevention of worsening HF compared to placebo.83 The most effective dose of relaxin (30 µg/kg/24 h) is being tested for its efficacy on dyspnoea relief and its intermediate-term outcomes in the ongoing phase III RELAX-AHF study. Relaxin might prove to be a novel pleiotropic vasodilator for the treatment of patients with AHF and preserved or elevated blood pressure.84
Istaroxime, a luso-inotropic agent
Given the limitations of current inotropic drugs, several novel agents with inotropic action are under investigation for the treatment of ADHF, including istaroxime and cardiac myosin activators.
Istaroxime is a new i.v. agent with inotropic and lusitropic properties related to modulation of calcium cycling through inhibition of the N+-K+-ATPase and simultaneously activation of the sarcoplasmatic reticular calcium adenosine triphosphate isoform 2a (SERCA 2a). Preclinical studies and clinical trials indicate that combining N+-K+-ATPase inhibition and SERCA2a activation may increase myocardium contractility (inotropy) and facilitate active relaxation (lusitropy), improving both systolic and diastolic functions, without increasing heart rate and oxygen consumption.85 HORIZON-HF is a randomized controlled trial that evaluated the short-term effects of istaroxime in patients hospitalized with AHF syndromes and left ventricular ejection fraction ≤35%. It demonstrated that istaroxime decreases pulmonary capillary wedge pressure, increases systolic blood pressure, and decreases diastolic stiffness. Nevertheless, istaroxime is far from a proven therapy at this stage and ongoing clinical trials will determine whether this agent lives up to its initial therapeutic promises.86
Omecamtiv mecarbil, a cardiac myosin activator
Omecamtiv mecarbil is a small-molecule, selective cardiac myosin activator, which accelerates the transition of myosin into the force-generating state without affecting myocyte calcium homeostasis. Animal models and initial clinical studies have shown that omecamtiv mecarbil improves cardiac function by increasing the duration of ejection without changing the rates of contraction and without increasing oxygen consumption.87 Teerlink is the chair of the executive committee for the ongoing randomized phase IIb trial, the Acute Treatment with Omecamtiv Mecarbil to increase Contractility-Acute Heart Failure (ATOMIC-AHF), which evaluates an i.v. formulation of this agent in AHF. However, phase III clinical trials are required before it becomes an established therapy.
Vasopressin antagonists
Arginine vasopressin (AVP) or antidiuretic hormone is secreted from the posterior pituitary gland in response to diminished arterial volume or hyperosmolality. It acts through three types of receptors: V1A, V1B, and V2. V2 receptors are located in the distal renal tubules and the collecting duct and cause vasoconstriction and water reabsorption through aquaporin channels in the tubules. In HF, secretion of AVP may be enhanced due to low blood pressure or decreased arterial volume. Excess AVP can cause low serum sodium levels. Selective V2 antagonists (vaptans), such as tolvaptan and conivaptan, can effectively induce aquaresis and increase the serum sodium in those that are hyponatraemic.88
Some studies have reported a strong aquaretic effect without renal impairment, in patients with ADHF treated with tolvaptan. In the ACTIV trial (the Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist trial), patients with AHF showed a greater reduction in body weight and urine output and a slight increase in serum sodium levels at 24 hours when treated with tolvaptan compared to those receiving placebo or standard therapy.89 The much larger trial EVEREST (Efficacy of Vasopressin Antagonist in Heart Failure Outcome Study with Tolvaptan) showed that the early administration of vasopressin antagonists decreased mean body weight and improved dyspnoea. Nevertheless, the long-term outcomes did not differ between vasopressin antagonist and the placebo groups. These data suggest that vaptans when initiated in the context of ADHF can modify kidney response to water retention. But still they do not favourably influence remodelling of heart and kidneys and have no effect on long-term mortality.90,91 A recent study by Udelson et al. showed that tolvaptan monotherapy without concomitant furosemide therapy reduced body weight when compared to placebo, without adverse effects on serum electrolytes during a sodium-restricted diet.92
Adenosine antagonists
Adenosine is produced in the renal tubules by the breakdown of ATP and ADP during the energy-requiring process of sodium excretion. In conditions such as HF, sodium excretion increases due to diuretic therapy and therefore adenosine concentrations rise, exert antinatriuretic properties, and serve as a regulator trying to restore the balance between energy supply and demand. The increased serum adenosine levels observed in HF can promote diuretic resistance and deterioration of renal function. When tubular glomerular filtration is impaired, adenosine is released and binds to type-A1 receptors causing constriction of the afferent arterioles. This deteriorates renal blood flow and GFR and enhances sodium reabsorption by the proximal tubules. A1 adenoside receptor antagonists are new promising agents that block adenosine type-A1 receptors, improve renal blood flow, facilitate diuresis, and increase sodium excretion.28
The efficacy of adenosine A1 receptor antagonists in the management of patients with HF has not been established yet. The results of the first clinical studies seem to be quite controversial. Gottlieb et al. showed that the addition of BG9719 (A1 adenoside antagonist) to furosemide in patients with HF and volume overload, significantly increased diuresis and prevented a decline in kidney function.26 On the other hand, the PROTECT study showed that rolofylline did not meet neither the primary efficacy endpoints (dyspnoea improvement) nor the secondary efficacy endpoints (death, cardiovascular or renal rehospitalization, persistent renal impairment), while the overall safety profiles of the placebo and rolofylline groups were similar (rolofylline was associated with higher incidence of seizure and a trend towards a higher incidence of stroke).93 In addition, the REACH UP trial, a multicentre, randomized, double-blind, placebo-controlled study, did not demonstrate any clear benefit of rolofylline on clinical status or renal function in patients with ADHF and recent or acute WRF. Although there were fewer deaths or rehospitalizations at 60 days in the rolofylline-treated patients, the numbers were small and did not reach statistical significance.94 Thus, further large-scale studies are required in order to evaluate their net effect on renal function and potential cardiovascular outcomes.
Conclusion
Volume overload is a major reason for hospital admission in patients with ADHF. Despite the substantial progress in both management and outcomes of patients with CHF, little has changed in the management and outcomes in ADHF. Diuretics alone or in combination with vasodilators or inotropes according to systolic blood pressure levels and peripheral perfusion status remains the cornerstone in the management of volume overload. In resistant cases, ultrafiltration can lead to effective removal of isotonic fluid preventing new episodes of acute decompensation. Because volume overload is the common clinical phenotype of a heterogenous group of conditions, it is of great importance to personalize therapeutic approach for each patient according to his/her symptoms, clinical presentation, haemodynamic status, and severity of the underlying disease. Finally, proper education of patients by primary care physicians and HF specialists providing instructions for daily weight measurements, regulation of fluid status, and dietary restrictions can prevent new episodes of volume overload and hospital admissions.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest: The authors declare that there is no conflict of interest.
References
- 1. Nieminen MS. Pharmacological options for acute heart failure syndromes: current treatments and unmet needs. Eur Heart J Suppl 2005; 7(Suppl B): B20–B24 [Google Scholar]
- 2. Dec GW. Management of acute decompensated heart failure. Curr Probl Cardiol 2007; 32(6): 321–366 [DOI] [PubMed] [Google Scholar]
- 3. Gheorghiade M, Filippatos G, De LL, et al. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med 2006; 119: S3–S10 [DOI] [PubMed] [Google Scholar]
- 4. The SOLVD Investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1992; 325: 293–302 [DOI] [PubMed] [Google Scholar]
- 5. Gottlieb SS, Abraham W, Butler J, et al. The prognostic importance of different definitions of worsening renal function in congestive heart failure. J Card Fail 2002; 8: 136–141 [DOI] [PubMed] [Google Scholar]
- 6. Nohria A, Tsang SW, Fang JC, et al. Clinical assessment of identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol 2003; 41: 1797–1804 [DOI] [PubMed] [Google Scholar]
- 7. Adams K, Fonarow G, Emerman C, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2005; 149: 209–216 [DOI] [PubMed] [Google Scholar]
- 8. Nieminen MS, Bohm M, Cowie MR, et al. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J 2005; 25: 384–416 [DOI] [PubMed] [Google Scholar]
- 9. Heywood T, Fonarow G, Costanzo MR, et al. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE data base. J Card Fail 2007; 13: 422–430 [DOI] [PubMed] [Google Scholar]
- 10. Heywood T. The cardiorenal syndrome: lessons from the ADHERE database and treatment options. Heart Fail Rev 2004; 9: 195–201 [DOI] [PubMed] [Google Scholar]
- 11. Dries DL, Exner DV, Domaski MJ, et al. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol 2000; 35: 681–689 [DOI] [PubMed] [Google Scholar]
- 12. Sarraf M, Masoumi A, Schrier R. Cardiorenal syndrome in acute decompensated heart failure. Clin J Am Soc Nephrol 2009; 4: 2013–2026 [DOI] [PubMed] [Google Scholar]
- 13. Testani J, McCauley B, Chen J, et al. Worsening renal function defined as an absolute increase in serum creatinine is a biased metric for the study of the cardio-renal interactions. Cardiology 2010; 116: 206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu PP. Cardiorenal syndrome in heart failure: a cardiologist’s perspective. Can J Cardiol 2008; 24 (Suppl B): 25B–29B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mullens W, Abrahams Z, Francis G, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009; 53: 589–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schrier RW. Body fluid volume regulation in health and disease: a unifying hypothesis. Ann Intern Med 1990; 113: 155–159 [DOI] [PubMed] [Google Scholar]
- 17. Schrier R. Role of diminished renal function in cardiovascular mortality. Marker or pathogenetic factor? J Am Coll Cardiol 2006; 47: 1–8 [DOI] [PubMed] [Google Scholar]
- 18. Schrier R, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med 1999; 341: 577–578 [DOI] [PubMed] [Google Scholar]
- 19. Brewster UC, Perazella MA. The renin-angiotensin-aldosterone system and the kidney: effects on kidney disease. Am J Med 2004; 116: 263–272 [DOI] [PubMed] [Google Scholar]
- 20. Hirsch AT, Pinto YM, Schunkert H, et al. Potential role of the tissue renin-angiotensin system in the pathophysiology of congestive heart failure. Am J Cardiol 1990; 66: 22D–30D [DOI] [PubMed] [Google Scholar]
- 21. Heymes C, Bentall JK, Ratajczak P, et al. Inreased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol 2003; 41: 2164–2171 [DOI] [PubMed] [Google Scholar]
- 22. Bongartz LG, Cramer MJ, Doevendans PA, et al. The severe cardiorenal syndrome: ‘Gyton revisited’. Eur Heart J 2005; 26: 11–17 [DOI] [PubMed] [Google Scholar]
- 23. Weber K. Mechanisms of disease: aldosterone in chronic heart failure. N Engl J Med 2001; 345: 1689–1697 [DOI] [PubMed] [Google Scholar]
- 24. Converse RL, Jacobsen TN, Toto RD, et al. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med 1992; 327: 1912–1918 [DOI] [PubMed] [Google Scholar]
- 25. Lee CR, Watkins ML, Patterson JH, et al. Vasopressin: a new target for the treatment of heart failure. Am Heart J 2003; 146: 9–18 [DOI] [PubMed] [Google Scholar]
- 26. Gottlieb SS, Brater DC, Thomas I, et al. BG9719 (CVT-124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy. Circulation 2002; 105: 1345–1353 [DOI] [PubMed] [Google Scholar]
- 27. Shlipak MG. Pharmacotherapy for heart failure in patients with renal insufficiency. Ann Intern Med 2003; 138: 917–924 [DOI] [PubMed] [Google Scholar]
- 28. Francis G. Acute decompensated heart failure: the cardiorenal syndrome. Clev Clin J Med 2006; 73 (Suppl 2): S8–S13 [DOI] [PubMed] [Google Scholar]
- 29. Howlett J. Current treatment options for early management in acute decompensated heart failure. Can J Cardiol 2008; 24 (Suppl B): 9B–14B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arnold JM, Howlett JG, Dorian P, et al. Canadian Cardiovascular Society Consensus Conference recommendations on heart failure update 2007: prevention, management during intercurrent illness or acute decompensation and use of biomarkers. Can J Cardiol 2007; 23: 21–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adams KF, Lindenfeld J, Arnold JMO, et al. ; Heart Failure Society of America. HFSA 2006 comprehensive heart failure guideline. J Card Fail 2006; 12: e1–e122 [DOI] [PubMed] [Google Scholar]
- 32. Lucas C, Johnson W, Hamilton MA, et al. Freedom from congestion predicts good survival despite previous class IV symptoms of heart failure. Am Heart J 2000; 140: 840–847 [DOI] [PubMed] [Google Scholar]
- 33. Bart B. Treatments of congestion in congestive heart failure: ultrafiltration is the only rational initial treatment of volume overload in decompensated heart failure. Circ Heart Fail 2009; 2: 499–504 [DOI] [PubMed] [Google Scholar]
- 34. Yancy CW, Lopatin M, Stevenson LW, et al. ; ADHERE Scientific Advisory Committee and Investigators. Clinical presentation, management and in- hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) database. J Am Coll Cardiol 2006; 47: 76–84 [DOI] [PubMed] [Google Scholar]
- 35. Salvador D, Ray N, Ramos G, et al. Continuous infusion versus bolus injection of loop diuretics in congestive heart failure. Cochrane Database Syst Rev 2004; 1: CD003178 [DOI] [PubMed] [Google Scholar]
- 36. Brater DC. Diuretic therapy. N Engl J Med 1998; 339: 387–395 [DOI] [PubMed] [Google Scholar]
- 37. Costanzo MR, Heywood JT, DeMarco T, et al. Impact of renal insufficiency and chronic diuretic therapy on outcome and resource utilization in patients with acute decompensated heart failure. J Am Coll Cardiol 2004; 43 (Suppl 1): A180 (abstract). [Google Scholar]
- 38. Geisberg C, Butler J. Addressing the challenges of cardiorenal syndrome. Clev Clin J Med 2006; 73(5): 485–491 [DOI] [PubMed] [Google Scholar]
- 39. Kramer BK, Schweda F, Riegger GA. Diuretic treatment and diuretic resistance in heart failure. Am J Med 1999; 106: 90–96 [DOI] [PubMed] [Google Scholar]
- 40. Felker G, Lee K, Bull D, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011; 364: 797–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ronco C, Haapio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol 2008; 52(19): 1527–1539 [DOI] [PubMed] [Google Scholar]
- 42. Testani J, Chen J, McCauley B, et al. Potential effects of aggressive decongestion during treatment of decompensated heart failure on renal function and survival. Circulation 2010; 122(3): 265–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hensen J, Abraham WT, Durr JA, et al. Aldosterone in congestive heart failure: analysis of determinants and role in sodium retention. Am J Nephrol 1991; 11: 441–446 [DOI] [PubMed] [Google Scholar]
- 44. Pitt B, Zannad F, Remme W, et al. ; for the Randomized Aldactone Evaluation Study Investigators. Randomized Aldactone Evaluation Study (RALES). N Engl J Med 1999; 341: 709–717 [DOI] [PubMed] [Google Scholar]
- 45. Pitt B, Bakris G, Ruilope LM, et al. ; EPHESUS Investigators. Serum potassium and clinical outcomes in the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS). Circulation 2008; 118: 1643–1650 [DOI] [PubMed] [Google Scholar]
- 46. Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine. Arch Intern Med 2000; 160: 685–693 [DOI] [PubMed] [Google Scholar]
- 47. Kittleson M, Hurwitz S, Shah MR, et al. Development of circulatory-renal limitations to angiotensin-converting enzyme inhibitors identifies patients with severe heart failure and early mortality. J Am Coll Cardiol 2003; 41: 2029–2035 [DOI] [PubMed] [Google Scholar]
- 48. Ljungman S., Kjekshus J, Swedberg K. Renal function in severe congestive heart failure during treatment with enalapril (the Cooperative North Scandinavian Enalapril Survival Study Trial). Am J Cardiol 1992; 70: 479–487 [DOI] [PubMed] [Google Scholar]
- 49. Butler J, Forman DE, Abraham WT, et al. Relationship between heart failure treatment and development of worsening renal function among hospitalised patients. Am Heart J 2004; 147: 331–338 [DOI] [PubMed] [Google Scholar]
- 50. Marik PE. Low-dose dopamine: a systematic review. Intensive Care Med 2002; 28: 877–883 [DOI] [PubMed] [Google Scholar]
- 51. Lauschke A, Teichgraber UK, Frei U. Low-dose dopamine worsens renal perfusion in patients with renal failure. Kidney Int 2006; 69: 1669–1674 [DOI] [PubMed] [Google Scholar]
- 52. Aziz E, Alviar C, Herzog E, et al. Continuous infusion of frusemide combined with low-dose dopamine compared to intermittent boluses in acutely decompensated heart failure is less nephrotoxic and carries a lower readmission at thirty days. Hellenic J Cardiol 2011; 52: 227–235 [PubMed] [Google Scholar]
- 53. Giamouzis G, Butler J, Starling RC, et al. Impact of dopamine infusion on renal function in hospitalized heart failure patients: results of the Dopamine in Acute Decompensated Heart Failure (DAD-HF) Trial. J Card Fail 2010; 16 (12): 922–930 [DOI] [PubMed] [Google Scholar]
- 54. Elkayam U, Tassisa G, Binanay C, et al. Use and impact of inotropes and vasodiltory therapy in hospitalized patients with severe heart failure. Am Heart J 2007; 153: 98–104 [DOI] [PubMed] [Google Scholar]
- 55. Felker GM, O’Connor CM. Inotropic therapy for heart failure: an evidence-based approach. Am Heart J 2001; 142: 393–401 [DOI] [PubMed] [Google Scholar]
- 56. Dec G. Acute decompensated heart failure. The shrinking role of inotropic therapy. J Am Coll Cardiol 2005; 46(1): 65–67 [DOI] [PubMed] [Google Scholar]
- 57. Parissis JT, Farmakis D, Nieminen M. Classical inotropes and new cardiac enhancers. Heart Fail Rev 2007; 12: 149–156 [DOI] [PubMed] [Google Scholar]
- 58. Zager AR, Johnson AC, Lund S, et al. Levosimendan protects against experimental endotoxemic acute renal failure. Am J Physiol Renal Physiol 2006; 290(6): F1453–F1462 [DOI] [PubMed] [Google Scholar]
- 59. Parissis J, Adreadou I, Bistola V, et al. Novel biological mechanisms of levosimendan and its effect on the failing heart. Expert Opin Investig Drugs 2008; 17: 1–8 [DOI] [PubMed] [Google Scholar]
- 60. Follath F, Cleland JG, Just H, et al. Efficacy and safety of intravenous levosimendan compared with dobutanine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet 2002; 360: 196–202 [DOI] [PubMed] [Google Scholar]
- 61. Moiseyev VS, Poder P, Andrejevs N, et al. Safety and efficacy of a novel calcium sensitizer, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction. A randomized, placebo-controlled, double-blind study (RUSSLAN). Eur Heart J 2002; 23: 1422–1432 [DOI] [PubMed] [Google Scholar]
- 62. Coletta AP, Cleland JG, Freemantle N, et al. Clinical trials update from the European Society of Cardiology Heart Failure meeting: SHAPE, BRING-UP 2 VAS, COLA II, FOSIDIAL, BETACAR, CASINO and meta-analysis of cardiac resynchronisation therapy. Eur J Heart Fail 2004; 6: 673–676 [DOI] [PubMed] [Google Scholar]
- 63. Cleland JG, Freemantle N, Coletta AP, et al. Clinical trials update from the American Heart Association: REPAIR-AMI, ASTAMI, JELIS, MEGA, REVIVE-II, SURVIVE and PROACTIVE. Eur J Heart Fail 2006; 8: 105–110 [DOI] [PubMed] [Google Scholar]
- 64. Cleland JG, Swedberg K, Poole-Wilson PA. Successes and failures of current treatment of heart failure. Lancet 1998; 352 (Suppl 1): SI19–SI28 [DOI] [PubMed] [Google Scholar]
- 65. Elkayam U, Bitar F, Akhter MW, et al. Intravenous nitroglycerin in the treatment of decompensated heart failure: potential benefits and limitations. J Cardiovasc Pharm Ther 2004; 9: 227–241 [DOI] [PubMed] [Google Scholar]
- 66. Heart Failure Society of America Executive summary: HFSA 2006 comprehensive heart failure practice guideline. J Card Fail 2006; 12: 10–38 [DOI] [PubMed] [Google Scholar]
- 67. Marcus LS, Hart D, Packer M, et al. Hemodynamic and renal excretory effects of human brain natriuretic peptide infusion in patients with congestive heart failure. Circulation 1996; 94: 3184–3189 [DOI] [PubMed] [Google Scholar]
- 68. Abraham WT, Lowes BD, Ferguson DA, et al. Systemic hemodynamic, neurohormonal and renal effects of a steady-state infusion of human brain natriuretic peptide in patients with hemodynamically decompensated heart failure. J Card Fail 1998; 4: 37–44 [DOI] [PubMed] [Google Scholar]
- 69. Publication Committee for the VMAC Investigators (Vasodilation in the Management of Acute CHF) Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA 2002; 287: 1531–1540 [DOI] [PubMed] [Google Scholar]
- 70. Yancy CW, Singh A. Potential applications of outpatient nesiritide infusions in patients with advanced heart failure and concomitant renal insufficiency (from the Follow-Up Serial Infusions of Nesiritide [FUSION I] trial. Am J Cardiol 2006; 98: 226–229 [DOI] [PubMed] [Google Scholar]
- 71. Yancy CW, Crum H, Massie BM, et al. Safety and efficacy of out patient nesiritide in patients with advanced heart failure. Results of the serial infusion of nesiritide (FUSION II) trial. Circ Heart Fail 2008; 1: 9–16 [DOI] [PubMed] [Google Scholar]
- 72. Gensch C, Hoppe U, Bohm M, et al. Late-breaking trials presented at the American Heart Association Congress in Chicago 2010. Clin Res Cardiol 2011; 100(1): 1–9 [DOI] [PubMed] [Google Scholar]
- 73. Jaski BE, Miller D. Ultrafiltration in decompensated heart failure. Curr Heart Fail Rep 2005; 2: 148–154 [DOI] [PubMed] [Google Scholar]
- 74. Marenzi G, Lauri G, Grazi M, et al. Circulatory response to fluid overload removal by extracorporeal ultrafiltration in refractory congestive heart failure. J Am Coll Cardiol 2001; 38: 963–968 [DOI] [PubMed] [Google Scholar]
- 75. Bart BA, Boyle A, Banks AJ, et al. Ultrafiltration versus usual care for hospitalized patients with heart failure: the Relief for Acutely Fluid-Overloaded Patients With Decompensated Congestive Heart Failure (RAPID-CHF) trial. J Am Coll Cardiol 2005; 46(11): 2043–2046 [DOI] [PubMed] [Google Scholar]
- 76. Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007; 49: 675–683 [DOI] [PubMed] [Google Scholar]
- 77. Giglioli C, Cecchi E, Chiostri M, et al. Effects of ULTRAfiltration vs. DIureticS on clinical, biohumoral and hemodynamic variables in patients with deCOmpensated heart failure: the ULTRADISCO study. Eur J Heart Fail 2011; 13(3): 337–346 [DOI] [PubMed] [Google Scholar]
- 78. Bart BA, Goldsmith SR, Lee KL, et al. Cardiorenal rescue study in acute decompensated heart failure: rationale and design of CARRESS-HF for the Heart Failure Clinical Research Network. J Card Fail 2012; 18(3): 176–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Marasco SF, Lucas G, McDonald M, et al. Review of ECMO (extra corporeal membrane oxygenation) support in critically ill adult patients. Heart Lung Circ 2008; 17(Suppl 4): S41–S47 [DOI] [PubMed] [Google Scholar]
- 80. Luss H, Mitrovic V, Seferovic PM, et al. Renal effects of ularatide in patients with decompensated heart failure. Am Heart J 2008; 155: 1012. e1–e8. [DOI] [PubMed] [Google Scholar]
- 81. Mitrovic V, Seferovic PM, Simeunovic D, et al. Haemodynamic and clinical effects of ularitide in decompensated heart failure. Eur Heart J 2006; 27: 2823–2832 [DOI] [PubMed] [Google Scholar]
- 82. Teichman SL, Unemori E, Dschietzig T, et al. Relaxin, a pleiotropic vasodilator for the treatment of heart failure. Heart Fail Rev 2009; 14: 321–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Metra M, Teerlink JR, Felker GM, et al. Dyspnoea and worsening heart failure in patients with acute heart failure: results from the Pre-RELAX-AHF study. Eur J Heart Fail 2010; 12: 1130–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ponikowski P, Metra M, Teerlink JR, et al. Design of the RELAXin in acute heart failure study. Am Heart J 2012; 163(2): 149–155 [DOI] [PubMed] [Google Scholar]
- 85. Gheorghiade M, Ambrosy AP, Ferrandi M, et al. Combining SERCA2a activation and Na-K ATPase inhibition: a promising new approach to managing acute heart failure syndromes with low cardiac output. Discov Med 2011; 12(63): 141–151 [PubMed] [Google Scholar]
- 86. Shah SJ, Blair JE, Filippatos GS, et al. ; HORIZON-HF Investigators. Effects of istaroxime on diastolic stiffness in acute heart failure syndromes: results from the Hemodynamic, Echocardiographic and Neurohormonal Effects of Istaroxime, a Novel Intravenous Inotropic and Lusitropic Agent: a Randomized Controlled Trial in Patients Hospitalized with Heart Failure (HORIZON-HF) trial. Am Heart J 2009; 157(6): 1035–1041 [DOI] [PubMed] [Google Scholar]
- 87. Cleland JG, Teerlink JR, Senior R, et al. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet 2011; 378(9792): 676–683 [DOI] [PubMed] [Google Scholar]
- 88. Nielson S, Chou CL, Marples D, et al. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci USA 1995; 92: 1013–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gheorghiade M, Gattis WA, O’Connor CM, et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial. JAMA 2004; 291: 1963–1971 [DOI] [PubMed] [Google Scholar]
- 90. Gheorghiade M, Konstam MA, Jr, Burnett JC, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST clinical status trials. JAMA 2007; 297: 1332–1343 . [DOI] [PubMed] [Google Scholar]
- 91. Konstam MA, Gheorghiade M, Jr, Burnett JC, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial. JAMA 2007; 297: 1319–1331 [DOI] [PubMed] [Google Scholar]
- 92. Udelson JE, Bilsker M, Hauptman PJ, et al. A multicenter, randomized, double-blind, placebo-controlled study of tolvaptan monotherapy compared to furosemide and the combination of tolvaptan and furosemide in patients with heart failure and systolic dysfunction. J Card Fail 2011; 17(12): 273–281 [DOI] [PubMed] [Google Scholar]
- 93. Weatherley BD, Cotter G, Dittrich HC, et al. ; for the PROTECT steering committee, Investigators and Coordinators. Design and rationale of the PROTECT study: a placebo-controlled randomized study of the selective A1 adenosine receptor antagonist rolofylline for patients hospitalized with acute decompensated heart failure and volume overload to assess treatment effect on congestion and renal function. J Card Fail 2010; 16: 25–35 [DOI] [PubMed] [Google Scholar]
- 94. Gottlieb SS, Givertz MM, Metra M, et al. The effects of adenosine A1 receptor antagonism in patients with acute decompensated heart failure and worsening renal function: the REACH UP study. J Card Fail 2010; 16(9): 714–719 [DOI] [PubMed] [Google Scholar]