Abstract
Aims:
Biomarkers are essential in the early detection of acute coronary syndromes (ACS). Serum extracellular vesicles are small vesicles in the plasma containing protein and RNA and have been shown to be involved in ACS-related processes like apoptosis and coagulation. Therefore, we hypothesized that serum extracellular vesicle protein levels are associated with ACS.
Methods and results:
Three serum extracellular vesicle proteins potentially associated with ACS were identified with differential Q-proteomics and were evaluated in 471 frozen serum samples of ACS-suspected patients presenting to the emergency department (30% of whom had an ACS). Protein levels were measured after vesicle isolation using ExoQuick. Mean serum extracellular vesicle concentration of the different proteins was compared between ACS and non-ACS patients. Selected proteins were tested in a univariate logistic regression model, as well as in a multivariate model to adjust for cardiovascular risk factors. A separate analysis was performed in men and women. In the multivariate logistic regression analysis, polygenic immunoglobulin receptor, (pIgR; OR 1.630, p=0.026), cystatin C (OR 1.641, p=0.021), and complement factor C5a (C5a, OR 1.495, p=0.025) were significantly associated with ACS, while total vesicle protein concentration was borderline significant. The association of the individual proteins with ACS was markedly stronger in men.
Conclusions:
These data show that serum extracellular vesicle pIgR, cystatin C, and C5a concentrations are independently associated with ACS and that there are pronounced gender differences. These observations should be validated in a large, prospective study to assess the potential role of vesicle content in the evaluation of patients suspected of having an ACS.
Keywords: ACS, acute coronary syndrome, biomarker, extracellular vesicle, protein
Introduction
Although therapy has improved markedly and mortality has declined the last decades, acute coronary syndromes (ACS) remain a major burden on society and on individual patients.1 Early reperfusion and adequate drug treatment are the cornerstones of ACS management, limiting cardiac dysfunction and subsequent morbidity and mortality. Early diagnosis and treatment are the most important predictors of long-term outcome.2 According to the recent guidelines of the ESC and the ACC/AHA,2–4 the diagnosis ACS is based on combined observations: clinical signs and symptoms, ECG, and blood biochemical markers. Although the application of high-sensitive troponin has improved the accuracy of the diagnosis, there are still patients presenting early after onset of symptoms in whom the diagnosis can’t be confirmed or rejected in the first 4–6 hours.5 Patients suspected of having an ACS but with normal troponin levels and no ST-segment deviation on the ECG have been shown to have a 3–7% risk of experiencing a myocardial infarction or death during 1 year of follow up.6 This group of patients would greatly benefit from new, early measurable biomarkers for ACS.
Extracellular vesicles are small vesicles in the serum (Figure 1), containing significant amounts of protein and RNA, which play a role in intercellular communication and carry information about cellular processes.7–10 Extracellular vesicles have been shown to be involved in ACS-related processes, like coagulation, tissue injury, and apoptosis.11–16 When cells are injured, the content and the function of the extracellular vesicles they excrete changes with the pathophysiological context in which they are released.17,18 Extracellular vesicles are actively secreted from numerous cell types, including cardiomyocytes,19 endothelial cells,20–22 platelets,21,23 and neutrophils.24 These cells participate in the pathophysiological processes causing ACS, and are the firsts to respond to myocardial ischaemia. We hypothesize that protein levels in or on the surface of circulating extracellular vesicles change after the occurrence of an acute cardiac event and therefore might carry information on the existence of an ACS. The role of vesicle protein concentrations as potential biomarkers for ACS remains unexplored thus far.
Figure 1.
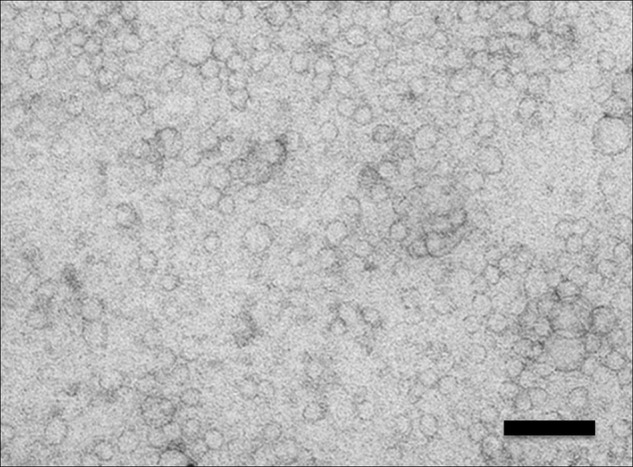
Electron microscopy picture of extracellular vesicles isolated with ExoQuick out of human serum. Extracellular vesicles are clearly visible with vesicles of different sizes, ranging from 20 to 80 nm. Magnification ×60,000. Bar, 100 nm.
Using serum samples from 471 patients with symptoms suggestive for ACS on the emergency department, we aimed to identify a potential association of extracellular vesicle proteins with ACS.
Methods
Patient cohort
FAME-ER (Fatty acid-binding protein in Acute Myocardial infarction Exclusion in the Emergency Room) is a prospective single-center cohort study among ACS-suspected patients presenting to the emergency department of a large regional teaching hospital in the Netherlands (Meander Medical Center, Amersfoort).25 Supplemental Material 1 (available online) gives a detailed description of the FAME-ER cohort.
Biomarker selection
Potential extracellular vesicle protein biomarkers were identified with differential quantitative proteomics comparing extracellular vesicles isolated by ultracentrifugation from a pooled serum sample of 30 patients diagnosed with ACS with extracellular vesicles from a pooled serum sample of 30 sex- and age-matched controls not found to have an ACS. Proteins that were found to have a different concentration between the groups were selected for validation in the individual patients of the whole cohort, using ingenuity pathway analysis and based on the two top ingenuity networks (see Supplemental Material 1 for a detailed description of quantitative proteomics and biomarker selection). This revealed 36 proteins from which three were selected, based on the availability of two antibodies and an antigen for Luminex.
Protein measurement
Extracellular vesicles were isolated from individual patient serum samples using ExoQuick exosome precipitation solution (System Biosciences). Extracellular vesicle concentrations of selected proteins were measured by Luminex-based multiplex panels.
Statistical analysis
All statistical analyses were performed using IBM SPSS version 20. Baseline characteristics were compared between patients with ACS and patients without ACS: continuous variables using Student’s t-test or Mann–Whitney U-test when appropriate, and dichotomous values using Pearson’s chi-squared test. Protein levels were skewed and therefore log transformed to ensure the best model fit. Mean extracellular vesicle protein concentrations were compared between ACS and non-ACS patients with Student’s t-test. Mean values were corrected for age and gender using UNIANOVA and compared between the ACS and non-ACS groups.
The proteins were tested in a univariate logistic regression model, as well as in a multivariate model corrected for risk factors for cardiovascular disease (CVD) (age, gender, hypertension, hypercholesterolaemia, diabetes mellitus, smoking (current and former smoking), and previous myocardial infarction (MI)). Subgroup analyses were performed for men and women, since the interaction terms of the individual proteins with gender were significant in the multivariate model (interaction terms of all markers p<0.05). The analysis has been repeated excluding the samples used for proteomic discovery.
Values are denoted as mean±SD, unless stated otherwise. p-values <0,05 were considered significant.
Results
Discovery of extracellular vesicle proteins associated with ACS
Frozen serum samples were available from 471 patients out of 541 patients in the whole cohort. Of these patients, 140 (30%) were diagnosed with an ACS. From the proteomics analysis, three potential biomarkers were selected: polygenic immunoglobulin receptor (pIgR), cystatin C, and complement factor C5a (see Supplemental Material 1 for a detailed description of protein selection). Total vesicle protein concentration was considered a potential independent biomarker and was analysed accordingly.
Baseline characteristics
In the ACS-diagnosed group, there were significantly more men (66 vs. 52%, p=0.005) and the average age was higher (67.7±12.6 vs. 60.1±14.5 years, p<0.001) (Table 1) than in the non-ACS group. ACS patients were more likely to have hypertension and hypercholesterolaemia, and more of them had experienced a previous MI. The time from onset of symptoms until arriving at the emergency department was the same in both groups. The non-ACS control group consisted of 51 patients with stable angina (15%), 24 with other cardiac diagnoses (8%), and 256 with non-cardiac diagnoses or in whom the diagnosis remained unclear (77%) (Supplemental Material 3 and Table S1).
Table 1.
Baseline characteristics of ACS and non-ACS patients.
| Characteristic (n=471) | Non-ACS (n=331) | ACS (n=140) | p-value |
|---|---|---|---|
| Male (n=469) | 171 (52) | 92 (66) | 0.005 |
| Age (n=465, years) | 60.1±14.5 | 67.7±12.6 | <0.001 |
| Duration of symptoms at presentation (n=435, hours) | 12.5±43.9 | 12.2±39.6 | 0.950 |
| Risk factors | |||
| Previous myocardial infarction (n=466) | 59 (18) | 40 (29) | 0.008 |
| Previous CABG (n=467) | 27 (8) | 19 (14) | 0.066 |
| Previous PCI (n=467) | 67 (20) | 30 (22) | 0.699 |
| Hypertension (n=467) | 121 (37) | 79 (58) | <0.001 |
| Hypercholesterolaemia (n=467) | 93 (28) | 56 (41) | 0.009 |
| Total cholesterol (n=262, mmol/l) | 4.89±1.34 | 4.86±1.29 | 0.854 |
| HDL cholesterol (n=229, mmol/l) | 1.27±0.36 | 1.27±0.30 | 0.953 |
| LDL cholesterol (n=208, mmol/l) | 2.93±1.26 | 2.79±1.18 | 0.438 |
| Triglycerides (n=255, mmol/l) | 1.49±0.91 | 1.66±1.07 | 0.190 |
| Diabetes mellitus (n=467) | 47 (14) | 25 (18) | 0.296 |
| Smoking (n=466) | 171 (52) | 70 (51) | 0.781 |
| Troponin I (n=471, µg/l) | 0.03±0.024 | 1.04±5.52 | <0.001 |
Values are n (%) or mean±SD.
ACS, acute coronary syndrome; CABG, coronary artery bypass graft; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PCI, percutaneous coronary intervention.
Mean protein concentration
The mean vesicle concentrations of pIgR (10.81±0.55 vs. 10.69±0.51 pg/ml, p=0.028) and cystatin C (11.58±0.54 vs. 11.32±0.56 pg/ml, p<0.001) were significantly higher in the ACS-diagnosed patients compared with the non-ACS patients (Table 2A). After correction for age and gender, also C5a and total extracellular vesicle protein concentration were significantly different between the groups (Table 2B), indicating an association with ACS independent of age and gender.
Table 2.
Mean concentrations of potential markers (non-ACS vs. ACS).
| Marker | Non-ACS (n=331) | ACS (n=140) | p-value |
|---|---|---|---|
| A. Absolute protein concentrations (log value±SD) | |||
| pIgR (pg/ml) | 10.69±0.51 | 10.81±0.55 | 0.028 |
| Cystatin C (pg/ml) | 11.32±0.56 | 11.58±0.54 | <0.001 |
| C5a (pg/ml) | 10.31±0.59 | 10.38±0.70 | 0.282 |
| Prot Conc (mg/ml) | 10.57±2.72 | 10.96±2.98 | 0.163 |
| B. Protein concentrations corrected for age and gender (UNIANOVA) (log value±SE) | |||
| pIgR (pg/ml) | 10.68±0.03 | 10.82±0.05 | 0.012 |
| Cystatin C (pg/ml) | 11.35±0.03 | 11.50±0.05 | 0.007 |
| C5a (pg/ml) | 10.29±0.03 | 10.43±0.05 | 0.023 |
| Prot Conc (mg/ml) | 10.52±0.16 | 11.10±0.25 | 0.049 |
Prot Conc, total extracellular vesicle protein concentration.
Logistic regression models
Consistent with the mean protein concentration findings, in a univariate logistic regression model, pIgR (OR 1.532, p=0.029) and cystatin C (OR 2.297, p=<0.001) were associated with ACS (Table 3). After correction for risk factors for CVD in a multivariate model, also C5a (OR 1.495, p=0.025) became significantly associated with ACS, in addition to pIgR (OR 1.630, p=0.026) and cystatin C (OR 1.641, p=0.021), with borderline significance for total protein concentration (OR 1.071, p=0.080) (Table 3).
Table 3.
Logistic regression analysis: all patients (n=471).
| Marker | Univariate |
Multivariatea
|
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| pIgR (pg/ml) | 1.532 | 1.044–2.248 | 0.029 | 1.630 | 1.060–2.507 | 0.026 |
| Cystatin C (pg/ml) | 2.297 | 1.597–3.306 | <0.001 | 1.641 | 1.076–2.502 | 0.021 |
| C5a (pg/ml) | 1.189 | 0.867–1.631 | 0.282 | 1.495 | 1.051–2.128 | 0.025 |
| Prot Conc (mg/ml) | 1.051 | 0.980–1.127 | 0.164 | 1.071 | 0.992–1.157 | 0.080 |
Adjusted for age, gender, previous MI, hypertension, hypercholesterolaemia, DM, and smoking (current and former).
Prot Conc, total extracellular vesicle protein concentration.
Gender
In the male subgroup (n=263), pIgR, cystatin C, and total protein concentration were strongly associated with ACS in a univariate logistic regression model, whereas C5a showed a non-significant trend. In the multivariate model corrected for risk factors, pIgR (OR 2.234, p=0.005), cystatin C (OR 1.914, p=0.025), C5a (OR 1.951, p=0.009), and total protein concentration (OR 1.165, p=0.007) all had a strong association with ACS: the associations were stronger in men than in the total group (Table 4). None of these markers was associated with ACS in the female subgroup (n=208) (Figure 2).
Table 4.
Multivariate logistic regression analysis: males (n=263).
| Marker | OR | 95% CI | Sign (p-value) |
|---|---|---|---|
| pIgR (pg/ml) | 2.234 | 1.272–3.921 | 0.005 |
| Cystatin C (pg/ml) | 1.914 | 1.085–3.376 | 0.025 |
| C5a (pg/ml) | 1.951 | 1.181–3.222 | 0.009 |
| Prot Conc (mg/ml) | 1.165 | 1.042–1.302 | 0.007 |
Adjusted for age, previous MI, hypertension, hypercholesterolaemia, DM, and smoking (current and former).
Prot Conc, total extracellular vesicle protein concentration.
Figure 2.
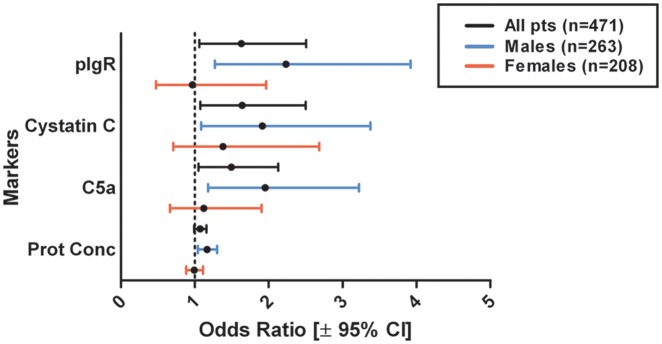
Forrest plot showing multivariate logistic regression analysis for ACS, corrected for age, gender (if applicable), previous MI, hypertension, hypercholesterolaemia, diabetes mellitus, and smoking. Prot Conc, extracellular vesicle protein concentration (mg/ml).
Analysis excluding proteomics samples
Multivariate analysis excluding the patients used in the discovery proteomics showed the same associations between extracellular vesicle proteins and ACS. Multivariate analysis corrected for the risk factors: pIgR (OR 1.617, p=0.040), cystatin C (OR 1.837, p=0.008), C5a (OR 1.569, p=0.018), and protein concentration (OR 1.092, p=0.032). Multivariate analysis in men only corrected for the risk factors: pIgR (OR 2.069, p=0.018), cystatin C (OR 2.288, p=0.009), C5a (OR 2.207, p=0.005), and protein concentration (OR 1.198, p=0.002).
Discussion
This is the first study to demonstrate that selected serum extracellular vesicle proteins carry diagnostic information on the presence of ACS. This holds true even when corrected for traditional risk factors for CVD, indicating that these proteins are independently associated with ACS. Although these proteins at this stage cannot compete with current biomarkers for ACS (Supplemental Material 4), these results demonstrate that vesicle proteins are associated with ACS and for this have the potential to function as biomarkers for ACS. This opens a new field of research. In the current study, pIgR, cystatin C, C5a, and total vesicle protein concentration were demonstrated to be associated with the presence of an ACS.
Polygenic immunoglobulin receptors are present in mucosal epithelium and function as transporters of IgA from the lamina propria to the mucosal lumen, and indirectly enhance the immune function of IgA.26 Their levels have recently been shown to be increased in sputum and blood of smokers and COPD patients.27 Their regulation is complex, involving inflammatory cytokines and activation by Toll-like receptor signalling.28 A role in tissue injury has not been described and remains to be studied. Yet, after correction for smoking, pIgR remained significantly associated with ACS, suggesting that this protein holds more information on ACS than smoking alone.
Cystatin C is a cysteine proteinase, produced by all nucleated cells. Serum levels are regarded as a strong marker of kidney function,29 but cystatin C is also associated with CVD independently of renal disease.30,31 Serum cystatin C has been established as a predictor of cardiovascular events and mortality in patients with non-ST-elevation ACS,32,33 ST-elevation myocardial infarction,34 and suspected ACS,35 but also in the general population36 and elderly persons.37 Although the predictive potential in CVD seems to be high, the diagnostic value of cystatin C for acute myocardial ischaemia remains unexplored so far.
Complement factor C5a is part of the terminal pathway of the complement system and is the most potent anaphylatoxin. Activation of C5a by cell damage or pathogens induces inflammation by chemotaxis of neutrophils and macrophages to the site of injury or infection.38,39 C5a has been shown to play a role in the early inflammation following myocardial ischaemia, contributing both to tissue repair and additional reperfusion injury.40 Plasma C5a levels are increased in patients with unstable angina compared with stable angina41 and increased plasma levels have been shown to be predictive of future cardiovascular events in patients with advanced atherosclerosis,42 making it an interesting potential marker of ACS.
Subgroup analyses revealed a striking difference between men and women. The correlation between concentration of the extracellular vesicle proteins studied and ACS was much stronger in men than in women. Total vesicle protein concentration had a strong association with ACS in men, but no association was found in women.
An increasing body of evidence indicates that symptoms, pathophysiology, and prognosis of ACS are markedly different between men and women.43 Although women (suspected of) having an ACS tend to have less obstructive coronary artery disease (CAD) and have a more preserved left ventricular function, they have a higher mortality and a worse prognosis after MI than men. Several studies implicate abnormal coronary reactivity, microvascular dysfunction, endothelial dysfunction, and distal microembolization in the absence of obstructive CAD as pathophysiological mechanisms underlying angina and ACS in women, in contrast with men in whom obstructive CAD is the predominant mechanism.44–46 These differences are probably driven by several factors, for example varying reproductive hormone levels and autonomic nervous system adrenergic pathways, in combination with a higher burden of pro-atherogenic risk factors in women.43 This may also explain the differences in vesicle protein expression found between men and women with an ACS in our study, which demonstrates the potential for gender-specific diagnostic and prognostic biomarkers of ACS.
Several studies have shown that plasma levels of extracellular vesicles originating from platelets47 and endothelial cells,47,48 but also the total level of procoagulant extracellular vesicles,49 are increased in patients with ACS. In our study, we now also find an association between total vesicle protein content and ACS, which might reflect the increased vesicle excretion in response to ischaemic cardiac events, in line with previous findings. The results of our study, the largest to date, confirm the role of extracellular vesicles in ACS and extend their potential use to the domain where diagnostic uncertainty is greatest, namely patients suspected of an ACS presenting to the emergency room.
Study limitations
Although the markers show a convincing association with ACS, they are not strong enough to compete with currently used blood biochemical markers (see Supplemental Material 4, Figure S1, and Table S2 for a comparison of markers with troponin I). Larger and prospective studies are warranted to evaluate the additional diagnostic value of vesicle proteins for ACS.
Extracellular vesicle protein discovery on patients that present early (within 3–6 hours after onset of symptoms) to the emergency room could be of additional value in identifying early diagnostic biomarkers for ACS. Unfortunately, the study is underpowered for subgroup analyses for the patients that would benefit most from novel diagnostic biomarkers, i.e. patients presenting early after the onset of symptoms, and patients with negative troponin at arrival and without ECG changes.
Although for discovery vesicles were isolated by ultracentrifugation and during validation by ExoQuick, this does not prove that proteins are indeed in or on vesicles. In order to show this, we identified the proteins in the floating vesicles on a sucrose gradient (see Supplemental Material 5 and Figure S2 for detailed description and results). pIgR, cystatin C, and C5a are all present in the floating fractions, showing that at least part of the protein measured is in or on extracellular vesicles. We cannot conclude from this experiment where the proteins are located: on the surface, in the vesicle membrane, or inside the vesicle.
The design of the FAME-ER cohort study did not consider isolation of extracellular vesicles, and therefore sample handling affecting vesicle numbers and content cannot be ruled out. However, experiments have shown that freezing and thawing the samples before vesicle isolation does not influence protein concentration (Supplemental Material 6 and Figure S3). Extracellular vesicles are preferably isolated from plasma instead of serum, since activation of platelets and other cells in serum might cause vesicle release after total blood coagulation. Since plasma samples were not available, serum samples were used instead of plasma.
Conclusions
Serum extracellular vesicle protein concentrations provide information regarding the presence of ACS and therefore have a potential role in the evaluation of patients suspected of having an ACS. There are marked differences in association of these markers with ACS between men and women, which may be a reflection of different pathophysiological mechanisms underlying ischaemic heart disease between genders. The detected differences between men and women in the current study ask for separate discovery in men and women. This can also provide novel gender-specific mechanistic insights into the pathophysiology of ischaemic cardiac events.
Supplementary Material
Acknowledgments
We gratefully acknowledge Hanna Inganas for her excellent technical assistance, and Louise Catanzariti and Sander van der Laan for their valuable scientific and statistical input.
Footnotes
Funding: This study was supported by the Netherlands Heart Foundation (grant number 2011T039, to LT).
Conflict of interest: The authors declare that there are no conflicts of interest.
References
- 1. World Health Organization Cardiovascular diseases (CVDs). Fact sheet no. 317. Available at: http://www.who.int/mediacentre/factsheets/fs317/en/index.html (Published September 2011, consulted May 2012).
- 2. Hamm CW, Bassand JP, Agewall S, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011; 32: 2999–3054 [DOI] [PubMed] [Google Scholar]
- 3. Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 Guidelines for the management of patients with un-stable angina/non-ST-elevation myocardial infarction – executive summary. J Am Coll Cardiol 2007; 50: 652–726 [Google Scholar]
- 4. O’Connor RE, Bossaert L, Arntz HR, et al. Acute Coronary Syndrome Chapter Collaborators. Part 9: acute coronary syndromes: 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation 2010; 122: S422–S465 [DOI] [PubMed] [Google Scholar]
- 5. Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009; 361: 858–867 [DOI] [PubMed] [Google Scholar]
- 6. Sanchis J, Bodí V, Núñez J, et al. New risk score for patients with acute chest pain, non-ST-segment deviation, and normal troponin concentrations. J Am Coll Cardiol 2005; 46: 443–449 [DOI] [PubMed] [Google Scholar]
- 7. Pap E, Pállinger É, Pásztói M, et al. Highlights of a new type of intercellular communication: microvesicle-based information transfer. Inflamm Res 2009; 58: 1–8 [DOI] [PubMed] [Google Scholar]
- 8. Simons M, Raposo G. Exosomes – vesicular carriers for intercellular communication. Curr Opin Cell Biol 2009; 21: 575–581 [DOI] [PubMed] [Google Scholar]
- 9. Camussi G, Deregibus MC, Bruno S, et al. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int 2010; 78: 838–848 [DOI] [PubMed] [Google Scholar]
- 10. Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res 2010; 107: 1047–1057 [DOI] [PubMed] [Google Scholar]
- 11. Morel O, Pereira B, Averous G, et al. Increased levels of procoagulant tissue factor-bearing microparticles within the occluded coronary artery of patients with ST-segment elevation myocardial infarction: role of endothelial damage and leukocyte activation. Atherosclerosis 2009; 204: 636–641 [DOI] [PubMed] [Google Scholar]
- 12. Owens AP, Mackman N. Microparticles in hemostasis and thrombosis. Circ Res 2011; 108: 1284–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shantsila E, Kamphuisen PW, Lip GYH. Circulating microparticles in cardiovascular disease: implications for atherogenesis and atherothrombosis. J Thromb Haemost 2010; 8: 2358–2368 [DOI] [PubMed] [Google Scholar]
- 14. Leroyer AS, Tedgui A, Boulanger CM. Role of microparticles in atherothrombosis. J Intern Medicine 2008; 263: 528–537 [DOI] [PubMed] [Google Scholar]
- 15. Morel O, Morel N, Jesel L, et al. Microparticles: a critical component in the nexus between inflammation, immunity, and thrombosis. Semin Immunopathol 2011; 33: 469–486 [DOI] [PubMed] [Google Scholar]
- 16. Tushuizen ME, Diamant M, Sturk A, et al. Cell-derived microparticles in the pathogenesis of cardiovascular disease: friend or foe? Arterioscl Thromb Vasc Biol 2010; 31: 4–9 [DOI] [PubMed] [Google Scholar]
- 17. Peterson DB, Sander T, Kaul S, et al. Comparative proteomic analysis of PAI-1 and TNF-alpha-derived endothelial microparticles. Proteomics 2008; 8: 2430–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simak J, Holada K, Vostal JG. Release of annexin V-binding membrane microparticles from cultured human umbilical vein endothelial cells after treatment with camptothecin. BMC Cell Biol 2002; 3: 11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Antoniak S, Boltzen U, Eisenreich A, et al. Regulation of cardiomyocyte full-length tissue factor expression and microparticle release under inflammatory conditions in vitro. J Thromb Haemost 2009; 7: 871–878 [DOI] [PubMed] [Google Scholar]
- 20. Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol 2010; 31: 27–33 [DOI] [PubMed] [Google Scholar]
- 21. Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev 2007; 21: 157–171 [DOI] [PubMed] [Google Scholar]
- 22. Combes V, Simon A-C, Grau G-E, et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest 1999; 104: 93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wolf P. The nature and significance of platelet products in human plasma. Brit J Haematol 1967; 13: 269–288 [DOI] [PubMed] [Google Scholar]
- 24. Mesri M, Altieri DC. Endothelial cell activation by leukocyte microparticles. J Immunol 1998; 161: 4382–4387 [PubMed] [Google Scholar]
- 25. Oerlemans MI, Mosterd A, Dekker MS, et al. Early assessment of acute coronary syndromes in the emergency department: the potential diagnostic value of circulating microRNAs. EMBO Mol Med 2012; 4: 1176–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wines BD, Hogarth PM. IgA receptors in health and disease. Tissue Antigens 2006; 68: 103–114 [DOI] [PubMed] [Google Scholar]
- 27. Ohlmeier S, Mazur W, Linja-aho A, et al. Sputum proteomics identifies elevated PIGR levels in smokers and mild- to-moderate COPD. J Proteome Res 2012; 11: 599–608 [DOI] [PubMed] [Google Scholar]
- 28. Asano M, Komiyama K. Polymeric immunoglobulin receptor. J Oral Sci 2011; 52: 147–156 [DOI] [PubMed] [Google Scholar]
- 29. Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis 2002; 40: 221–226 [DOI] [PubMed] [Google Scholar]
- 30. Koc M, Batur MK, Karaarslan O. Clinical utility of serum cystatin C in predicting coronary artery disease. Cardiol J 2010; 17: 374–380 [PubMed] [Google Scholar]
- 31. Kiyosue A, Hirata Y, Ando J, et al. Plasma cystatin C concentration reflects the severity of coronary artery disease in patients without chronic kidney disease. Circ J 2010; 74: 2441–2447 [DOI] [PubMed] [Google Scholar]
- 32. Windhausen F, Hirsch A, Fischer J, et al. ; for the Invasive versus Conservative Treatment in Unstable Coronary Syndromes (ICTUS) Investigators. Cystatin C for enhancement of risk stratification in non-ST elevation acute coronary syndrome patients with an increased troponin T. Clin Chem 2009; 55: 1118–1125 [DOI] [PubMed] [Google Scholar]
- 33. Ristiniemi N, Lund J, Tertti R, et al. Cystatin C as a predictor of all-cause mortality and myocardial infarction in patients with non-ST-elevation acute coronary syndrome. Clin Biochem 2012: 1–6 [DOI] [PubMed] [Google Scholar]
- 34. Silva D, Cortez-Dias N, Jorge C, et al. Cystatin C as prognostic biomarker in ST-segment elevation acute myocardial infarction. Am J Cardiol 2012; 109: 1431–1438 [DOI] [PubMed] [Google Scholar]
- 35. Jernberg T, Lindahl B, James S, et al. Cystatin C: a novel predictor of outcome in suspected or confirmed non-ST-elevation acute coronary syndrome. Circulation 2004; 110: 2342–2348 [DOI] [PubMed] [Google Scholar]
- 36. Toft I, Solbu M, Kronborg J, et al. Cystatin C as risk factor for cardiovascular events and all-cause mortality in the general population. The Tromso Study. Nephrol Dial Transplant 2012; 27: 2780–2787 [DOI] [PubMed] [Google Scholar]
- 37. Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 2005; 352: 2049–2060 [DOI] [PubMed] [Google Scholar]
- 38. Vakeva AP, Agah A, Rollins SA, et al. Myocardial infarction and apoptosis after myocardial ischemia and reperfusion. Circulation 1998; 97: 2259–2267 [DOI] [PubMed] [Google Scholar]
- 39. Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res 2002; 53: 31–47 [DOI] [PubMed] [Google Scholar]
- 40. Timmers L, Pasterkamp G, De Hoog VC, et al. The innate immune response in reperfused myocardium. Cardiovasc Res 2012; 94: 276–283 [DOI] [PubMed] [Google Scholar]
- 41. Kostner KM, Fahti RB, Case C, et al. Inflammation, complement activation and endothelial function in stable and unstable coronary artery disease. Clin Chim Acta 2006; 365: 129–134 [DOI] [PubMed] [Google Scholar]
- 42. Speidl WS, Exner M, Amighi J, et al. Complement component C5a predicts future cardiovascular events in patients with advanced atherosclerosis. Eur Heart J 2005; 26: 2294–2299 [DOI] [PubMed] [Google Scholar]
- 43. Shaw LJ, Bugiardini R, Merz CNB. Women and ischemic heart disease. J Am Coll Cardiol 2009; 54: 1561–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shaw LJ, Bairey Merz CN, Pepine CJ, et al. Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) StudyPart I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol 2006; 47: S4–S20 [DOI] [PubMed] [Google Scholar]
- 45. Merz CNB, Shaw LJ, Reis SE, et al. Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) StudyPart II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol 2006; 47: S21–S29 [DOI] [PubMed] [Google Scholar]
- 46. Gehrie ER, Reynolds HR, Chen AY, et al. Characterization and outcomes of women and men with non–ST-segment elevation myocardial infarction and nonobstructive coronary artery disease: results from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines (CRUSADE) quality improvement initiative. Am Heart J 2009; 158: 688–694 [DOI] [PubMed] [Google Scholar]
- 47. VanWijk MJ, VanBavel E, Sturk A, et al. Microparticles in cardiovascular diseases. Cardiovasc Res 2003; 59: 277–287 [DOI] [PubMed] [Google Scholar]
- 48. Bernal-Mizrachi L, Jy W, Jimenez J, et al. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am Heart J 2003; 145: 962–970 [DOI] [PubMed] [Google Scholar]
- 49. Mallat Z, Benamer H, Hugel B, et al. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation 2000; 101: 841–843 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


