Abstract
Aims:
Negative T waves in precordial leads often occur in patients with acute coronary syndrome (ACS), but are also found in acute pulmonary embolism (APE) and Takotsubo cardiomyopathy (TC). Because the clinical features of these two diseases mimic those of ACS, differential diagnosis is essential to select an appropriate treatment strategy improve outcomes. This study aimed to clarify the differences in negative T waves among ACS, APE and TC.
Methods and results:
We studied admission ECGs in 300 patients (198 patients with ACS caused by the left anterior descending coronary artery disease, 81 with APE and 21 with TC). All patients were admitted within 48 h from symptom onset and had negative T waves ≥1.0 mm without ST-segment elevation in leads V1 to V4. The number and maximal amplitude of negative T waves were greatest in patients with TC, followed by in those with ACS, and were lowest in patients with APE (p < 0.001, respectively). The prevalence of negative T waves significantly differed in all 12 leads among the three groups (p < 0.01, respectively). Negative T waves in both leads III and V1 identified APE with 90% sensitivity and 97% specificity. Negative T waves in lead –aVR (i.e., positive T waves in lead aVR) and no negative T waves in lead V1 identified TC with 95% sensitivity and 97% specificity. These values represented the highest diagnostic accuracies.
Conclusion:
The distributions of negative T waves differed among ACS, APE and TC, and these differences were useful for differentiating among these three diseases.
Keywords: Electrocardiogram, acute coronary syndrome, pulmonary embolism, Takotsubo cardiomyopathy
Introduction
Negative T waves are common electrocardiographic changes in patients with non-ST-segment elevation acute coronary syndrome (ACS).1-4 In particular, negative T waves in the precordial leads suggest severe ischemia of the left ventricular anterior wall due to a critical stenosis of the left anterior descending coronary artery (LAD).5-7 However, this electrocardiographic finding is also frequently observed in patients with acute pulmonary embolism (APE), especially in those at risk for adverse outcomes.8-12 Symptoms of APE, such as chest pain/discomfort or dyspnea, are often difficult to differentiate from those of ACS. Misdiagnosis of APE as ACS has been linked to preventable deaths in patients with APE.13,14 Furthermore, Takotsubo cardiomyopathy (TC) is a recently recognized novel cardiac syndrome characterized by new electrocardiographic abnormalities (ST-segment elevation, negative T waves), elevated cardiac enzymes and transient left ventricular apical ballooning without obstructive coronary disease. Symptoms of TC, such as chest pain/discomfort or dyspnea, have been also shown to be similar to those of ACS.15-17 In most patients with TC, left ventricular dysfunction improves rapidly and dramatically, and the prognosis is generally considered favorable.15-17 There is a risk of misdiagnosing TC as ACS in patients with negative T waves on initial electrocardiogram (ECG), potentially leading to inappropriate treatment at presentation and subsequently.
APE and TC should thus be included in the differential diagnosis of ACS in patients who have precordial negative T waves at initial presentation. Prompt differentiation among these three diseases is essential to ensure selection of an appropriate management strategy and thus improve outcomes. Cardiac troponin is a highly sensitive and specific marker of myocardial damage, and its role in risk stratification is well established in ACS.5 However, elevated cardiac troponin levels are found in patients with severe APE10,18,19 and are most commonly elevated in TC,15,17 suggesting limited value for differentiating among these three diseases. Echocardiography is likely to be useful for differential diagnosis, but has several technical limitations, and fully assessable echocardiographic images are often not obtained. Furthermore, the results of echocardiographic evaluation are not always readily available at the time of presentation. The 12-lead ECG is a simple, prompt, inexpensive, and most widely available initial clinical diagnostic examination. This study sought to assess the value of negative T waves for differentiating among ACS, APE and TC.
Methods
We retrospectively studied 300 consecutive patients (198 with ACS, 81 with APE and 21 with TC) who were admitted to our coronary care unit between May 1998 and June 2011 and fulfilled the following criteria: (1) admission within 48 h after the onset of symptoms such as chest pain/discomfort, dyspnea or other ischemic symptoms;5 (2) no conditions precluding the evaluation of ST-segment changes on ECG (i.e. complete left or right bundle branch block, left ventricular hypertrophy, ventricular pacing or receiving drugs with potential effects on ECG); (3) no obvious past history of cardiopulmonary disease; and (4) fully assessable ECG on admission with negative T waves of at least 1.0 mm in two or more contiguous precordial leads (V1 to V4). Patients with new ST-segment elevation of at least 2.0 mm in two contiguous precordial leads on admission ECG were excluded. The study was approved by the Ethics Committee of our institution, and all subjects gave informed consent.
Patients with ACS who had an unstable pattern of symptoms, including rest, new-onset, or increasing angina were studied.5 All patients underwent cardiac catheterization during hospitalization. The culprit lesion was defined as the lesion associated with angiographic findings suggesting local thrombus, the most severe lesion, or both, and it was confirmed to be located in the LAD. The diagnosis of APE was confirmed by one or more of the following examinations: pulmonary angiography, lung perfusion scintigraphy or computed tomographic scan. The patients with TC showed the following features:15,17 (1) transient hypokinesis, akinesis or dyskinesis of the left ventricular apical segment (and midventricular segment) with regional wall-motion abnormalities extending beyond a single epicardial vascular distribution; (2) the absence of significant (>50%) obstructive coronary artery disease or angiographic evidence of acute plaque rupture; (3) new electrocardiographic abnormalities (T wave inversions); and (4) the absence of pheochromocytoma or myocarditis. All patients with TC underwent cardiac catheterization or coronary computed tomography angiography during hospitalization.
Twelve-lead ECGs were recorded on admission at a paper speed of 25 mm/s and an amplification of 10 mm/mV. All ECGs were examined by a single cardiologist who was blinded to all other clinical data. ST-segment deviation was measured manually to the nearest 0.5 mm at the J point. A negative T wave was considered present if the depth was at least 1.0 mm. In this study, the anatomically contiguous Cabrera sequence (III, aVF, II, –aVR, I and aVL) was used to display the limb leads, as recommended in current international recommendations for the clinical interpretation of ECG.6 We also analyzed the following admission electrocardiographic findings, previously shown to be associated with APE:8,9 (1) P pulmonale, P waves ≥2.5 mm in limb leads or >1.5 mm in lead V1; (2) right axis deviation, QRS electrical axis >90°; (3) left axis deviation, QRS electrical axis ≤–30°; (4) S1S2S3 pattern, the presence of S waves ≥1.5 mm in leads I, II and III; (5) S1Q3T3 pattern, the presence of S waves in lead I and Q waves in lead III, each having amplitudes >1.5 mm, in association with negative T waves in lead III; (6) low voltage, greatest overall deflection of the QRS complex ≤5.0 mm in all limb leads; and (7) clockwise rotation, a shift in the transition zone (R=S) in the precordial leads to V5 or beyond.
A qualitative assay for cardiac-specific troponin T (Roche Diagnostics, Tokyo, Japan; detection limit, 0.1 ng/ml of cardiac-specific troponin T) was performed on admission. Troponin T ≥0.1 ng/ml was defined as positive.
Statistical analysis
Continuous data are expressed as mean values ± standard deviation, and categorical data are expressed as numbers and percentages. Analysis of variance was used to compare continuous variables. Chi-square analysis was used to compare categorical variables. Differences were considered statistically significant at p < 0.05. The data were analyzed using SPSS, version 20.0 (SPSS, Inc., Chicago, IL).
Results
The baseline characteristics of the subjects are shown in Table 1. Patients with TC tended to be older and less frequently admitted within 24 h from symptom onset, but the differences did not reach statistical significance. Patients with ACS were more likely to be men and had a higher systolic blood pressure on admission as well as higher prevalences of diabetes mellitus, hypertension, hyperlipidemia and smoking. Heart rate on admission was highest in patients with APE, followed by those with TC and was lowest in patients with ACS. Patients with TC had more frequently positive troponin T on admission. In patients with ACS, 34% were multivessel disease, 75% were proximal LAD disease, and 25% were distal LAD disease. Severe stenosis of ≥90% was observed in 68% of patients with proximal LAD disease, and in 88% of those with distal LAD disease.
Table 1.
Baseline characteristics
| ACS (n=198) | APE (n=81) | TC (n=21) | p value | |
|---|---|---|---|---|
| Age (years) | 67 ± 10 | 64 ± 14 | 70 ± 12 | 0.05 |
| Men (%) | 122 (62) | 26 (32) | 5 (24) | <0.001 |
| Admission within 24 h from symptom onset (%) | 152 (77) | 60 (74) | 12 (57) | 0.14 |
| Systolic blood pressure on admission (mmHg) | 151 ± 26 | 123 ± 28 | 126 ± 17 | <0.001 |
| Heart rate on admission (beats/minute) | 71 ± 14 | 95 ± 19 | 85 ± 18 | <0.001 |
| Diabetes mellitus (%) | 47 (24) | 9 (11) | 3 (14) | 0.045 |
| Hypertension (%) | 128 (65) | 33 (41) | 9 (43) | 0.001 |
| Hyperlipidemia (%) | 90 (46) | 9 (11) | 4 (19) | <0.001 |
| Smoking (%) | 94 (48) | 6 (7) | 3 (14) | <0.001 |
| Positive troponin T on admission (%) | 89 (45) | 31 (50)* | 18 (86) | 0.002 |
Data are presented as mean values + SD or numbers (percentages) of patients. *Available in 62 patients.
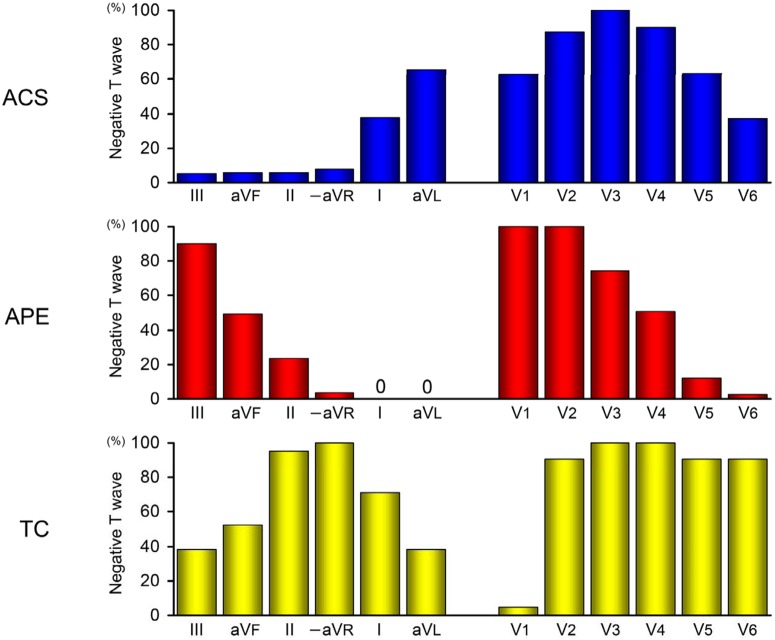
Electrocardiographic findings on admission are presented in Table 2. APE was more frequently associated with P pulmonale, S1S2S3 pattern and clockwise rotation. Right axis deviation and S1Q3T3 pattern were observed in only APE. Low voltage was more frequent in APE and TC. However, the frequencies of these findings were low. There was no significant difference in left axis deviation among the three groups. ACS was more frequently associated with ST-segment depression as well as greater summed ST-segment depression. The number and maximal amplitude of negative T waves were greatest in patients with TC, followed by those with ACS and were lowest in patients with APE. The prevalence of negative T waves in the three groups is shown in Figure 1. The frequencies of negative T waves significantly differed in each of the 12 leads among the 3 groups (p = 0.004 for lead V2 and p < 0.001 for the remaining 11 leads). In the limb and precordial leads, the distribution of negative T waves obviously differed among the three groups. In the limb leads, negative T waves were frequently observed in leads I and aVL, particularly in the latter, and were rare in inferior leads and lead –aVR in patients with ACS. In contrast, the prevalence of negative T waves gradually decreased from leads III to –aVR, and negative T waves were not found in lead I or aVL in patients with APE, whereas a high prevalence of negative T waves centered around lead –aVR in patients with TC. In the precordial leads, the distribution of negative T waves centered around lead V3 in patients with ACS. In contrast, negative T waves were consistently observed in leads V1 and V2, and their prevalence gradually decreased from leads V3 to V6 in patients with APE, whereas a high prevalence of negative T waves was noted in precordial leads except for lead V1 in patients with TC. Negative T waves in both leads III and V1 were present in 90% of patients with APE, but only in 3% of patients with ACS or TC (p < 0.001). Negative T waves in lead –aVR (i.e. positive T waves in lead aVR) and no negative T waves in lead V1 were observed in 95% of patients with TC in contrast to only 3% of patients with ACS or APE (p < 0.001). Table 3 shows the sensitivities, specificities, positive and negative predictive values and predictive accuracies of electrocardiographic findings for the diagnosis of APE. P pulmonale, right and left axis deviation, S1S2S3 and S1Q3T3 patterns, low voltage and clockwise rotation were specific, but not sensitive for APE. No negative T waves in lead I, aVL or V6 and negative T waves in lead V1 were very sensitive for APE, but the specificities of these findings were relatively low. Negative T waves in lead III was highly predictive of APE; furthermore, the diagnostic accuracy of this finding combined with negative T waves in lead V1 was very high, representing the highest predictive accuracy. Table 4 shows the sensitivities, specificities, positive and negative predictive values and predictive accuracies of electrocardiographic findings for the diagnosis of TC. Negative T waves in lead –aVR (i.e. positive T waves in lead aVR) and no negative T waves in lead V1 were highly predictive of TC, but the positive predictive values of these findings were low. However, the combination of these two findings resulted in the highest ability to differentiate TC. For other continuous electrocardiographic variables not shown in the table, no cut-off points were found to discriminate among the three groups. Figure 2 shows representative ECGs for one patient from each group.
Table 2.
Electrocardiographic findings
| ACS (n=198) | APE (n=81) | TC (n=21) | p value | |
|---|---|---|---|---|
| P pulmonale (%) | 8 (4) | 10 (12) | 0 | 0.014 |
| Right axis deviation (%) | 0 | 6 (7) | 0 | <0.001 |
| Left axis deviation (%) | 12 (6) | 5 (6) | 3 (14) | 0.35 |
| S1S2S3 pattern (%) | 3 (2) | 11 (14) | 1 (5) | <0.001 |
| S1Q3T3 pattern (%) | 0 | 20 (25) | 0 | <0.001 |
| Low voltage (%) | 8 (4) | 23 (28) | 6 (29) | <0.001 |
| Clockwise rotation (%) | 1 (1) | 21 (26) | 1 (5) | <0.001 |
| ST-segment depression ≥0.5 mm (%) | 115 (58) | 27 (33) | 6 (29) | <0.001 |
| Summed ST-segment depression (mm) | 1.7 ± 2.3 | 0.9 ± 2.2 | 0.9 ± 1.8 | 0.017 |
| Number of negative T waves | 5.7 ± 1.6 | 4.8 ± 1.8 | 8.6 ± 1.8 | <0.001 |
| Maximal negative T wave (mm) | 4.6 ± 3.4 | 3.4 ± 1.8 | 7.0 ± 3.0 | <0.001 |
Data are presented as mean values ± SD or numbers (percentages) of patients.
Figure 1.
Prevalence of negative T waves in patients with ACS, APE and TC.
Table 3.
Predictive values of electrocardiographic variables for the diagnosis of APE.
| Predictor | Sensitivity | Specificity | PPV | NPV | Predictive accuracy |
|---|---|---|---|---|---|
| P pulmonale | 12%** | 96% | 56%** | 75%** | 74%** |
| Right axis deviation | 7%** | 100%* | 100% | 74%** | 75%** |
| Left axis deviation | 6%** | 93%* | 25%** | 73%** | 70%** |
| S1S2S3 pattern | 14%** | 98% | 73%* | 75%** | 75%** |
| S1Q3T3 pattern | 25%** | 100%* | 100% | 78%** | 80%** |
| Low voltage | 28%** | 94% | 62%** | 78%** | 76%** |
| Clockwise rotation | 26%** | 99% | 91% | 78%** | 79%** |
| Neg T in lead III | 90% | 92%* | 80% | 96% | 91% |
| No Neg T in lead I | 100%** | 41%** | 39%** | 100% | 57%** |
| No Neg T in lead aVL | 100%** | 63%** | 50%** | 100%* | 73%** |
| Neg T in lead V1 | 100%** | 43%** | 39%** | 100% | 58%** |
| No NegT in lead V6 | 98% | 43%** | 39%** | 98% | 57%** |
| Neg T in both leads III and V1 | 90% | 97% | 92% | 96% | 95% |
Neg T, negative T wave; Pos T, positive T wave; PPV, positive predictive value; NPV, negative predictive value.
p < 0.05, **p < 0.01 versus Neg T in both leads III and V1.
Table 4.
Predictive values of electrocardiographic variables for the diagnosis of TC.
| Predictor | Sensitivity | Specificity | PPV | NPV | Predictive accuracy |
|---|---|---|---|---|---|
| Pos T in lead aVR (= Neg T in lead -aVR) | 100% | 93%# | 53% | 100% | 94%# |
| No Neg T in lead V1 | 95% | 73%## | 21%## | 99.5% | 75%## |
| Pos T in lead aVR and no Neg T in lead V1 | 95% | 97% | 74% | 99.6% | 97% |
Neg T, negative T wave; Pos T, positive T wave; PPV, positive predictive value; NPV, negative predictive value.
p < 0.05, ## p < 0.01 versus Pos T in lead aVR and no Neg T in lead V1.
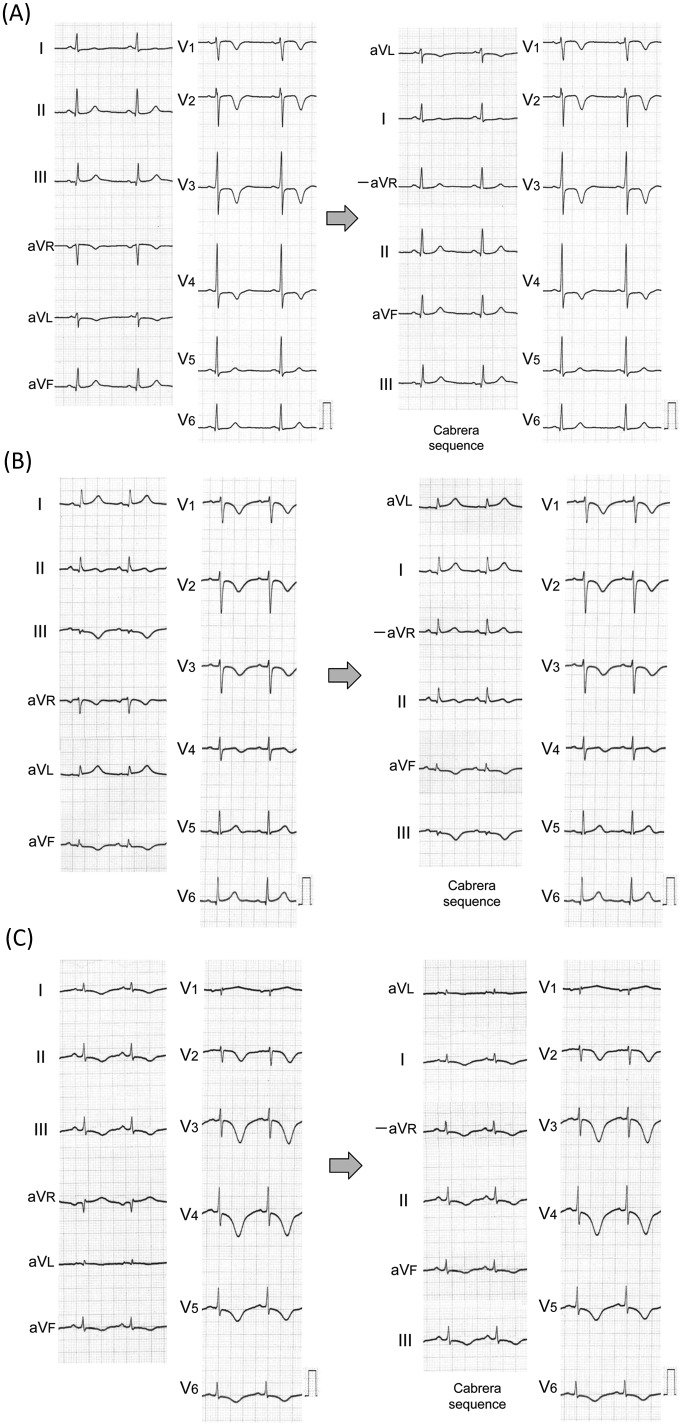
Figure 2.
Representative ECGs of ACS, APE and TC.
(A) ACS. Negative T waves were observed in leads aVL and V1 to V4. When the limb leads were displayed according to the anatomically contiguous Cabrera sequence, negative T waves were seen in only lead aVL, which faces the upper lateral region of the left ventricle. Coronary angiography revealed 90% stenosis of the proximal lesion of the LAD. (B) APE. Negative T waves were observed in leads III, aVF and V1 to V4. When the limb leads were displayed according to the Cabrera sequence, the amplitude of negative T waves was greatest in lead III, which faces the inferior region of the right ventricle, and smaller in lead aVF. T wave was slightly inverted in lead II. A computed tomographic scan of the chest showed multiple filling defects in the main right and left pulmonary arteries. Lung perfusion scintigraphy revealed filling defects in the right upper, right middle and left upper lung fields. (C) TC. Negative T waves were observed in leads I, II, III, aVF and V2 to V6. In lead aVR, positive T waves were observed. When the limb leads were displayed according to the Cabrera sequence, negative T waves were broadly distributed in all leads except for lead aVL, which faces the upper lateral region of the left ventricle. Echocardiography showed transient akinesis of the left ventricular apical and mid-ventricular segments, and coronary angiography revealed no significant coronary stenosis.
Discussion
This study included only patients with ACS caused by LAD disease, APE and TC who were admitted within 48 h from symptom onset and had precordial negative T waves without ST-segment elevation at presentation. In this setting, the distribution of negative T waves obviously differed among these three diseases, and these differences were useful for differential diagnosis. Negative T waves in both leads III and V1 strongly suggested APE, and negative T waves in lead –aVR (i.e. positive T waves in lead aVR) combined with no negative T waves in lead V1 strongly suggested TC. The clinical presentations of these three diseases often overlap, but these electrocardiographic criteria could simply but accurately differentiate APE or TC from ACS. The reasons why negative T waves are differently distributed among ACS, APE and TC are uncertain, but may reflect the differences in underlying electrophysiologic mechanisms among the three diseases.
Negative T waves in ACS
In patients with non-ST-segment elevation ACS, T wave changes have been shown to be more common than ST-segment changes.1,3 Negative T waves can actually be preceded by transient ST-segment elevation, which is present in leads facing the site of ischemia.6 Therefore, the ischemia-related artery and its perfusion territory may be predicted on the basis of the distribution of negative T waves. In patients with ACS caused by LAD disease in this study, negative T waves were distributed primarily around leads V2 to V4 in the precordial leads, facing the anterior region of the left ventricle, and in lead aVL in the limb leads, facing the lateral region of the left ventricle. Negative T waves in lead V1 were observed in 63% of these patients. Lead V1 is considered to reflect the right paraseptal region, often supplied by the septal branch of the LAD. Negative T waves in this lead may suggest severe ischemia in the interventricular septum caused by more proximal LAD disease.20 In this study, the majority (75%) of patients with ACS had proximal LAD disease. Conversely, the prevalence of negative T waves in lead –aVR and inferior leads was very low. Lead –aVR (+30°) bridges the gap between lead I (0°) and lead II (60°); in other words, lead –aVR faces the apical region of the left ventricle.6 The perfusion territory of the LAD might not extend to the inferior as well as the apical regions of the left ventricle,21 resulting in less negative T waves in leads facing these regions.
Negative T waves in APE
The electrocardiographic manifestations of APE include rhythm disturbances and changes in P waves, QRS complexes or T waves.8 These abnormalities have been shown to be highly variable and frequently transient.8 In the present study, electrocardiographic findings associated with APE, such as P pulmonale, right and left axis deviation, S1S2S3 and S1Q3T3 patterns, low voltage and clockwise rotation were specific, but not sensitive for APE. On the other hand, negative T waves are known to be the most common, persistent change in patients with APE.8,10 Previous studies have suggested that severe ischemia of the right ventricle may result from an acute right ventricular pressure overload, impaired coronary blood flow and hypoxia caused by APE, possibly leading to negative T waves.8,12 In the present study, negative T waves in leads III, V1 and V2 were very common in patients with APE. Lead III faces the inferior region of the right ventricle, and leads V1 and V2 face the anterior region of the right ventricle. With increasing severity of right heart failure and dilation of the right ventricle towards the left owing to limited pericardial expansion, negative T waves are thought to move towards the left, i.e. from leads III to aVF to II in the limb leads and from leads V1 to V6 in the precordial leads. Negative T waves were rare in leads –aVR, V5 and V6, and were not found in leads I and aVL. These findings are probably ascribed to the fact that dilation of the right ventricle in APE rarely extends to the regions faced by these leads.
We have previously shown that negative T waves in both leads III and V1 could differentiate APE from ACS in patients who had precordial negative T waves at presentation.9 The results of the present study confirm and extend our previous findings. This simple electrocardiographic criterion may help to correctly differentiate APE from not only ACS, but also TC.
Negative T waves in TC
In TC, negative T waves commonly appear after the resolution of initial ST-segment elevation.15 Previous studies have reported that negative T waves are already found on ECG at presentation in an appreciable number of patients with TC.16,17 Electrocardiographic changes in TC have been shown to be similar to those in anterior acute myocardial infarction.15,22-24 The development of negative T waves after reperfused acute myocardial infarction has been attributed to viable but sympathetically denervated myocardium, because sympathetic denervation delays repolarization.25 We have recently shown that negative T waves progressively developed in both TC and reperfused anterior acute myocardial infarction during the subacute phase and were especially prominent in the former, suggesting that TC might be associated with more viable but sympathetically denervated myocardium.24 In the present study, TC was also associated with a greater amplitude and higher prevalence of negative T waves, as compared with ACS and APE. In addition, negative T waves were more broadly distributed around lead –aVR in the limb leads and the precordial leads except lead V1. These findings are probably ascribed to the fact that wall motion abnormalities in TC are centered around the apical region of the left ventricle faced by lead –aVR and less frequently extend to the regions faced by lead V1, i.e. the right ventricular anterior region as well as the right paraseptal region.26-28 Moreover, less negative T waves in lead V1 may be attributed to another reason: TC, but not ACS caused by LAD disease or APE, is usually associated with wall motion abnormalities in the posterolateral region,29 resulting in negative T waves in this region. This finding is reflected in the appearance of positive T waves in the opposing lead V1. We have previously shown that during the acute phase, TC is characterized by ST-segment elevation in lead –aVR and no ST-segment elevation in lead V1.23 During the subacute phase, it is plausible that these findings were reflected in negative T waves in lead –aVR and no negative T waves in lead V1.24 The underlying reasons remain speculative, but these electrocardiographic findings most accurately predicted TC in the present study.
Extent of negative T waves
In patients with non-ST-segment elevation ACS, previous studies showed that merely the presence of negative T waves has little prognostic value as compared with ST-segment depression.4 However, quantitative T wave analysis can provide additional predictive information about clinical outcomes. The number of leads with negative T waves of ≥6, indicating more extensive ischemia, was associated with adverse outcomes in several large clinical trials.2,3 In patients with APE, negative T waves in precordial leads (V1 to V4) have been shown to correlate with worse outcomes.10 We have previously demonstrated that an increasing number of leads with negative T waves is associated with poorer short-term clinical outcomes in patients with APE.11 Thus, patients with diffuse negative T waves are thought to be at high risk in ACS or APE. However, in the present study, the distribution of negative T waves was most extensive in patients with TC, whose prognosis is generally considered favorable. These findings may strengthen the clinical importance of differentiating among these three diseases in patients who present with precordial negative T waves.
Limitations
This study was retrospective and performed at a single center. Furthermore, we studied a small number of patients who met strict inclusion criteria to ensure a homogenous group of subjects. Consequently, our findings may not be able to be extrapolated to a general group of patients with ACS, APE and TC. Caution is required because we retrospectively studied only ECGs with precordial negative T waves. Furthermore, clinically significant coronary stenosis may have been present in some of the patients with APE. However, the diagnosis of APE was based on standardized criteria in all patients. Therefore, further diagnostic workup, particularly invasive coronary angiography to exclude concomitant coronary artery disease, was not deemed appropriate. Further prospective studies in larger numbers of patients are required to confirm our findings.
Conclusion
In patients with ACS, APE and TC who had precordial negative T waves without ST-segment elevation on ECG at presentation, focusing on T wave changes in leads III, aVR and V1 can help to differentiate among these three diseases.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest: The authors declare no conflicts of interest in preparing this article.
References
- 1. O’Donoghue M, Boden WE, Braunwald E, et al. Early invasive vs conservative treatment strategies in women and men with unstable angina and non-ST-segment elevation myocardial infarction: a meta-analysis. JAMA 2008; 300: 71–80 [DOI] [PubMed] [Google Scholar]
- 2. Damman P, Holmvang L, Tijssen JG, et al. Usefulness of the admission electrocardiogram to predict long-term outcomes after non-ST-elevation acute coronary syndrome (from the FRISC II, ICTUS, and RITA-3 [FIR] Trials). Am J Cardiol 2012; 109: 6–12 [DOI] [PubMed] [Google Scholar]
- 3. Jacobsen MD, Wagner GS, Holmvang L, et al. Quantitative T-wave analysis predicts 1 year prognosis and benefit from early invasive treatment in the FRISC II study population. Eur Heart J 2005; 26: 112–118 [DOI] [PubMed] [Google Scholar]
- 4. Goodman SG, Bozovich GE, Tan M, et al. for the Efficacy and Safety of Subcutaneous Enoxaparin in Non-Q-Wave Coronary Events Study Group The greatest benefit of enoxaparin over unfractionated heparin in acute coronary syndromes is achieved in patients presenting with ST-segment changes: the Enoxaparin in Non-Q-Wave Coronary Events (ESSENCE) Electrocardiogram Core Laboratory Substudy. Am Heart J 2006; 151: 791–797 [DOI] [PubMed] [Google Scholar]
- 5. Anderson JL, Adams CD, Antman EM, et al. for the 2011 WRITING GROUP MEMBERS; ACCF/AHA TASK FORCE MEMBERS 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011; 123: e426–e579 [DOI] [PubMed] [Google Scholar]
- 6. Wagner GS, Macfarlane P, Wellens H, et al. for the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology, American College of Cardiology Foundation and Heart Rhythm Society AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part VI: acute ischemia/infarction: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation 2009; 119: e262–e270 [DOI] [PubMed] [Google Scholar]
- 7. Renkin J, Wijns W, Ladha Z, Col J. Reversal of segmental hypokinesis by coronary angioplasty in patients with unstable angina, persistent T wave inversion, and left anterior descending coronary artery stenosis. Additional evidence for myocardial stunning in humans. Circulation 1990; 82: 913–921 [DOI] [PubMed] [Google Scholar]
- 8. Stein PD, Dalen JE, McIntyre KM, Sasahara AA, Wenger NK, Willis PW. The electrocardiogram in acute pulmonary embolism. Prog Cardiovasc Dis 1975; 17: 247–257 [DOI] [PubMed] [Google Scholar]
- 9. Kosuge M, Kimura K, Ishikawa T, et al. Electrocardiographic differentiation between acute pulmonary embolism and acute coronary syndromes on the basis of negative T waves. Am J Cardiol 2007; 99: 817–821 [DOI] [PubMed] [Google Scholar]
- 10. Jaff MR, McMurtry MS, Archer SL, et al. for the American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, American Heart Association Council on Peripheral Vascular Disease and American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123: 1788–1830 [DOI] [PubMed] [Google Scholar]
- 11. Kosuge M, Kimura K, Ishikawa T, et al. Prognostic significance of inverted T waves in patients with acute pulmonary embolism. Circ J 2006; 70: 750–755 [DOI] [PubMed] [Google Scholar]
- 12. Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100: 598–603 [DOI] [PubMed] [Google Scholar]
- 13. Fedullo PF, Tapson VF. The evaluation of suspected pulmonary embolism. N Engl J Med 2003; 349: 1247–1256 [DOI] [PubMed] [Google Scholar]
- 14. Sanchez-Recalde A, Galeote G, Sanchez-Aquino R. Acute massive pulmonary thromboembolism simulating anterolateral myocardial ischemia. Heart 2004; 90: 1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bybee KA, Kara T, Prasad A, et al. Transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med 2004; 141: 858–865 [DOI] [PubMed] [Google Scholar]
- 16. Dib C, Asirvatham S, Elesber A, Rihal C, Friedman P, Prasad A. Clinical correlates and prognostic significance of electrocardiographic abnormalities in apical ballooning syndrome (Takotsubo/stress-induced cardiomyopathy). Am Heart J 2009; 157: 933–938 [DOI] [PubMed] [Google Scholar]
- 17. Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (Takotsubo) cardiomyopathy. JAMA 2011; 306: 277–286 [DOI] [PubMed] [Google Scholar]
- 18. Lankeit M, Jiménez D, Kostrubiec M, et al. Predictive value of the high-sensitivity troponin T assay and the simplified Pulmonary Embolism Severity Index in hemodynamically stable patients with acute pulmonary embolism: a prospective validation study. Circulation 2011; 124: 2716–2724 [DOI] [PubMed] [Google Scholar]
- 19. Giannitsis E, Müller-Bardorff M, Kurowski V, et al. Independent prognostic value of cardiac troponin T in patients with confirmed pulmonary embolism. Circulation 2000; 102: 211–217 [DOI] [PubMed] [Google Scholar]
- 20. Engelen DJ, Gorgels AP, Cheriex EC, et al. Value of the electrocardiogram in localizing the occlusion site in the left anterior descending coronary artery in acute anterior myocardial infarction. J Am Coll Cardiol 1999; 34: 389–395 [DOI] [PubMed] [Google Scholar]
- 21. Sapin PM, Musselman DR, Dehmer GJ, Cascio WE. Implications of inferior ST-segment elevation accompanying anterior wall acute myocardial infarction for the angiographic morphology of the left anterior descending coronary artery morphology and site of occlusion. Am J Cardiol 1992; 69: 860–865 [DOI] [PubMed] [Google Scholar]
- 22. Kurisu S, Inoue I, Kawagoe T, et al. Time course of electrocardiographic changes in patients with tako-tsubo syndrome: comparison with acute myocardial infarction with minimal enzymatic release. Circ J 2004; 68: 77–81 [DOI] [PubMed] [Google Scholar]
- 23. Kosuge M, Ebina T, Hibi K, et al. Simple and accurate electrocardiographic criteria to differentiate Takotsubo cardiomyopathy from anterior acute myocardial infarction. J Am Coll Cardiol 2010;55: 2514–2516 [DOI] [PubMed] [Google Scholar]
- 24. Kosuge M, Ebina T, Hibi K, et al. Differences in negative T waves between Takotsubo cardiomyopathy and reperfused anterior acute myocardial infarction. Circ J 2012; 76: 462–468 [DOI] [PubMed] [Google Scholar]
- 25. Matetzky S, Barabash GI, Shahar A, et al. Early T wave inversion after thrombolytic therapy predicts better coronary perfusion: clinical and angiographic study. J Am Coll Cardiol 1994; 24: 378–383 [DOI] [PubMed] [Google Scholar]
- 26. Haghi D, Athanasiadis A, Papavassiliu T, et al. Right ventricular involvement in Takotsubo cardiomyopathy. Eur Heart J 2006; 27: 2433–2439 [DOI] [PubMed] [Google Scholar]
- 27. Ben-Gal T, Sclarovsky S, Herz I, et al. Importance of the conal branch of the right coronary artery in patients with acute anterior wall myocardial infarction: electrocardiographic and angiographic correlation. J Am Coll Cardiol 1997; 29: 506–511 [DOI] [PubMed] [Google Scholar]
- 28. Geft IL, Shah PK, Rodriguez L, et al. ST elevations in leads V1 to V5 may be caused by right coronary artery occlusion and acute right ventricular infarction. Am J Cardiol 1984; 53: 991–996 [DOI] [PubMed] [Google Scholar]
- 29. Patel SM, Lennon RJ, Prasad A. Regional wall motion abnormality in apical ballooning syndrome (Takotsubo/stress cardiomyopathy): importance of biplane left ventriculography for differentiating from spontaneously aborted anterior myocardial infarction. Int J Cardiovasc Imaging 2012; 28: 687–694 [DOI] [PubMed] [Google Scholar]