Abstract
Spontaneous coronary artery dissection is a rare cause of acute presentations to the catheter laboratory. Often, the angiographic findings are subtle and may be mistaken for a plaque rupture. We descibe a case where repeat presentation revealed the diagnosis of recurrent spontaneous coronary artery dissection.
Keywords: Acute coronary syndrome, coronary angiography, myocardial infarction
Introduction
Spontaneous coronary artery dissection (SCAD) is a rare event. It may never present clinically or only become apparent as an incidental finding. Commonly, the dissection plane can restrict coronary flow and be a cause of myocardial ischaemia, presenting with symptoms, signs, and diagnostic findings of an acute coronary syndrome. The clinician needs to be alert to the possible diagnosis or it may be missed. We present a case of woman, with no predisposing condition, where repeat presentations with ischaemic events were only diagnosed as recurrent SCAD when serial angiography was able to demonstrate healing. The literature is also discussed.
Case presentation
A 56-year-old woman was admitted to our institution following a 30-min history of severe central chest pain that was not relieved by sublingual nitrates. She reported the sudden, unexpected death of her partner 12 hours prior to the onset of pain. Her initial ECG revealed lateral ST-segment elevation with reciprocal changes in the inferior leads.
In accordance with standard practice, she underwent emergent coronary angiography with a view to perform primary angioplasty. As with the operator’s standard practice, each coronary injection was preceded by intracoronary administration of 500 µg isosorbide dinitrate. The left main stem and left anterior descending artery were angiographically normal. The circumflex/obtuse marginal system was severely spastic in its proximal course with angiographic evidence of spontaneous dissection. TIMI-3 flow was maintained in the distal vessel (Figure 1). The right coronary artery was dominant and angiographically normal. Left ventricular function was maintained.
Figure 1.
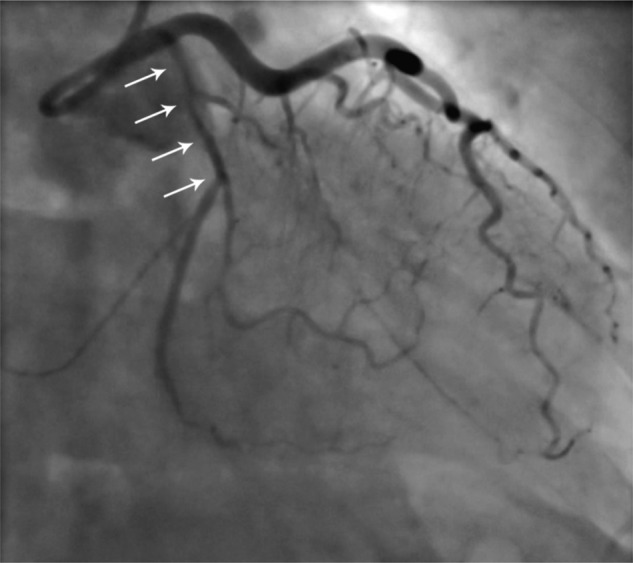
Coronary angiogram (right anterior oblique caudal projection) in October 2010, demonstrating dissection of the proximal circumflex vessel (white arrows), with extension into the vessel beyond.
She had a background history of hypertension and a family history of coronary artery disease. She was a non-smoker and denied cocaine use. Enquiry revealed that this was her third presentation with similar symptoms.
Two years prior she had presented with cardiac chest pain, evolved septal T-wave inversion, and raised cardiac markers. At the time, coronary angiography revealed a distally tapered left anterior descending artery but unobstructed circumflex and right coronary arteries (Figure 2). No suggestion of spasm was seen. Due to the presumed small size of the target vessel on angiography, the decision was made not to undertake intervention and to manage the patient conservatively. The presumed plaque rupture was medically treated with dual antiplatelet therapy, an angiotensin-converting enzyme inhibitor, and a beta-blocker. The diagnosis of coronary dissection was not considered. She made a good recovery.
Figure 2.
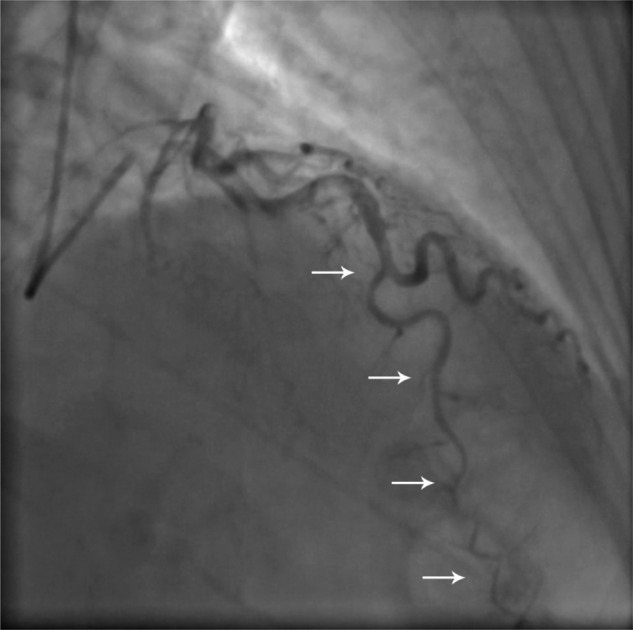
Coronary angiogram (right anterior oblique cranial projection) in August 2008, demonstrating dissection of the left anterior descending artery in its midcourse (white arrows).
Nineteen months later she presented for the second time with chest pain and inferior ST-elevation. Thrombolysis had failed to resolve her symptoms or ECG changes and emergency angiography was arranged with a view to proceeding with rescue angioplasty, which at that time was at a separate institution. This demonstrated functional occlusion of a small posterior left ventricular branch of the right coronary artery with no evidence of spasm (Figure 3). Images of the previous angiogram were not available at the time, but the left anterior descending artery disease noted previously was not seen. In light of the presumed small size of vessel, management at that time had also been conservative, again with no mention of possible spontaneous dissection.
Figure 3.
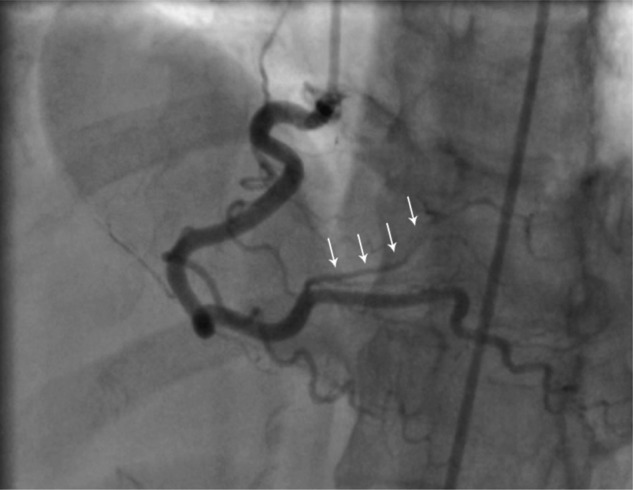
Coronary angiogram (left anterior oblique cranial projection) in March 2010 of the right coronary artery, demonstrating dissection of the posterior lateral branch (white arrows).
With this historical information available, and also with the knowledge of previous angiographic appearances during earlier presentations, a decision was made to manage this latest presentation medically with high-dose calcium channel blockers, in addition to continuation with dual antiplatelet therapy. She made an uneventful recovery. Cardiac biomarkers 24 h after presentation revealed a peak creatine kinase of 1000 IU/l and troponin I of 35.3 µg/l.
Follow-up diagnostic coronary angiography was undertaken 8 weeks later. The circumflex dissection had resolved and there was no evidence of residual disease in other vessels (Figures 4–6). Left ventriculography was normal. She had made a full clinical recovery with no residual symptoms.
Figure 4.
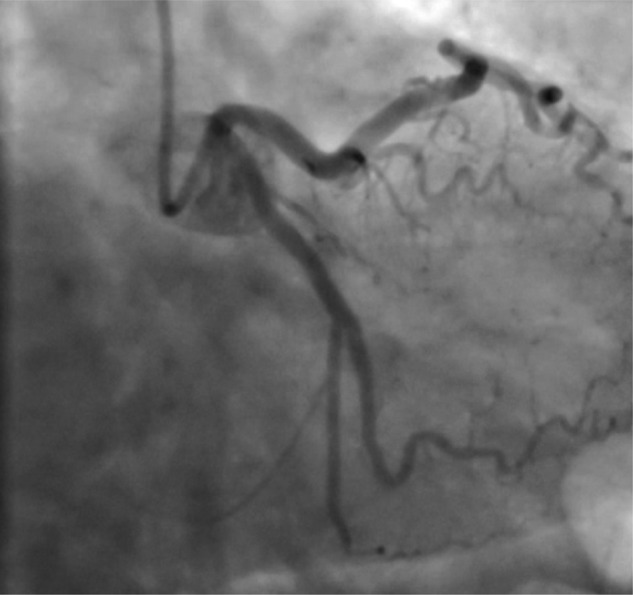
Coronary angiogram in December 2010, demonstrating healing of the circumflex artery dissection
Figure 6.
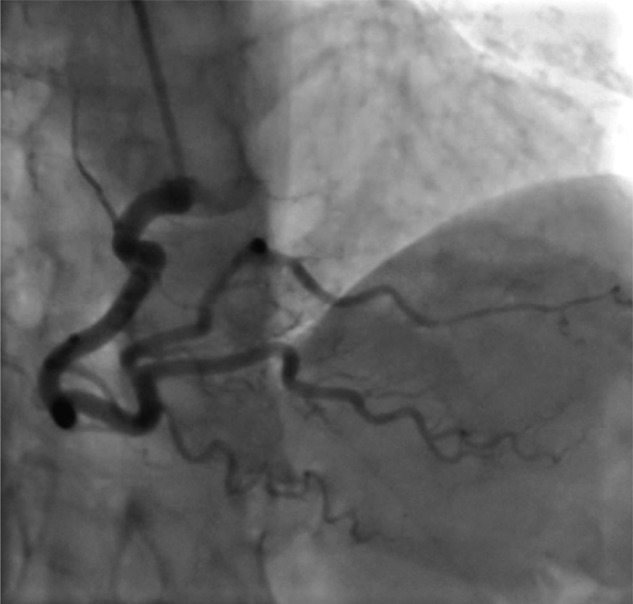
Coronary angiogram in December 2010, demonstrating healing of the right coronary artery dissection
Figure 5.
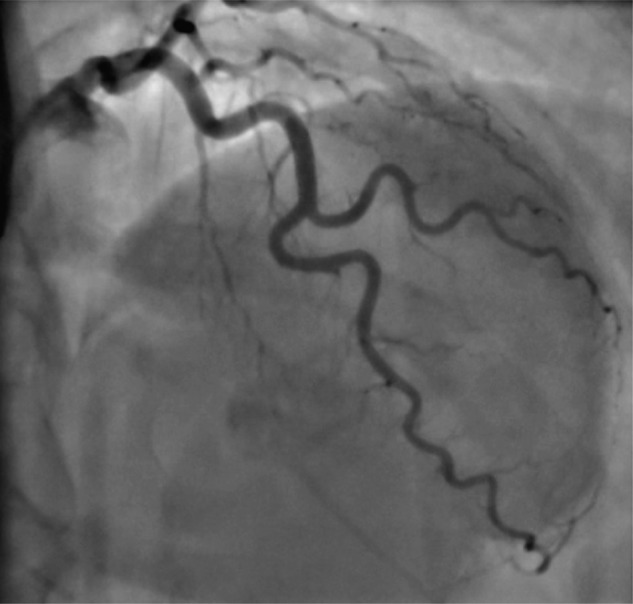
Coronary angiogram in December 2010, demonstrating healing of the left anterior descending artery dissection.
Discussion
Coronary dissection results in separation of the layers of the arterial wall. The false lumen, created by a dissection plane, can be between the intima and media, or the media and adventitia. Compression of the vessel lumen, resulting from haemorrhage and haematoma between the outer media and external elastic lamina, has long been documented in the pathological literature.1 This rare condition as a cause for presentation with myocardial ischaemic syndromes is increasingly recognized as such patients undergo coronary angiography early in the course of their management. The angiographic findings can be graded A–F according to the National Heart, Lung, and Blood Institute classification system developed by the Coronary Angioplasty Registry: type A and B dissections demonstrate filling defects on contrast injection but have no or minimal persistence of contrast after the dye has cleared; type C dissections appear as dye staining in an extraluminal cap; type D as a spiral luminal defect; type E as persistent luminal defects; and type F as total luminal occlusion.2 A long eccentric stenosis may be thought to be plaque, when in actual fact the angiographic findings may be due to SCAD and intramural haematoma/thrombus may actually be present, particularly when present in an otherwise normal coronary tree. The clinician needs to be alert to this fact, especially in a patient that does not fit a typical coronary presentation. Intravascular ultrasound can be particularly helpful in these circumstances, allowing differentiation between atherosclerotic plaque and coronary dissection with intramural haematoma.3,4
SCAD was first described following a post mortem on a woman with sudden cardiac death.5 Since that time, several case reports and reviews have discussed the diagnosis. The true incidence is unknown, not least because not all cases presenting with a compatible history are investigated with the gold standard coronary angiography. Of all patients who are referred for angiography, spontaneous dissection is present in between 0.07% and 1.1%, with a more recent database analysis suggesting an incidence of up to 10% in women <50 years old presenting with ACS.6–8
The commonest associated pathologies of SCAD are atherosclerosis and peripartum vascular changes. Hypertension has been seen in both pregnant and non-pregnant cases, although a causal association is not clear.9 Other causes are connective tissue disease, systemic lupus erythematosus vasculitis, cocaine abuse, vigorous exercise, and prolonged sneezing.10 Coronary artery spasm has been associated with SCAD.11
The incidence of recurrent SCAD is unknown. In a retrospective review of cases associated with pregnancy, 20% surviving the initial event had evidence of further episodes at follow up.12 The first case of non-pregnancy-related recurrent SCAD was reported following the post mortem of a 40-year-old man.13 A further case reports a 45-year-old woman with a history of medically managed inferior infarction, secondary to right coronary artery dissection, presenting 3 years later with SCAD of the left anterior descending artery. Although no risk factors for SCAD were identified in that case, beta-blockers had been discontinued 2 weeks earlier.14 A third such case reports a 30-year-old female suffering an anterolateral myocardial infarction caused by spontaneous left main stem and left anterior descending artery dissection, managed with coronary artery bypass grafting (CABG) with venous conduits after internal mammary grafts had spontaneously dissected. Histopathology of the internal mammary arteries showed cystic medial necrosis. She had 2 further presentations relating to dissection affecting native coronary vessels.15 A recent case series of spontaneous dissection reports recurrent dissection occurring in up to 50% of cases within 1 month of initial presentation, although no clear events occurring chronically.16
No specific management guidance exists and this must be individualized to each case. Strategies include medical therapy, percutaneous coronary intervention or CABG surgery. Angiographic resolution is frequently reported following medical management, which includes heparin, antiplatelets, and anti-ischaemic medication such as beta-blockers and nitrates. Fibrinolytics should be avoided. Percutaneous coronary intervention can be considered where there is marked major vessel involvement and ongoing ischaemia; appropriate technical care is needed to avoid operating in the false lumen or propagating the dissection distally. Multivessel or left main SCAD should be managed with CABG.10 Coronary artery spasm can be managed with calcium channel blockers.17
Conclusion
Our case report of recurrent spontaneous coronary artery dissection in three different coronary arteries in a 56-year-old-woman is important for a number of reasons. First, it serves to remind us that not all acute chest pain presentations are due to plaque rupture. Second, it highlights the need to gather as much historical clinical information as is possible, even during emergency treatment, before embarking on a course of management. Third, it illustrates that, rarely, SCAD can be recurrent in different coronary territories in the same individual, but often complete healing is possible.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors report no conflict of interest.
References
- 1. Lovitt WV, Jr, Corzine WJ., Jr Dissecting intramural hemorrhage of anterior descending branch of left coronary artery. AMA Arch Pathol 1952; 54: 458–462 [PubMed] [Google Scholar]
- 2. Coronary artery angiographic changes after PTCA: manual of operations. NHLBI PTCA Registry 1985–6; 9 [Google Scholar]
- 3. Maehara A, Mintz GS, Castagna MT, et al. Intravascular ultrasound assessment of spontaneous coronary artery dissection. Am J Cardiol 2002; 89: 466–468 [DOI] [PubMed] [Google Scholar]
- 4. Arnold JR, West NE, van Gaal WJ, et al. The role of intravascular ultrasound in the management of spontaneous coronary artery dissection. Cardiovasc Ultrasound 2008; 6: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pretty HC. Dissecting aneurysm of coronary artery in a woman aged 42: rupture. BMJ 1931; 1: 667 [Google Scholar]
- 6. Mortensen KH, Thuesen L, Kristensen IB, et al. Spontaneous coronary artery dissection: a Western Denmark Heart Registry study. Catheter Cardiovasc Interv 2009; 74: 710–717 [DOI] [PubMed] [Google Scholar]
- 7. Hering D, Piper C, Hohmann C, et al. Prospective study of the incidence, pathogenesis and therapy of spontaneous, by coronary angiography diagnosed coronary artery dissection. Z Kardiol 1998; 87: 961–970 [DOI] [PubMed] [Google Scholar]
- 8. Vanzetto G, Berger-Coz E, Barone-Rochette G, et al. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J Cardiothorac Surg 2009; 35: 250–254 [DOI] [PubMed] [Google Scholar]
- 9. Basso C, Morgagni GL, Thiene G. Spontaneous coronary artery dissection: a neglected cause of acute myocardial ischemia and sudden death. Heart 1996; 75: 451–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vrints CJM. Spontaneous coronary artery dissection. Heart 2010; 96: 801–808 [DOI] [PubMed] [Google Scholar]
- 11. Mark DB, Kong Y, Whalen RE. Variant angina and spontaneous coronary artery dissection. Am J Cardiol 1985; 56: 485–486 [DOI] [PubMed] [Google Scholar]
- 12. Koller PT, Cliffe CM, Ridley DJ. Immunosuppressive therapy for peripartum-type spontaneous coronary artery dissection: case report and review. Clin Cardiol 1998; 21: 40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van der Bel-Kahn J. Recurrent primary coronary artery dissecting aneurysm (hematoma). Am J Clin Pathol 1982; 78: 394–398 [DOI] [PubMed] [Google Scholar]
- 14. Eddinger J, Dietz WA. Recurrent spontaneous coronary artery dissection. Catheter Cardiovasc Interv 2005; 66: 566–569 [DOI] [PubMed] [Google Scholar]
- 15. Harris JL, Brereton J, Lim CS, et al. Recurrent spontaneous coronary artery dissection: a case report and review of the literature. Int J Angiol 2007; 16: 109–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kansara P, Graham S. Spontaneous coronary artery dissection: case series with extended follow up. J Invasive Cardiol 2011; 23: 76–80 [PubMed] [Google Scholar]
- 17. Kamran M, Guptan A, Bogal M. Spontaneous coronary artery dissection: case series and review. J Invasive Cardiol 2008; 20: 553–559 [PubMed] [Google Scholar]


