Abstract
Cardiotoxicity is a rare but serious complication of hydroxychloroquine, a 4-aminoquinoline increasingly used in the treatment of rheumatological disorders. We describe typical clinical, echocardiographic, and histological features of this rare condition according to the currently available literature, illustrated with a recent new biopsy-proven case of hydroxychloroquine cardiotoxicity in a 52-year-old female with rheumatoid arthritis. Presentation in this case was of a rapidly progressive decompensated biventricular cardiomyopathy associated with recurrent biomarker elevations, conduction system disease, and possibly neuromyotoxicity. Death occurred suddenly 2 months after diagnosis despite drug discontinuation and clinical improvement. The potential role of cardiac magnetic resonance delayed gadolinium enhancement imaging in the prognosis of this toxic cardiomyopathy is also introduced. This case-based literature review highlights that, although rare, hydroxychloroquine cardiotoxicity can be fatal, particularly if irreversible histopathological changes have occurred prior to drug discontinuation. Given this, regular screening with 12-lead electrocardiography and transthoracic echocardiography to detect conduction system disease and/or biventricular morphological or functional changes should be considered in hydroxychloroquine-treated patients in addition to recommended ophthalmological screening.
Keywords: Cardiomyopathy, cardiotoxicity, endomyocardial biopsy, heart failure, hydroxychloroquine
Introduction
Hydroxychloroquine (HCQ) is a 4-aminoquinoline which differs from chloroquine (CQ) in the addition of a hydroxyl group. Initially used as antimalarials, both drugs have now become mainstays in the management of rheumatic diseases principally systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA).1,2 Evidence suggesting HCQ is less toxic than CQ has led to its increased use.3 Retinal toxicity is the most well-recognized complication of long-term use of these agents, but less frequently cardiotoxicity and neuromyotoxicity can also occur. To date, up to 70 cases of cardiotoxicity have been reported in the literature, although less than half of these have been proven on endomyocardial biopsy. In the past, CQ has been predominantly implicated,4–10 but more recently several reports of HCQ-induced cardiomyopathy have emerged,5,11–20 likely reflecting its increased prevalence of use. Reported cases have occurred predominantly in SLE including discoid lupus rather than RA; more recently, a case has also been reported in scleroderma.14 To our knowledge, only four other cases of biopsy-proven HCQ cardiotoxicity in patients with isolated RA have been reported.5,16,19 We present an additional case in a 52-year-old female with a history of connective tissue disease and venous thromboembolism presenting with refractory New York Heart Association (NYHA) functional class 2–3 dyspnoea and intermittent chest pain over a 2-year period in association with recurrent biomarker elevations, conduction system disease on 12-lead electrocardiograph (ECG), and rapidly evolving non-ischaemic pattern biventricular dysfunction.
Case report
A 52-year-old woman was admitted to our tertiary referral hospital in September 2010 for further investigation and work up of progressively worsening NYHA class 2–3 dyspnoea over a period of 4–6 weeks, associated with four-pillow orthopnoea, episodic paroxysmal nocturnal dyspnoea, and profound fatigue. She had also noted three episodes of chest pain unrelated to exertion. She had been extensively investigated for similar symptoms leading to three hospital admissions over the previous 2-year period. Background medical history was significant for a number of conditions, including RA (since age 18), venous thromboembolism (deep venous thrombosis and pulmonary embolism complicating a left total knee replacement in January 2009), hypertension, and chronic kidney disease (renal biopsy had not been performed). Medications included warfarin, HCQ (400 mg daily since 1995), maintenance-dose oral steroids (prednisolone 5–10 mg), and bisoprolol 5 mg once daily. She was an ex-smoker. Family history was negative for ischaemic heart disease, cardiomyopathy, or other inherited conditions.
On physical examination, she was haemodynamically stable, in sinus rhythm, and had no overt clinical evidence of heart failure. She had a proximal myopathy predominantly affecting the upper limbs. Troponin I and creatinine kinase – both intermittently elevated over the previous 2-year period – were once again elevated at 0.73 µg/l and 577 U/l respectively. B-type natriuretic peptide was 1560 pg/ml. 12-lead ECG showed a new complete left bundle branch block since her last hospitalization 5 months previously. Echocardiography, performed on a number of occasions over the previous 2-year period, had repeatedly shown preserved biventricular systolic function associated with concentric left ventricular (LV) hypertrophy. Repeat echocardiography now showed global LV systolic dysfunction with an LV ejection fraction of 30–35%, at least mild concentric LV hypertrophy, restrictive stage diastolic dysfunction, and reduced right ventricular (RV) function. No significant valvular regurgitation was present. Repeat left heart catheterization reaffirmed the absence of significant coronary artery disease with TIMI 3 flow in all vessels. Left ventriculogram confirmed at least moderately reduced LV systolic function and elevated LV end-diastolic pressure. Invasive haemodynamic studies revealed significantly reduced cardiac output (2.0 l/min) and elevated pulmonary vascular resistance (4.5 Wood units). Cardiac magnetic resonance (CMR) imaging – performed several months previously – showed normal LV volumes with biventricular hypertrophy (LV mass index 114 g/m2) with overall preserved LV function and moderately reduced RV function. Post gadolinium, patchy areas of delayed gadolinium enhancement (DGE) were found in a non-ischaemic distribution in the septum and mid-wall as well as the apical anterior wall.
Respiratory work up ruled out new pulmonary thromboembolic episodes or significant parenchymal lung disease. RV endomyocardial biopsy was subsequently performed. Histology revealed myocyte hypertrophy with focal areas of myocyte damage and marked interstitial fibrosis. In addition, vacuoles were noted in the cytoplasm of some cardiomyocytes, some containing central nuclei. Further processing by electron microscopy showed cytoplasmic granules, some of which contained curvilinear character and occasional myelin like configuration – so called ‘myeloid bodies’ – confirming the diagnosis of HCQ-induced cardiomyopathy. The drug was immediately discontinued and standard guidelines-based heart failure treatment was commenced.
On review 1 month later, she had improved by ≥1 NYHA class without any recurrence of acute heart failure or other symptoms. 12-lead ECG and echocardiography remained unchanged. Further discussion on options such as device therapy and transplant evaluation were deferred pending further optimization of her heart failure treatment. Thirteen days later, the patient died suddenly at home. Post-mortem examination was not carried out.
Discussion
Antimalarial-related cardiotoxicity most commonly manifests clinically as a restrictive or dilated cardiomyopathy or with conduction system abnormalities including atrioventricular block and bundle branch block.4–7,9–14,16–20 The most frequent presenting symptoms relate to decompensated left or biventricular failure as illustrated above. However, also as in our case, non-specific chest discomfort may be a presenting or co-existent feature12,14,16 as can presyncope associated with conduction disease12,14 or atrial arrhythmias.11 One patient presented with a generalized tonic-clonic seizure in the setting of sinus arrest.16 In-hospital ventricular arrhythmias in the setting of a prolonged QT interval have been described;18,21 in both cases, QT interval shortened following discontinuation of the drug.
Clinical features of other toxicities may also be present. A review by Costedoat-Chalumeau et al. in 20077 noted out of 25 patients with CQ- or HCQ-related congestive heart failure, associated toxicity was present in 15 (myopathy in 12, neuropathy in five, and retinopathy in six). In a new case reported by the same authors, concerning severe cardiotoxicity leading to heart transplantation due to use of both HCQ and CQ over a 9-year period, retinal toxicity and neuromyotoxicity were also present.7 The most common manifestation of neuromyotoxicity is a bilateral progressive proximal weakness of the lower extremities with variable polyneuropathy.7,11 Proximal myopathy was present in this case, although muscle biopsy to confirm antimalarial toxicity as the cause of this finding had not been performed. Retinopathy, the most well-known complication of these drugs, may manifest as a spectrum of changes from asymptomatic and reversible pigment changes to visual loss persisting or progressing after drug discontinuation.22,23
Prognosis in antimalarial cardiotoxicity can vary from death to cardiac transplantation to partial or complete improvement in cardiac function.5,7,8,10,11,13–20,24–26 Two case reports have described successful orthotopic heart transplantation.7,10 Indication in both cases was refractory congestive heart failure 3 months after drug discontinuation. In the review by Costedoat-Chalumeau et al.,7 of the 25 congestive heart failure cases described, death occurred in 11 (46%) and partial or complete improvement was seen in eight out of 12 cases where the drug was discontinued. Meanwhile regression of conduction disease appears to be rare – out of 15 patients with conduction disorders in which the causative agent was discontinued, only three had resolution of the disturbance.7 Considering cases published since this review,13–20 death occurred in two patients. Both were middle-aged females with a long-standing history of SLE taking 400 mg HCQ daily for >10 years; both died shortly after presentation with acute heart failure despite drug discontinuation.14,17 Partial or complete improvement in symptoms and/or echocardiographic parameters (ranging from 3 months to 1 year) occurred in the remainder of cases;13–16,18–20 of note, complete histological improvement was also documented in one of these cases.20
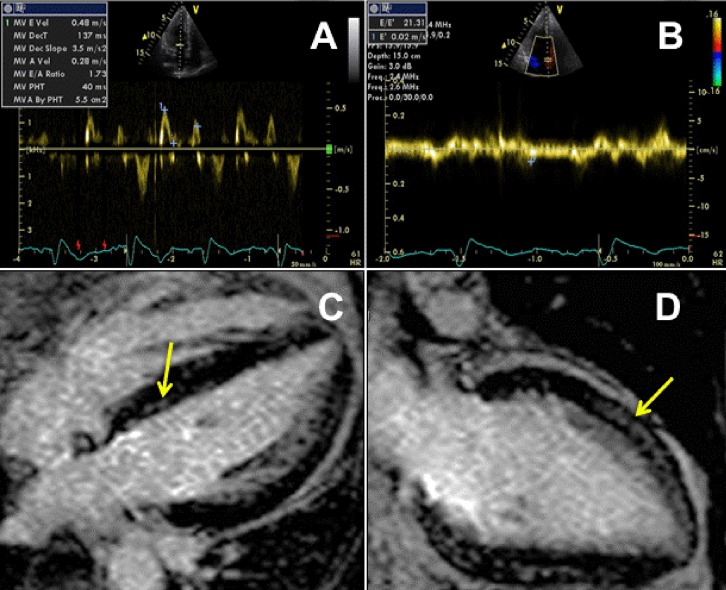
Echocardiography plays a key adjunctive role in the diagnosis of HCQ cardiotoxicity. Diffusely thickened ventricular walls on transthoracic echocardiography are one of the hallmarks of this form of cardiomyopathy7,12,13,17–20 and were seen from earlier on in our patient’s course. In one report where the patient went on to undergo successful orthotopic heart transplantation, interventricular septal thickness in the explanted heart measured 20 mm.7 Biatrial enlargement13,14,16–18 and restrictive physiology13,16,19 are frequently associated (Figure 1a and b). Such findings, in the absence of significant systolic dysfunction, may be the predominant structural abnormalities seen on echocardiogram13,16,18 or may precede the development of systolic dysfunction as in this case.11,12 In cases with systolic dysfunction, LV ejection fraction is frequently severely reduced.7,11,14,17,20 Although ventricular chamber sizes are frequently normal as in our case, LV and/or RV dilatation have been reported.11,14,18,20 Mitral regurgitation of varying degrees has been reported with one recent case study documenting severe mitral regurgitation likely due to papillary muscle hypertrophy and tethering.12,13,16,18
Figure 1.
Findings on imaging modalities in support of the diagnosis of hydroxychloroquine cardiomyopathy. (a) Pulse-wave Doppler of mitral inflow on 2-dimensional transthoracic echocardiography shows increased E-wave peak velocity to A-wave peak velocity ratio (E/A) of >1.5 associated with reduced deceleration time of <160 m/s suggestive of restrictive physiology. (b) Pulse-wave tissue Doppler imaging at the septal mitral annulus shows significantly reduced early diastolic relaxation velocity (0.02 m/s) confirming restrictive pattern diastolic dysfunction. In addition, markedly elevated E/E′(ratio of mitral inflow E-wave peak velocity to tissue Doppler early diastolic velocity) of >15 is shown, suggestive of significantly elevated left ventricular filling pressures. (c and d) Cardiac magnetic resonance 4-chamber (c) and 2-chamber (d) delayed gadolinium enhancement imaging illustrating areas of patchy enhancement throughout the mid-wall, particularly in the septum and apical anterior walls (arrows).
CMR may serve as an increasingly important diagnostic modality in antimalarial cardiotoxicity for a number of reasons. Firstly, it is the reference standard for the assessment of LV and RV function and morphology, the latter also commonly abnormal in antimalarial cardiotoxicity.7,11–13,17,18 Secondly, CMR also plays a primary role in the exclusion of differential diagnoses. Although rare, myocarditis is one of the recognized cardiovascular sequelae of RA27 and is clearly visualized on CMR as a hyperintense area or areas on T2-weighted MRI sequences, not seen in this case. Restrictive cardiomyopathy in particular cardiac amyloidosis was an important differential diagnosis in our case due to both the past history and echocardiographic findings; however, CMR did not show the typical imaging features suggestive of cardiac amyloid deposition. Another infiltrative cardiomyopathy, cardiac sarcoidosis, was also considered given that it has been associated with conduction system disease on presentation, female predominance, and a higher incidence of abnormal wall thickness, all relevant features to this case.28 However, once again, CMR, shown to be more than twice as sensitive for diagnosing cardiac sarcoidosis than current consensus criteria29 did not show any evidence of the typical ‘punched out’ appearance associated with this cardiomyopathy. Lastly, CMR may also play a role in prognosis through its assessment of the degree of fibrosis as determined by DGE imaging. The presence of DGE in non-ischaemic cardiomyopathies predicts an 8-fold increased risk of an adverse cardiac outcome.30 In the current case, patchy areas of diffuse DGE were present in a non-ischaemic distribution in the septum, mid-wall, and apical anterior wall predating the development of LV systolic dysfunction (Figure 1c and d); the patient was admitted with decompensated heart failure 4 months later with subsequent biopsy confirming marked interstitial fibrosis. Sudden death, most likely due to a malignant ventricular arrhythmia, occurred less than 2 months later. The possibility of CMR as a potential prognostic indicator and/or monitoring tool in future cases of HCQ and other toxic cardiomyopathies – perhaps suggesting earlier referral for device therapy and/or cardiac transplantation if fibrosis persists despite drug discontinuation – requires further research.
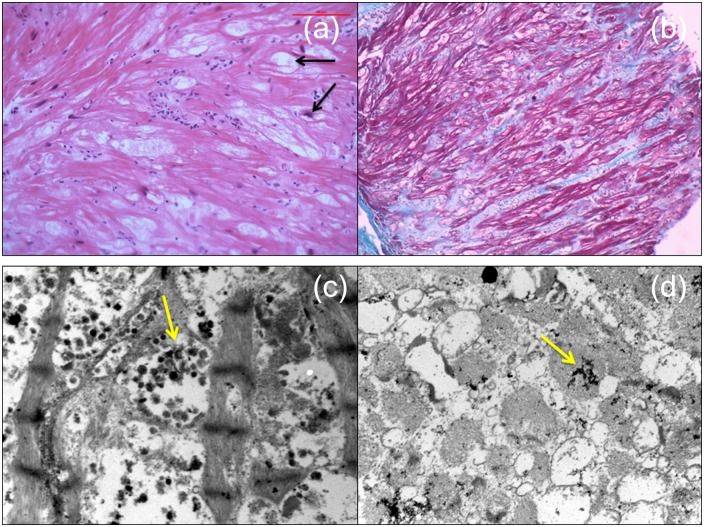
Endomyocardial biopsy was the key diagnostic test in this case. Histology is essential to provide an accurate diagnosis and exclude differential diagnoses, including amyloidosis, myocarditis, and sarcoidosis. The pathological findings in HCQ cardiotoxicity were first reported by Piette et al.31 and include enlarged and vacuolated cells on light microscopy and the presence of myelinoid and curvilinear bodies – thought to represent abnormal lysosomes – within cardiac myocytes on transmission electron microscopy (Figure 2). Curvilinear bodies in particular appear to be the most specific histological indicator of antimalarial-related cardiotoxicity and these structures are not seen in differential diagnoses of some of the other pathological findings, such as other toxic cardiomyopathies or Fabry’s disease.4,5,8,25 Histopathological findings may persist for years after drug discontinuation.26 However, one recent case report noted that, within 6 months of drug withdrawal, cardiomyocytes were reduced in size and devoid of intracellular vacuoles and myelin and curvilinear bodies were replaced by new contractile elements.20 Similar histological findings may be seen in skeletal muscle biopsies if neuromyotoxicity is present and may be diagnostic in cases where cardiac biopsy is contraindicated due to the severity of the patient’s condition.24
Figure 2.
Histological findings from right ventricular endomyocardial biopsy on light microscopy (top panels) and electron microscopy (bottom panels). (a) Myocyte hypertrophy and focal myocyte damage is demonstrated with vacuolization of the some of the cardiomyocytes. Some of these vacuoles contain central nuclei (arrows). (b) Masson’s trichrome stain highlights marked interstitial fibrosis (green/blue staining). (c and d) Electron microscopy showed cytoplasmic inclusion bodies: rounded aggregates of dark osmiophilic staining small bodies, some of which contain curvilinear and lamellar character (so-called ‘myelin bodies’) (arrows).
Risk factors for the development of HCQ-induced cardiotoxicity are thought to include older age, female sex, longer duration of therapy (>10 years), elevated per-kilogram daily dose, pre-existing cardiac disease, and renal insufficiency.8,11,26,32 In the previously noted review,7 treatment duration prior to the diagnosis of cardiomyopathy ranged from 3 months to 27 years (mean 10 years). A more recent case describes a 30-year duration of therapy prior to the development of clinical heart failure.13 A wide range of cumulative dosage of antimalarial drugs in heart failure cases was also noted (270–9125 g). Guidelines for dosing of both CQ and HCQ are based on a large cohort study of 900 rheumatoid patients looking at risk for developing retinal toxicity.33 A safe daily dose where only reversible asymptomatic pigment changes were observed was defined as 6.0–6.5 mg/kg/day for HCQ. Our patient, 58 kg at the time of her last admission, would have been above this cut-off at 6.9 mg/kg/day. However, serious retinal toxicity level from this study was only reported from the level of 7.8 mg/kg/day.
Regarding presence of pre-existing cardiac disease, as noted above there were several factors in this case which may have contributed to myocardial and/or electrical dysfunction, including hypertension, venous thromboembolism, and chronic steroid use. It is also important to note that RA itself can be associated with a variety of cardiac manifestations,34 some of which, including myocarditis as previously noted, could present with similar features to those noted in our case. Rheumatoid vasculitis is typically seen in long standing ‘burnt-out’ RA, such as seems the case in this patient; one series of 50 patients documented cardiac manifestations including pericarditis and myocardial infarction in approximately one-third of patients.35 However, myocardial infarctions directly resulting from rheumatoid-associated coronary arteritis are rare, especially in patients without documented evidence of vasculitis in other major organs.36–38 Meanwhile, RA is associated with a higher prevalence of symptomatic heart failure compared with individuals without RA which is not explained by traditional risk factors or clinical ischaemic disease, suggesting that chronic rheumatoid inflammation may account for the increased susceptibility.39,40
Impairment in renal function has been proposed as a mechanism for toxicity given that these drugs undergo renal as well as hepatic excretion.32 Of note, many of the more recently described HCQ-induced cardiotoxicity cases occurred in patients with lupus nephritis.11,12,14,18 Although she did not have SLE, the patient in this case had known chronic kidney disease.
In conclusion, we have presented, to our knowledge, only the fifth reported biopsy-proven case of HCQ cardiotoxicity, presenting as a rapidly evolving non-ischaemic biventricular dysfunction in a 52-year-old female with RA and multiple other comorbidities on long-term HCQ therapy. Although rare, the current case and the accompanying clinically orientated literature review highlights that antimalarial cardiotoxicity can be a fatal diagnosis with cardiac dysfunction persisting despite drug discontinuation. Given this, and the potential for reversibility, regular screening with 12-lead ECG and transthoracic echocardiography should be considered in HCQ- or CQ-treated patients in addition to ophthalmological screening, particularly if prolonged duration of treatment or other manifestations of toxicity.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest: The authors report no conflicts of interest.
References
- 1. Morand EF, McCloud PI, Littlejohn GO. Continuation of long term treatment with hydroxychloroquine in systemic lupus erythematosus and rheumatoid arthritis. Ann Rheum Dis 1992; 51: 1318–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson RJ. Hydroxychloroquine therapy in rheumatic diseases. Ann Rheum Dis 1995; 44: 6–7 [PubMed] [Google Scholar]
- 3. Felson DT, Anderson JJ, Meenan RF. The comparative efficacy and toxicity of second-line drugs in rheumatoid arthritis. Results of two metaanalyses. Ann Rheum Dis 1990; 33: 1449–1461 [DOI] [PubMed] [Google Scholar]
- 4. McAllister HA, Jr., Ferrans VJ, Hall RJ, et al. Chloroquine-induced cardiomyopathy. Arch Pathol Lab Med 1987; 111: 953–956 [PubMed] [Google Scholar]
- 5. Roos JM, Aubry MC, Edwards WD. Chloroquine cardiotoxicity: clinicopathologic features in three patients and comparison with three patients with Fabry disease. Cardiovasc Pathol 2002; 11: 277–283 [DOI] [PubMed] [Google Scholar]
- 6. Veinot JP, Mai KT, Zarychanski R. Chloroquine related cardiac toxicity. J Rheumatol 1998; 25: 1221–1225 [PubMed] [Google Scholar]
- 7. Costedoat-Chalumeau N, Hulot JS, Amoura Z, et al. Cardiomyopathy related to antimalarial therapy with illustrative case report. Cardiology 2007; 107: 73–80 [DOI] [PubMed] [Google Scholar]
- 8. Baguet JP, Tremel F, Fabre M. Chloroquine cardiomyopathy with conduction disorders. Heart 1999; 81: 221–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reuss-Borst M, Berner B, Wulf G, et al. Complete heart block as a rare complication of treatment with chloroquine. J Rheumatol 1999; 26: 1394–1395 [PubMed] [Google Scholar]
- 10. Freihage JH, Patel NC, Jacobs WR, et al. Heart transplantation in a patient with chloroquine-induced cardiomyopathy. J Heart Lung Transplant 2004; 23: 252–255 [DOI] [PubMed] [Google Scholar]
- 11. Nord JE, Shah PK, Rinaldi RZ, et al. Hydroxychloroquine cardiotoxicity in systemic lupus erythematosus: a report of 2 cases and review of the literature. Semin Ann Rheum Dis 2004; 33: 336–351 [DOI] [PubMed] [Google Scholar]
- 12. Keating RJ, Bhatia S, Amin S, et al. Hydroxychloroquine-induced cardiotoxicity in a 39-year-old woman with systemic lupus erythematosus and systolic dysfunction. J Am Soc Echocardiogr 2005; 18: 981. [DOI] [PubMed] [Google Scholar]
- 13. Cotroneo J, Sleik KM, Rene Rodriguez E, et al. Hydroxychloroquine-induced restrictive cardiomyopathy. Eur J Echocardiogr 2007; 8: 247–251 [DOI] [PubMed] [Google Scholar]
- 14. Soong TR, Barouch LA, Champion HC, et al. New clinical and ultrastructural findings in hydroxychloroquine-induced cardiomyopathy – a report of 2 cases. Hum Pathol 2007; 38: 1858–1863 [DOI] [PubMed] [Google Scholar]
- 15. Manohar VA, Moder KG, Edwards WD, et al. Restrictive cardiomyopathy secondary to hydroxychloroquine therapy. J Rheumatol 2009; 36: 440–441 [DOI] [PubMed] [Google Scholar]
- 16. Lee JH, Chung WB, Kang JH, et al. A case of chloroquine-induced cardiomyopathy that presented as sick sinus syndrome. Korean Circ J 2010; 40: 604–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muthukrishnan P, Roukoz H, Grafton G, et al. Hydroxychloroquine-induced cardiomyopathy: a case report. Circ Heart Fail 2011; 4: e7–e8 [DOI] [PubMed] [Google Scholar]
- 18. Newton-Cheh C, Lin AE, Baggish AL, et al. Case records of the Massachusetts General Hospital. Case 11-2011. A 47-year-old man with systemic lupus erythematosus and heart failure. N Engl J Med 2011; 364: 1450–1460 [DOI] [PubMed] [Google Scholar]
- 19. Hartmann M, Meek IL, van Houwelingen GK, et al. Acute left ventricular failure in a patient with hydroxychloroquine-induced cardiomyopathy. Neth Heart J 2011; 19: 482–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frustaci A, Morgante E, Antuzzi D, et al. Inhibition of cardiomyocyte lysosomal activity in hydroxychloroquine cardiomyopathy. Int J Cardiol 2012; 157: 117–119 [DOI] [PubMed] [Google Scholar]
- 21. Chen CY, Wang FL, Lin CC. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin Toxicol (Phila) 2006; 44: 173–175 [DOI] [PubMed] [Google Scholar]
- 22. Rynes RI. Ophthalmologic safety of long-term hydroxychloroquine sulfate treatment. Am J Med 1983; 75: 35–39 [DOI] [PubMed] [Google Scholar]
- 23. Maksymowych W, Russell AS. Antimalarials in rheumatology: efficacy and safety. Semin Ann Rheum Dis 1987; 16: 206–221 [DOI] [PubMed] [Google Scholar]
- 24. Estes ML, Ewing-Wilson D, Chou SM, et al. Chloroquine neuromyotoxicity. Clinical and pathologic perspective. Am J Med 1987; 82: 447–455 [DOI] [PubMed] [Google Scholar]
- 25. Ratliff NB, Estes ML, Myles JL, et al. Diagnosis of chloroquine cardiomyopathy by endomyocardial biopsy. N Engl J Med 1987; 316: 191–193 [DOI] [PubMed] [Google Scholar]
- 26. August C, Holzhausen HJ, Schmoldt A, et al. Histological and ultrastructural findings in chloroquine-induced cardiomyopathy. J Mol Med (Berl) 1995; 73: 73–77 [DOI] [PubMed] [Google Scholar]
- 27. Mutru O, Laakso M, Isomaki H, et al. Cardiovascular mortality in patients with rheumatoid arthritis. Cardiology 1989; 76: 71–77 [DOI] [PubMed] [Google Scholar]
- 28. Kim JS, Judson MA, Donnino R, et al. Cardiac sarcoidosis. Am Heart J 2009; 157: 9–21 [DOI] [PubMed] [Google Scholar]
- 29. Patel MR, Cawley PJ, Heitner JF, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation 2009; 120: 1969–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu KC, Weiss RG, Thiemann DR, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol 2008; 51: 2414–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Piette JC, Guillevin L, Chapelon C, et al. Chloroquine cardiotoxicity. N Engl J Med 1987; 317: 710–711 [DOI] [PubMed] [Google Scholar]
- 32. Mackenzie AH. Pharmacologic actions of 4-aminoquinoline compounds. Am J Med 1983; 75: 5–10 [DOI] [PubMed] [Google Scholar]
- 33. Mackenzie AH. Dose refinements in long-term therapy of rheumatoid arthritis with antimalarials. Am J Med 1983; 75: 40–45 [DOI] [PubMed] [Google Scholar]
- 34. Voskuyl AE. The heart and cardiovascular manifestations in rheumatoid arthritis. Rheumatology (Oxford) 2006; 45 Suppl 4: iv 4–7 [DOI] [PubMed] [Google Scholar]
- 35. Scott DG, Bacon PA, Tribe CR. Systemic rheumatoid vasculitis: a clinical and laboratory study of 50 cases. Medicine (Baltimore) 1981; 60: 288–297 [PubMed] [Google Scholar]
- 36. Sokoloff L. The heart in rheumatoid arthritis. Am Heart J 1953; 45: 635–643 [DOI] [PubMed] [Google Scholar]
- 37. Cruickshank B. The arteritis of rheumatoid arthritis. Ann Rheum Dis 1954; 13: 136–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Albada-Kuipers GA, Bruijn JA, Westedt ML, et al. Coronary arteritis complicating rheumatoid arthritis. Ann Rheum Dis 1986; 45: 963–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nicola PJ, Maradit-Kremers H, Roger VL, et al. The risk of congestive heart failure in rheumatoid arthritis: a population-based study over 46 years. Ann Rheum Dis 2005; 52: 412–420 [DOI] [PubMed] [Google Scholar]
- 40. Crowson CS, Nicola PJ, Kremers HM, et al. How much of the increased incidence of heart failure in rheumatoid arthritis is attributable to traditional cardiovascular risk factors and ischemic heart disease? Ann Rheum Dis 2005; 52: 3039–3044 [DOI] [PubMed] [Google Scholar]