Abstract
This case vignette relates the unknown association between systemic capillary leak syndrome, namely Clarkson’s syndrome, and acute cardiac dysfunction. ‘Central extra-corporeal life support (ECLS)’ was needed for the management of an intractable cardiogenic shock. The acute cardiac condition completely resolved within few days. Pathology showed diffuse interstitial edema within the myocardium suggestive of cardiac involvement of the disease.
Keywords: Systemic capillary leak syndrome, cardiogenic shock
Introduction
Systemic capillary leak syndrome (SCLS) or Clarkson’s syndrome is a rare, unexplained and severe condition1 characterized by acute episodes of generalized edema associated with hypotension, hemoconcentration and hypoproteinemia. A sudden increase in capillary permeability that produces extravasations of fluid and plasmatic proteins into the extravascular space is the hallmark of this disease. Vascular endothelial growth factor and angiopoietin-2 were recently found elevated in sera samples from SCLS patients during acute attacks but not in remission sera. These cytokines are thought to promote transient microvascular endothelial barrier breakdown.2 Prophylactic treatment of the acute attacks is supportive and relies on adequate fluid resuscitation. Prophylactic treatment with β2-agonists or intravenous immunoglobulin have been found to reduce the frequency and severity of attacks.3 Visceral localization of the disease has not been thoroughly described yet. We, here, describe the first case of cardiogenic shock in a young patient with a history of repetitive hypovolemic shock related to Clarkson’s syndrome. Severe left ventricular (LV) systolic dysfunction completely recovered with extra-corporeal life support (ECLS). Myocardial biopsies provided findings highly suggestive of myocardial involvement of the Clarkson’s syndrome.
Case description
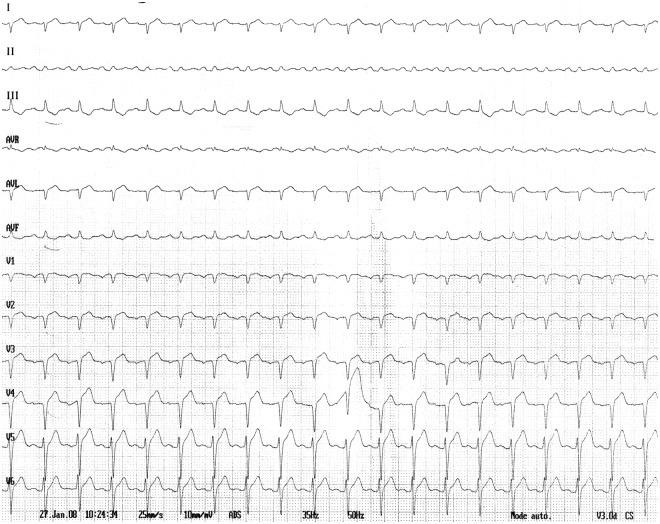
A 41-year-old man with a history of SCLS evolving for several years was admitted to the intensive care unit (ICU) because of chest pain associated with electrocardiogram changes. Over the previous days he was treated with intravenous human immunoglobulins, as usual for a mild exacerbation of the disease. On admission systolic blood pressure was 100 mm Hg, heart rate 90 beats per minute and body temperature 37°C. The electrocardiogram showed ST-segment elevation in anterior leads and QS pattern in leads V1–V4 without reciprocal changes (Figure 1). Chest X-ray was normal. Left cardiac catheterism was carried out immediately and showed normal coronary arteries.
Figure 1.
Electrocardiogram at admission shows low voltage with ST-segment elevation in anterior leads and QS pattern in leads V1–V4 without reciprocal changes.
Transthoracic echocardiogram revealed severely reduced global LV systolic function (LV ejection fraction 20%) associated with right ventricular systolic dysfunction (Video 1(A),1(B)). Of note cardiac chambers were not enlarged but LV wall thickness was mildly increased (septal and posterior LV wall end-diastolic thicknesses were measured at 14 mm). Results of laboratory tests (Table 1) disclosed a mild cardiac troponin I increase (4.85 ng/L, N <0.1). One hour after admission, the patient developed circulatory collapse (systolic blood pressure at 60 mm Hg with cold extremities) requiring administration of dobutamine and norepinephrine in increasing doses. As the low output state was unresponsive to plasma expanders and inotropic therapy, the patient underwent emergency mechanical cardiac support with a ECLS (Figure 2). Through a median sternotomy, a complete circulatory support was achieved with a complete discharge of left and right cardiac chambers. Two cannulae were inserted into the left ventricle through the left atrium and into the right atrium respectively. The blood was drained to an oxygenator and then returned to the ascending aorta in order to achieve an antegrade systemic perfusion. Cardiac index was maintained over 2.2 l/min/m2. While LV ejection fraction was less than 10% at postoperative day 1, LV ejection fraction increased unexpectedly to near normal values at postoperative day 2 (Video 2). After ECLS removal at Day 7 the patient slowly recovered and received broadspectrum antimicrobial therapy for a fever of unknown origin. Delayed enhancement cardiac magnetic resonance imaging did not detect any myocardial necrosis. The patient was finally discharged at Day 17. Electrocardiogram at discharge showed increase in voltage, normalization of QRS pattern and repolarization (Figure 3). Transthoracic echocardiography examination (apical four chamber view) at discharge showed near normalization of LV function (Video 3).
Table 1.
Laboratory data on admission in intensive care unit.
| B-type natriuretic peptide (pg/mL) | 1828 (N<100) |
| Cardiac troponin I (ng/mL) | 4.85 (N<0.05) |
| Creatine phosphokinase (UI/L) | 493 (N<195) |
| C-reactive protein (mg/L) | 31 (N<6) |
| Creatinine (mg/L) | 14 (N<13) |
| Urea (g/L) | 0.6 (N<0.5) |
| Sodium (mmol/L) | 131 (N>135) |
| Glucose (g/L) | 1.12 (N<1.0) |
| Aspartate transaminase (UI/L) | 41 (N<40) |
| Alanine transaminase (UI/L) | 16 (N<40) |
| Leucocytes (10.9/L) | 12.42 (N:4.0–10.0) |
| Hemoglobin (g/dL) | 14.6 (N:13.0–17.0) |
| Platelets (10.9/L) | 203 (N:150–400) |
| Fibrinogen (g/L) | 4.4 (N:2–4) |
| D-dimers (ng/mL) | 502 (N<500) |
| Total cholesterol (g/L) | 1.34 (N<2.4) |
| Triglycerides (g/L) | 4.5 (N<1.5) |
| Thyroid-stimulating hormone (microUI/mL) | 3.4 (N: 0.4–3.6) |
| Procalcitonin (ng/mL) | 0.1 (N<2) |
Figure 2.
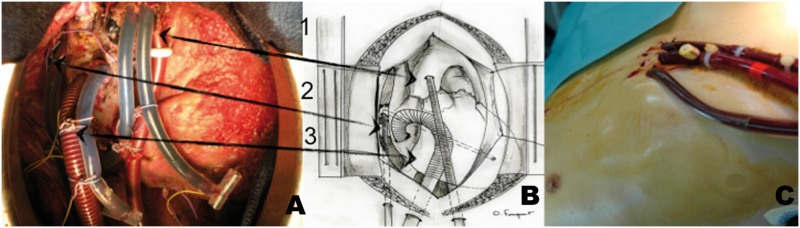
(A) and (B) ‘Central extra-corporeal life support (ECLS)’ perioperative view (1-aorta, 2-left ventricle cannula implanted through the right pulmonary vein and the mitral valve, 3-venous cannula implanted in the right atrium). (C) ‘Central extra-corporeal life support (ECLS)’ aspect after closure of the chest.
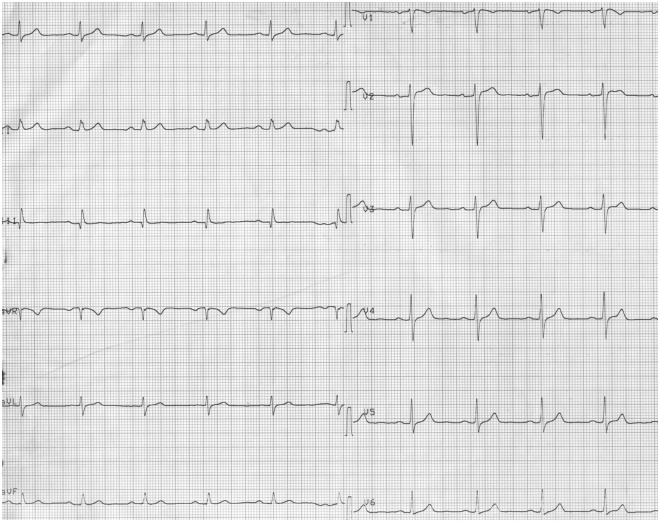
Figure 3.
Electrocardiogram at discharge showed increase in voltage, normalization of QRS pattern and repolarization.
Two myocardial biopsies were sampled during ECLS implantation, one from the interventricular septum and the other from the left ventricle free wall. Histopathological examination found diffuse interstitial edema without inflammatory infiltrates and myocyte necrosis (Figure 4(A) and (B)). Stress exercise echocardiography performed two months later did not reveal any abnormality. While the patient had several crises during four years of follow-up he did not experience any evidence of LV failure. In order to prevent the risk of heart failure recurrence due to the CLS, it was decided to maintained intravenous injection of immunoglobulin at a dose of 1g/kg every 4–6 weeks. The outcome was uneventful over 48 months follow-up except for a mild episode of hemoconcentration.
Figure 4.
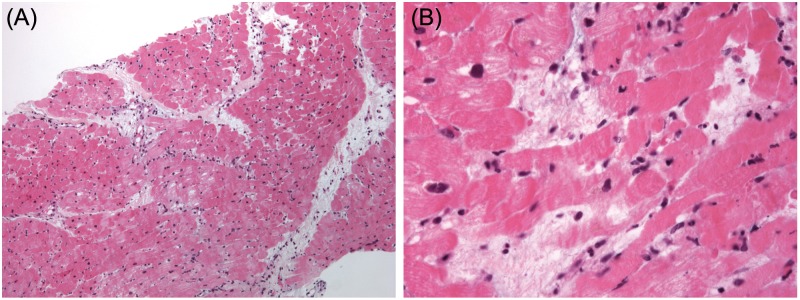
(A) Endomyocardial biopsy specimen. The microphotograph shows diffuse interstitial edema, without inflammatory infiltrates and myocyte necrosis. Contraction bands are artefactual. Interstitial edema results in myocardial wall thickening. Magnification: x 100; stain: hematoxylin-eosin. (B) Endomyocardial biopsy specimen. At higher magnification, dissociation of myocytes junctions is visible. Consequently, interstitial edema caused by capillary permeability results in systolic dysfunction. There are no inflammatory infiltrates nor myocyte necrosis which explains reversibility of myocardial dysfunction. Contraction bands are artefactual. Magnification: x 400; stain: hematoxylin-eosin.
Discussion
Myocardial involvement in Clarkson’s syndrome has been previously reported in only two cases on the basis of echocardiographic findings.4,5 Claessens et al. observed reversible biventricular wall thickening, while contractility remained normal.4 In contrast, both myocardial wall thickening and severe systolic dysfunction were observed in our case. Endomyocardial biopsies showed diffuse interstitial edema, without inflammatory infiltrates and myocyte necrosis. Of note, endomyocardial biopsies were performed because cardiac failure was believed to have been related to myocarditis. Histological findings and clinical course allowed the ruling out of both a myocarditis and a shock-related myocardial infarction. Enlargement of interstitial spaces is frequent in endomyocardial biopsies because of artefactual changes. However, in our case this could be correlated to wall thickening. Dissociation of myocytes junctions caused by edema resulted in systolic dysfunction.
The extreme severity of the cardiac dysfunction required an urgent mechanical cardiac support with an ECLS. Modified ‘central’ implantation of ECLS is performed in our institution because it allows for better left ventricle decompression and thereby prevents pulmonary edema that is a potential complication of peripheral ECLS. This modified technique of cardiac assistance also precludes limb ischemia which is another severe drawback of peripheral ECLS.
Lastly, immunoglobulin was administered intravenously just prior to the circulatory collapse of our patient and might be involved in its pathogenesis. However, there are no other reports in the literature to suggest that this is likely.3 Of note, after discharge immunoglobulin infusions were administered in our patient on a monthly basis without any cardiac complications over a 48-month period.
To our knowledge this is the first case of cardiogenic shock associated with Clarkson’s syndrome, likely due to a sudden increase of extravascular fluid within the myocardial wall. Endomyocardial biopsies proved useful in ruling out a myocarditis. In addition, this case also illustrates successful aggressive management, using ECLS, of a life-threatening organ dysfunction.
Supplementary Material
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest: None declared.
References
- 1. Kapoor P, Greipp PT, Schaefer EW, et al. Idiopathic systemic capillary leak syndrome (Clarkson’s disease): the Mayo clinic experience. Mayo Clin Proc 2010; 85: 905–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xie Z, Ghosh CC, Patel R, et al. Vascular endothelial hyperpermeability induces the clinical symptoms of Clarkson disease (the systemic capillary leak syndrome). Blood 2012; 119: 4321–4332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gousseff M, Arnaud L, Lambert M, et al. Capillary leak syndrome registry. The systemic capillary leak syndrome: a case series of 28 patients from a European registry. Ann Intern Med 2011; 154: 464–471 [DOI] [PubMed] [Google Scholar]
- 4. Claessens YE, Joly LM, Cariou A, et al. Acute reversible cardiac involvement associated with systemic capillary leak syndrome. Intensive Care Med 1999; 25: 334–335 [DOI] [PubMed] [Google Scholar]
- 5. Guillaume M, Tolsma V, Colombe B, et al. Idiopathic systemic capillary leak syndrome: a case report with cardiac involvement. Rev Med Interne 2011; 32: e69–71 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.