Abstract
Background:
The role of venous blood gases as an alternative to arterial blood gases in patients with severe acute heart failure has not been established.
Objective:
To assess the correlation between arterial and peripheral venous blood gases together with pulse-oximetry (SpO2), as well as to estimate arterial values from venous samples in the first hours upon admission of patients with acute cardiogenic pulmonary oedema.
Methods:
Simultaneous venous and arterial blood samples were extracted on admission and over the next 1, 2, 3, 4, and 10 hours. SpO2 was also registered at the same intervals.
Results:
A total of 178 pairs of samples were obtained from 34 consecutive patients with acute cardiogenic pulmonary oedema. Arterial and venous blood gases followed a parallel course in the first hours, showing high correlation rates at all time intervals. Venous samples underestimated pH (mean difference −0.028) and overestimated CO2 (+5.1 mmHg) and bicarbonate (+1 mEq/l). Conversely, SpO2 tended to underestimate SaO2 (mean±SD: 93.1±9.1 vs. 94.2±8.4). Applying simple mathematical formulae based on these differences, arterial values were empirically calculated from venous samples, showing acceptable agreement in the Bland−Altman test. Likewise, a venous pH <7.32, pCO2 >51.3 mmHg, and bicarbonate <22.8 mEq/l could fairly identify arterial acidosis, either respiratory or metabolic, with a test accuracy of 92, 68, and 91%, respectively.
Conclusions:
In patients with cardiogenic pulmonary oedema, arterial blood gas disturbances may be estimated from peripheral venous samples. By monitoring SpO2 simultaneously, arterial punctures could often be avoided.
Keywords: Acute heart failure, arterial blood gases, pulmonary oedema, oxygen saturation, venous blood gases
Introduction
Arterial blood gas analysis is essential in the management of critically ill patients. However, it requires invasive and sometimes painful techniques that are not risk free for patients or hospital staff. Alternatively, venous blood gases have shown a good correlation with arterial blood gases in many clinical situations1–6 and specifically in diabetic ketoacidosis,7,8 exacerbation of chronic respiratory failure,9–12 uraemic acidosis,6 high altitudes,13 and in patients ventilated for acute respiratory failure14 or multiple trauma.15 Furthermore, in some clinical scenarios with severe circulatory failure, like haemorrhagic shock or while performing cardiopulmonary resuscitation, venous values have shown to reflect tissue perfusion more accurately than arterial ones.16,17
Cardiogenic acute pulmonary oedema is a critical situation with simultaneous respiratory and circulatory failure. Measuring arterial blood gases at presentation helps to assess the severity of the process, giving information about the degree of tissue hypoperfusion (pH, bicarbonate, base excess) and respiratory failure (pCO2, pO2, SaO2), which may establish indications for specific therapies, like inotropic support or mechanical ventilation.
The value of peripheral venous blood gases in patients with acute pulmonary oedema has not been well studied. The aim of this study was to determine the correlation and agreement between arterial and venous blood gases, as well as arterial oxygen saturation (SaO2) and SpO2 in these patients, at different intervals after admission. Secondary objectives were to find a simple formula to be applied at bedside to easily estimate arterial values from venous samples in these patients and additionally, which venous values might more accurately predict arterial acid–base disturbances.
Methods
Forty consecutive patients with cardiogenic pulmonary oedema were studied prospectively as a part of a randomized clinical trial assessing the efficacy of noninvasive ventilation in the treatment of acute pulmonary oedema.18 The study was approved by the Ethics Committee of the hospital, and informed consent was obtained from all the patients.
Inclusion criteria were: dyspnoea of sudden onset, signs of pulmonary oedema upon physical examination, congestion on chest radiograph and hypoxaemia, defined as oxygen saturation measured by pulse-oximetry (SpO2) below 90%, or a PaO2/FiO2 ratio below 300 mmHg. Patients with immediate tracheal intubation at presentation, cardiogenic shock (systolic blood pressure <90 mmHg), severe chronic obstructive pulmonary disease or chronic renal failure (serum creatinine level >265 µmol/l), pneumonia, and acute myocardial infarction receiving reperfusion therapy were excluded.
Patients were immediately transferred to the ICU for monitoring. The management protocol was previously described.18 Patients received initial standard medical treatment with intravenous morphine (4 mg), furosemide (40 mg), and nitroglycerin. Half of the patients were randomly assigned to receive conventional oxygen therapy (Ventimask) or up to 4 hours of noninvasive ventilation (NIV) with pressure support (average 15.2±2.4 cmH2O) and positive end expiratory pressure (5 cmH2O). NIV was delivered by a ventilator (Puritan Bennett 7200, CA, USA) through a oronasal mask (Respironics, Murrysville, USA), obtaining tidal volumes (mean±SD) that ranged from 531±143 ml at study entry to 627±137 ml at 240 minutes. The initial FiO2 was 0.5, which was increased, according to the response, up to 1 in patients treated with NIV or to 0.85–0.90 with a Monaghan reservoir mask in those assigned to conventional treatment.
In addition to a peripheral venous line, arterial catheter was inserted for blood pressure monitoring and blood extractions. Arterial and peripheral venous samples were taken simultaneously during the first hours: on admission (0 minutes) and at 60, 120, 180, 240, and 600 minutes. Measurement of pH, pCO2, bicarbonate, and oxygen saturation by co-oximetry (SaO2 or SvO2) was made for each sample, using an Instrumentation Laboratory Blood Gas Analyzer IL1620, which was located in the emergency laboratory. Blood samples were taken through a heparinized syringe and were carried from the ICU to the laboratory; pH, pCO2, and pO2 were directly measured, whereas actual plasma bicarbonate (HCO3–), SaO2, and other additional parameters were derived mathematically.
At the same intervals that blood samples were taken, simultaneous measurement of oxygen saturation by pulse-oximetry (SpO2) was obtained by Nellcor Pulse-oximeters (N560 or N600) with standard finger sensors.
Because intubation was an end point of the study, measurements taken after intubation were not registered.
The number of patients was enough for the purpose of the study. Taking into account a probability of type I error equal to 0.05, a sample of 34 pairs of measurements led to a statistical power equal to 0.9994 in the estimation of an observed correlation coefficient of 0.7.
Data was analysed using the Pearson correlation coefficient. Receiver operating characteristic (ROC) curves were plotted to set the best value that identified acidosis (arterial pH <7.35), either metabolic (arterial bicarbonate <21 mmHg) or respiratory (defined by hypercapnia: arterial pCO2>45 mmHg), as well as alkalosis (arterial pH >7.45) and hypocapnia (arterial pCO2 <36 mmHg). The limit range for these definitions was taken from our institutional laboratory. In order to establish a simple empiric formula to be used in clinical practice, arterial blood gases were estimated from venous samples, adding a coefficient to each of the venous values. These coefficients were calculated by rounding the mean of the differences between arterial and venous values to the nearest exact decimal value. The agreement of these ‘empirically calculated arterial values’ and the two measurements of oxygen saturation were tested using the bias plot (Bland−Altman) method.
General data was analysed using IBM SPSS Statistics version 19, whereas the Bland−Altman test was analysed with MedCalc trial software (Mariakerke, Belgium).
Results
From 40 patients with cardiogenic pulmonary oedema initially recruited, 34 were finally included in the study. Six patients were excluded, for final diagnoses other than acute heart failure (n=3) or incomplete data (n=3). The baseline characteristics of the patients are summarized in Table 1.
Table 1.
Characteristics of the patients.
| Characteristic | Patients (n=34) |
|---|---|
| Demographics | |
| Age (years) | 76.9±8.7 |
| Male | 50 |
| Prior diagnosis | |
| Hypertension | 78 |
| Diabetes mellitus | 43 |
| COPD | 27 |
| CAD | 60 |
| Atrial fibrillation | 24 |
| LVEF (%) | 43.6±13 |
| Measurements on admission | |
| Systolic BP (mmHg) | 172.2±43.2 |
| Diastolic BP (mmHg) | 94.4±26.4 |
| Heart rate (bpm) | 109.7±22 |
| Respiratory rate (breaths/min) | 37.6±7.5 |
| APACHE II score | 16±5.8 |
| Lactacidaemia (mg/dl) | 27.1±18.9 |
Values are mean±SD or %. COPD, chronic obstructive pulmonary disease; CAD, coronary artery disease; LVEF, left ventricular ejection fraction; APACHE, Acute Physiology And Chronic Health Evaluation at 24 hours; BP, blood pressure; lactacidaemia, venous acid lactic levels.
A total of 178 pairs of samples were obtained. Average arterial and venous blood gases with their correlation coefficients, on admission and for the whole series, are shown in Table 2.
Table 2.
Blood gas and pulse-oximetry measurements.
| Measurement | Arterial | Venous | Pulse-oximetry | Coefficient |
|---|---|---|---|---|
| On admission (n=34) | ||||
| pH | 7.27±0.1 | 7.24±0.09 | 0.79 | |
| CO2 (mmHg) | 51.6±15.2 | 56.8±17.6 | 0.70 | |
| Bicarbonate (mEq/l) | 24.4±4.3 | 26.3±4.9 | 0.81 | |
| Base excess | −2.2±5 | −2.4±4.6 | 0.90 | |
| Oxygen saturation | 82.4±11.5 | – | 81.4±11.5 | 0.87 |
| PaO2/FiO2 (mmHg) | 140.9±67 | – | ||
| Overall (n=178) | ||||
| pH | 7.35±0.09 | 7.32±0.09 | 0.92 | |
| CO2 (mmHg) | 46.1±11 | 51.2±13.2 | 0.86 | |
| Bicarbonate (mEq/l) | 25.3±4.8 | 26.3±4.7 | 0.84 | |
| Base excess | −0.05±4.8 | −0.11±4.8 | 0.90 | |
| Oxygen saturation | 94.2±8.4 | – | 93.1±9.1 | 0.83 |
Values are mean±SD. Coefficient, Pearson correlation coefficient; FiO2, fraction of inspired oxygen.
Compared to arterial gases, venous samples underestimated pH and overestimated pCO2 and bicarbonate. Mean differences between venous and arterial were: pH −0.028, pCO2 5.1 mmHg, and bicarbonate 1 mEq/l. Correlation was lower for all parameters on admission, with the exception of bicarbonate and SpO2 that remained substantially stable at all intervals.
Oxygen saturation as well as arterial and venous blood gases showed a parallel course at all time points (Figure 1). Oxygen saturation reached normal values in the first hour, while pH needed several hours to normalize.
Figure 1.
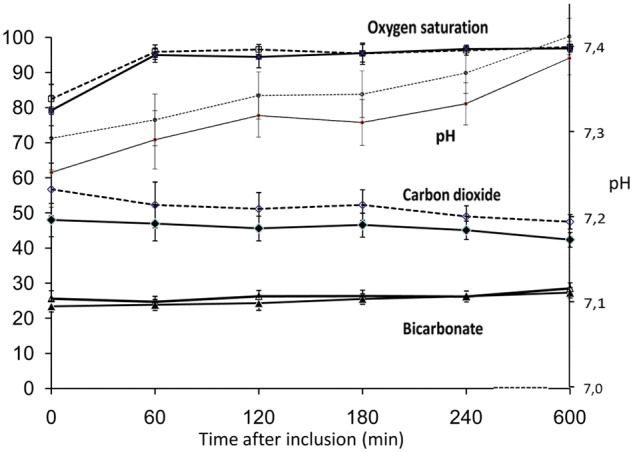
Oxygen Saturation, pH, carbon dioxide, and bicarbonate at different study times
Values are (mean ± SEM) (standard error of the mean) for: oxygen saturation (SaO2, white squares; SpO2, black squares); pH (arterial, white circles; venous, black circles); carbon dioxide (arterial, black diamonds; venous, white diamonds); and bicarbonate (arterial, white triangles; venous, black triangles). Left Y-axis is equivalent to: % oxygen saturation, mmHg CO2, and mEq/l bicarbonate.
On admission, the incidence of arterial acidosis was 73.5%, alkalosis 5.9%, hypercapnia 47.1%, and hypocapnia 14.7%. ROC curves were plotted to set the venous value that best identified arterial acidosis, either respiratory or metabolic. The range for these definitions was previously reported in the methods. When the whole series was considered (Figure 2), the cutoff (mean±SE) for acidosis was a venous pH of 7.32±0.08, with sensitivity 95%, specificity 89%, and test accuracy 92%. The cutoff for hypercapnia was a venous pCO2 of 51.3±0.32 mmHg, with sensitivity 81%, specificity 78%, and test accuracy 68%. The cutoff for metabolic acidosis was venous bicarbonate of 22.8±0.03 mEq/l, with sensitivity 94%, specificity 91%, and test accuracy 91%.
Figure 2.
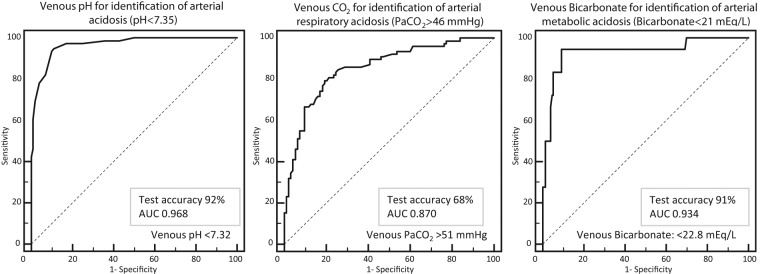
ROC curves for identification of (A) arterial acidosis, (B) hypercapnia, and (C) metabolic acidosis from venous samples.
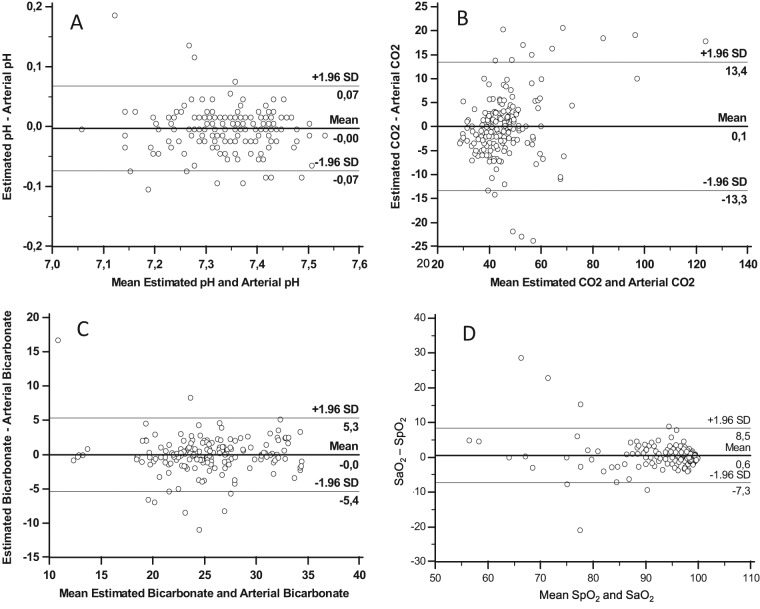
The bias plot for agreement (Bland−Altman) for venous and arterial blood gases showed a mean bias for pH of −0.028 (95% CI −0.042 to 0.099), for CO2 +5.1 mmHg (−18.4 to 8.3 mmHg), and for bicarbonate +1 mEq/l (−5.3 to 5.4 mEq/l). As described in the methods, arterial blood gases were empirically estimated according to these differences, by adding 0.03 to venous pH, subtracting 5 mmHg from venous CO2, and subtracting 1 mEq/l from venous bicarbonate. After this empirical adjustment, the Bland−Altman test showed greater agreement: mean bias difference for pH −0.002 (95% CI −0.072 to 0.069), for CO2 0.1 (−13.4 to 13.3), and for bicarbonate −0.01 (−5.3 to 5.4), as shown in Figure 3. The Bland−Altman plot for SaO2 and SpO2 is also included in this figure, showing a mean bias 0.6 (−7.3 to 8.5).
Figure 3.
Bias (Bland−Altman) plots for agreement of (A) ‘estimated arterial’ and arterial pH, (B) ‘estimated arterial’ and arterial pCO2, (C) ‘estimated arterial’ and arterial bicarbonate, and (D) SaO2 and SpO2.
The ‘estimated’ arterial values were empirically obtained from peripheral venous samples (see text).
When applying this empirical adjustment in the identification of arterial hypercapnia on admission, venous blood samples only misclassified three patients (8%).
Discussion
This is the first study that assesses the correlation between serial arterial and venous blood gases in the setting of acute pulmonary oedema, providing detailed information about the evolution of these parameters throughout the first hours. Previous studies have analysed the correlation in other clinical scenarios.1–17
We observed good correlation and agreement between arterial and venous blood gases for the values of pH, bicarbonate, and base excess, being somewhat lower for pCO2.
The study found slightly lower precision (SD of the mean of the differences) than other authors in the comparison between SpO2 and SaO2.19,20 This could be explained by the fact that blood samples were analysed out of the ICU. Likewise, the highest deviation was found in the lower values.20,21 In spite of this, the small differences support the use of pulse-oximetry as a method to assess oxygenation, especially when the saturation is not extremely low (i.e. >80%).
Overall, the best correlation between arterial and venous samples was seen for pH. This is clinically relevant because severe acidosis (arterial pH <7.25) has been described to be predictor of needing for ventilatory support in patients with cardiogenic pulmonary oedema.22 The high correlation rate found in pH has also been described in other acute clinical scenarios.1–13 However, this correlation decreased in hypotensive or severely hypoperfused patients, who have also shown large differences in pCO2 and bicarbonate.14,15 Since we excluded such patients, we cannot transfer the results to other patients with acute heart failure showing hypotension or cardiogenic shock.
Regarding pCO2, the arterio-venous differences observed were wider and the agreement was substantially lower than for pH. Similar results have been reported for patients with acute respiratory disease and chronic obstructive pulmonary disease in the emergency department7,8. In spite of this, venous pCO2 has been proposed as a screening method to detect arterial hypercapnia in this setting.8
In patients with pulmonary oedema we tried to estimate arterial pCO2 from the venous samples (by subtracting 5 mmHg) with acceptable accuracy in most cases. By applying this calculation, 92% of the patients on admission would have been correctly classified regarding hypercapnia. Alternatively, the cutoff of venous pCO2 proposed in this study (51 mmHg) could be used. Identification of hypercapnia is important because it may be an indication for NIV17 and is predictive of the success of this technique.23,24
The empirical formulae proposed in the present study to estimate arterial values from the venous samples, showed an acceptable agreement in the Bland-Altman analysis, especially for pH and bicarbonate. These empirical formulae may be quickly and easily remembered at bedside. Other authors have advocated the use of regression equations,10 which may be difficult to apply in an emergency setting. Toftegaard et al.,25 with the help of mathematical models, calculated arterial values by simulating the transport of venous blood back through the tissues, using respiratory quotient until simulated arterial oxygenation matched that measured by pulse-oximetry. This method showed excellent agreement and described better accuracy using peripheral venous than central venous blood samples.26 These findings may reinforce the use of peripheral samples as opposed to central venous or arterialized capillary samples from the earlobe or the finger.27
This study has several limitations. Although the number of paired samples was acceptable, they were obtained from a small series of patients with sequential determinations. Regarding the increase in the correlation coefficients when stabilization was progressively achieved, our results may be overrated because the majority of data was obtained after admission. In addition, the fact that the gas analyser was located in the emergency department laboratory, instead of at bedside, could have affected some measurements.
In contrast to more accurate regression equations or mathematical models, we proposed very simplified formulae. Such simplicity potentially resulted in less accuracy. Furthermore, these results should be further validated in a prospective study. Finally, the exclusion of patients with ST-segment elevation acute myocardial infarction, or those with hypotension or shock, might limit the application of the method in a subgroup of patients with acute heart failure syndromes.
However, since a venous line is generally inserted for blood analysis and intravenous treatment in all patients admitted for pulmonary oedema, the matter of course inclusion of venous blood gas testing would be worthwhile, and the results of this study support the use of venous samples, together with pulse-oximetry, as a useful approach to arterial blood gases in these patients.
Acknowledgments
The authors thank the following: Mitsi Ito, language review; Montse Martin, statistics; Arantxa Mas, comments; Josep Ballús, Rosario Cañizares, and Rubén Manresa, data.
Footnotes
Funding: This study was partially financed by the Fondo de Investigación Sanitaria (FIS Grant 96/1134), Ministerio de Sanidad y Consumo.
Conflict of interest: The authors declare they do not have any conflict of interest.
References
- 1. Gennis PR, Skovron ML, Aronson ST, et al. The usefulness of peripheral venous blood in estimating acid–base status in acutely ill patients. Ann Emerg Med 1985; 14: 845–849 [DOI] [PubMed] [Google Scholar]
- 2. Kelly AM, McAlpine R, Kyle E. Venous pH can safely replace arterial pH in the initial evaluation of patients in the emergency department. Emerg Med J 2001; 18: 340–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kelly AM, McAlpine R, Kyle E. Agreement between bicarbonate measured on arterial and venous blood gases. Emerg Med Australas 2004; 16: 407–409 [DOI] [PubMed] [Google Scholar]
- 4. Middleton P, Kelly AM, Brown J, et al. Agreement between arterial and central venous values for pH, bicarbonate, base excess, and lactate. Emerg Med J 2006; 23: 622–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walkey AJ, Farber HW, O’Donnell C, et al. The accuracy of the central venous blood gas for acid–base monitoring. J Intensive Care Med 2010; 25: 104–110 [DOI] [PubMed] [Google Scholar]
- 6. Treger R, Pirouz S, Kamangar N, et al. Agreement between central venous and arterial blood gas measurements in the intensive care unit. Clin J Am Soc Nephrol 2010; 5: 390–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brandenburg MA, Dire DJ. Comparison of arterial and venous blood gas values in the initial emergency department evaluation of patients with diabetic ketoacidosis. Ann Emerg Med 1998; 31: 459–465 [DOI] [PubMed] [Google Scholar]
- 8. Gokel Y, Paydas S, Koseoglu Z, et al. Comparison of blood gas and acid–base measurements in arterial and venous blood samples in patients with uremic acidosis and diabetic ketoacidosis in the emergency room. Am J Nephrol 2000; 20: 319–323 [DOI] [PubMed] [Google Scholar]
- 9. Kelly AM, Kyle E, McAlpine R. Venous pCO2 and pH can be used to screen for significant hypercarbia in emergency patients with acute respiratory disease. J Emerg Med 2002; 22: 15–19 [DOI] [PubMed] [Google Scholar]
- 10. Ak A, Ogun CO, Bayir A, et al. Prediction of arterial blood gas values from venous blood gas values in patients with acute exacerbation of chronic obstructive pulmonary disease. Tohoku J Exp Med 2006; 210: 285–290 [DOI] [PubMed] [Google Scholar]
- 11. Kelly AM, Kerr D, Middleton P. Validation of venous pCO2 to screen for arterial hypercarbia in patients with chronic obstructive airways disease. J Emerg Med 2005; 28: 377–379 [DOI] [PubMed] [Google Scholar]
- 12. Razi E, Moosavi GA. Comparison of arterial and venous blood gases analysis in patients with exacerbation of chronic obstructive pulmonary disease. Saudi Med J 2007; 28: 862–865 [PubMed] [Google Scholar]
- 13. Koul PA, Khan UH, Wani AA, et al. Comparison and agreement between venous and arterial gas analysis in cardiopulmonary patients in Kashmir valley of the Indian subcontinent. Ann Thorac Med 2011; 6: 33–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chu YC, Chen CZ, Lee CH, et al. Prediction of arterial blood gas values from venous blood gas values in patients with acute respiratory failure receiving mechanical ventilation. J Formos Med Assoc 2003; 102: 539–543 [PubMed] [Google Scholar]
- 15. Malinoski DJ, Todd SR, Slone S, et al. Correlation of central venous and arterial blood gas measurements in mechanically ventilated trauma patients. Arch Surg 2005; 140: 1122–1125 [DOI] [PubMed] [Google Scholar]
- 16. Weil MH, Rackow EC, Trevino R, et al. Difference in acid–base state between venous and arterial blood during cardiopulmonary resuscitation. N Engl J Med 1986; 315: 153–156 [DOI] [PubMed] [Google Scholar]
- 17. Adrogué HJ, Rashad MN, Gorin AB, et al. Assessing acid–base status in circulatory failure. Differences between arterial and central venous blood. N Engl J Med 1989; 320: 1312–1316 [DOI] [PubMed] [Google Scholar]
- 18. Masip J, Betbesé AJ, Páez J, et al. Non-invasive pressure support ventilation versus conventional oxygen therapy in acute cardiogenic pulmonary oedema: a randomised trial. Lancet 2000; 356: 2126–2132 [DOI] [PubMed] [Google Scholar]
- 19. Van de, Louw A, Cracco C, Cerf C, et al. Accuracy of pulse oximetry in the intensive care unit. Intensive Care Med 2001; 27; 1606–1613 [DOI] [PubMed] [Google Scholar]
- 20. Jensen LA, Onyskiw JE, Prasad NG. Meta-analysis of arterial oxygen saturation monitoring by pulse oximetry in adults. Heart Lung 1998; 27: 387–408 [DOI] [PubMed] [Google Scholar]
- 21. Perkins GD, McAuley DF, Giles F, et al. Do changes in pulse oxymeter oxygen saturation predict equivalent changes in arterial oxygen saturation? Crit Care 2003; 7: R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Masip J, Páez J, Merino M, et al. Risk factors for intubation as a guide for noninvasive ventilation in patients with severe acute cardiogenic pulmonary edema. Intensive Care Med 2003; 29: 1921–1928 [DOI] [PubMed] [Google Scholar]
- 23. Rusterholtz T, Kempf J, Berton C, et al. Noninvasive pressure support ventilation with face mask in patients with acute pulmonary edema. Intensive Care Med 1999; 25: 21–28 [DOI] [PubMed] [Google Scholar]
- 24. Rodriguez L, Carrillo A, Melgarejo A, et al. Predictive factors related to success of non invasive ventilation and mortality in the treatment of acute cardiogenic pulmonary edema. Med Clin (Barc) 2005; 124: 126–131 [DOI] [PubMed] [Google Scholar]
- 25. Toftegaard M, Rees SE, Andreassen S. Evaluation of a method for converting venous values of acid–base and oxygenation status to arterial values. Emerg Med J 2009; 26: 268–272 [DOI] [PubMed] [Google Scholar]
- 26. Toftegaard M, Rees SE, Andreassen S. Correlation between acid–base parameters measured in arterial blood and venous blood sampled peripherally, from vena cavae superior, and from the pulmonary artery. Eur J Emerg Med 2008; 15: 86–91 [DOI] [PubMed] [Google Scholar]
- 27. Zavorsky GS, Cao J, Mayo NE, et al. Arterial versus capillary blood gases: a meta-analysis. Respir Physiol Neurobiol 2007; 155: 268–279 [DOI] [PubMed] [Google Scholar]