Abstract
Background:
Anaemia is associated with an increased risk for morbidity and mortality in ST-elevation myocardial infarction (STEMI) patients. While several physiological mechanisms have been proposed to explain this association, decreased receipt of guidelines-based care may also contribute. We examined the relationship between admission haemoglobin (Hgb) level, receipt of ACC/AHA guidelines-based treatments, and in-hospital outcomes among STEMI patients. We also evaluated whether administration of these treatments modified the association between anaemia and in-hospital mortality in this group.
Methods and results:
We analysed data from 92,686 patients diagnosed with STEMI included in the NCDR ACTION Registry-GWTG database from January 2007 to March 2011. Patients were stratified by initial Hgb value: 83.1% (n=77,035) were classified as non-anaemic (Hgb >13.0 g/dl for men, >12.0 g/dl for women), 11.6% (n=10,710) as mildly anaemic (11.1−13.0 g/dl for men, 11.1−12.0 g/dl for women), 4.4% (n=4059) as moderately anaemic (9.1−11.0 g/dl), and 1.0% (n=882) as severely anaemic (<9.0 g/dl). Anaemia was associated with a significantly increased prevalence of other baseline comorbidities and decreased odds of receiving several class I recommended pharmacological treatments (heparin, beta-blockers, and angiotensin-converting enzyme inhibitors, p<0.01). The overall use of reperfusion therapy (fibrinolytic therapy and/or percutaneous coronary intervention) was also lower in anaemic vs. non-anaemic patients (p<0.01). Anaemia was associated higher in-hospital mortality risk, which remained significant after adjustment for use of guidelines-recommended therapies and interventions (p<0.01).
Conclusions:
In a national sample of STEMI patients, anaemia on presentation was associated with decreased receipt of ACC/AHA guidelines-based care and higher in-hospital mortality. However, the higher mortality rates could not be fully explained by differences in in-hospital treatment.
Keywords: Anaemia, guidelines, outcomes, ST-segment myocardial infarction (STEMI), treatment
Introduction
Anaemia is a common comorbidity among patients presenting with ST-elevation myocardial infarction (STEMI), and its presence is associated with significantly increased major adverse cardiovascular events.1 Likewise, anaemia prior to percutaneous coronary intervention (PCI) and coronary artery bypass graft (CABG) surgery is also common and associated with increased morbidity and mortality.2 Several physiological mechanisms have been proposed to explain the correlation of anaemia with adverse outcomes during myocardial ischaemia.3 However, decreased guidelines-based treatment administration, as has been shown in acute coronary syndrome (ACS) patients with other comorbidities, may also contribute to the poorer outcomes observed in anaemic STEMI patients.4,5,6 The primary aim of this study was to investigate whether there was a significant association between admission haemoglobin (Hgb) level, ACC/AHA guidelines-based therapy administration, and in-hospital mortality among patients treated for STEMI in a national US registry. The secondary aim was to examine whether administration of these treatments modified the association between anaemia and in-hospital mortality in this group.
Methods
Study population
We performed an observational analysis using data from the NCDR ACTION Registry-GWTG, a nationally representative, voluntary, quality-improvement AMI registry that receives data from over 600 participating hospitals throughout the USA. Details on the data collection process have been previously reported.7 All patients with the diagnosis of STEMI were identified in the database from 1 January 2007 to 31 March 2011, producing a starting population of 113,305 patients from 597 facilities. Diagnostic criteria for STEMI were: (1) ischaemic symptoms at rest, lasting ≥10 minutes, occurring within 72 hours prior to admission; and (2) ECG changes associated with STEMI (new LBBB or persistent ST-segment elevation ≥1 mm in 2 or more contiguous electrocardiographic leads).
Patients were excluded sequentially based on: data captured using the short data collection form (n=8152), missing data (n=1558), patients cared for at centres without access to both cardiothoracic surgery and catheterization lab facilities (n=3613), home warfarin use (n=3048), Hgb values considered to be extreme (<5 or >20 g/dl, n=140), and patients with time from arrival to initial Hgb value of ≥6 hours (n=4108). This left a study population of 92,686 patients from 458 sites (Figure 1).
Figure 1.
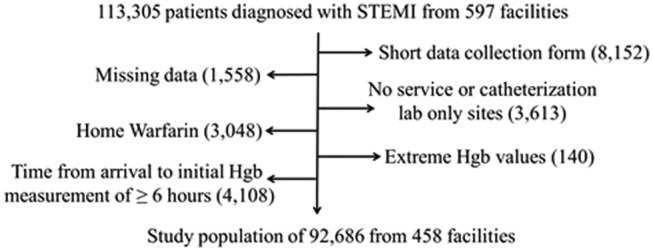
The study population.
113,305 patients met criteria for STEMI in the ACTION Registry-GWTG. After exclusions, the 92,686 patients were included in the study sample.
The study population was stratified into four categories based on the first Hgb measurement obtained at the treating facility using the World Health Organization classification of anaemia: >13.0 g/dl for men and >12.0 g/dl for women (non-anaemic), 11.1−13.0 g/dl for men and 11.1−12.0 g/dl for women (mildly anaemic), 9.1−11.0 g/dl (moderately anaemic), and <9.0 g/dl (severely anaemic).8 Baseline characteristics, patient presentations, in-hospital treatment, and all-cause in-hospital mortality were compared between groups. The treatment data used for this analysis included selected class I recommendations from the 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients with STEMI and the 2008 ACC/AHA Performance Measures for Adults with ST-Elevation and Non-ST-Elevation Myocardial Infarction (Table 1).9,10 These guidelines are similar to the ESC 2008 guidelines for the management of acute myocardial infarction in patients presenting with persistent ST-segment elevation, except that the ESC guidelines do not support the use of fondaparinux for primary PCI patients (class III recommendation).11 In addition, we considered use of glycoprotein IIb-IIIa inhibitors. Patients were considered to have received a pharmacological intervention if it was administered within 24 hours of presentation and a non-pharmacological intervention if it was performed at any point during the index hospital stay. Patients were candidates for angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARBs) if they had an in-hospital documented left ventricular ejection fraction of <40%. Patients with documented contra-indications to a medication or procedure were excluded from the denominator eligible for that intervention. Initial and peak troponin ratio values were defined as the initial or peak value divided by the local laboratory upper limit of normal.
Table 1.
Select Class I recommendations from the 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients with STEMI and the 2008 ACC/AHA Performance Measures for Adults with STEMI.
| 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients with ST-Elevation Myocardial Infarction |
|---|
| Aspirin at arrival |
| Evaluation of LVEF |
| ACEI or ARB for LVEF <40% |
| Door-to-needle time ≤ 30 minutes for fibrinolytic therapy |
| Door-to-balloon time ≤ 90 minutes for primary PCI |
| Reperfusion therapy (receiving either fibrinolysis or primary PCI) |
| 2008 ACC/AHA Performance Measures for Adults with ST-Elevation and Non-ST-Elevation Myocardial Infarction Class I Recommendations |
| Aspirin should be chewed by patients who have not taken aspirin before presentation; the initial dose should be 162−325 mg |
| Oral beta-blocker therapy should be initiated in the first 24 hours for patients without contraindications |
| A loading dose of thienopyridines recommended for STEMI patients in whom PCI is planned |
| For patients preceding to primary PCI who have been treated with aspirin and a thienopyridine, recommended supportive anticoagulant regimens include the following: unfractionated heparin, low-molecular-weight heparin, bivalirudin, or fondaparinux |
| STEMI patients presenting to a hospital with PCI capability should be treated with primary PCI within 90 minutes of first medical contact |
| STEMI patients presenting to a hospital without PCI capability and who cannot be transferred to a PCI centre and undergo PCI within 90 minutes of first medical contact should be treated with fibrinolytic therapy within 30 minutes of hospital presentation |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-II receptor blocker; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention.
Statistical methods
Baseline characteristics, medication use, and in-hospital outcomes were compared across anaemia categories. Medians with interquartile ranges were presented for continuous variables, while percentages and counts were presented for discrete variables. Anaemia categories were treated as ordinal variables. All discrete variables were compared using chi-squares rank-based group means score statistics and all continuous variables were compared using Spearman’s rank correlations. To explore the association between pharmacological and non-pharmacological treatment use and anaemia categories, the logistic generalized estimating equations (GEE) method with exchangeable working correlation matrix was used to account for within-hospital clustering. This method produces odds ratios (95% confidence interval) similar to those from ordinary logistic regression, but variances are adjusted for the correlation of outcomes within a hospital site.12 Similarly, the GEE method was used to investigate the unadjusted and adjusted relationships between in-hospital mortality and anaemia categories. Adjusted analyses included covariates from the validated ACTION-GWTG in-hospital mortality model.13 In addition, individual treatments were included for the in-hospital mortality analyses and interactions between anaemia and each treatment were tested. GEE modelling was used to test for linear trend by fitting anaemia as an ordinal variable in the model. For all of the adjusted analyses, each of the anaemia groups was compared to the non-anaemic group. The significance level used for all hypothesis testing was a two-sided p<0.05. The rate of missing data from this database is low at ≤5% across all variables. For model adjustment, missing values of continuous variables were set to the variable median and missing categorical variables were set to the most frequent group.
The Duke Clinical Research Institute served as the data analysis centre and had an agreement to analyse the aggregate de-identified data for research purposes. All participating institutions were required to comply with local regulatory and privacy guidelines and, if required, to secure institutional review board approval. Because data were used primarily at the local site for quality improvement, sites were granted a waiver of informed consent under the common rule. This study complies with the Declaration of Helsinki and was approved by the Institutional Review Board at Wake Forest Health Sciences for waiver of consent and HIPPA authorization.
All analyses were performed using SAS software (version 9.2; SAS Institute, Cary, North Carolina, USA).
Results
Patient population
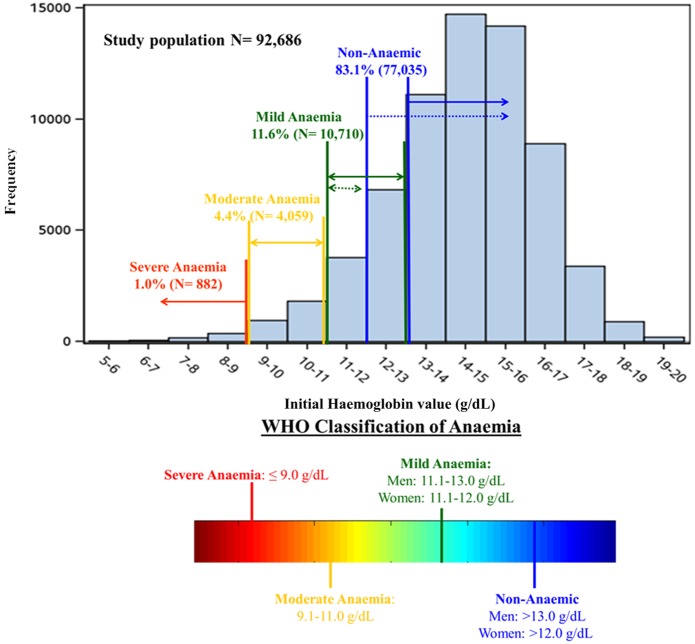
Overall, 83.1% (n=77,035) of patients were non-anaemic, 11.6% (n=10,710) mildly anaemic, 4.4% (n=4059) moderately anaemic, and 1.0% (n=882) severely anaemic (Figure 2). Decreasing levels of admission haemoglobin were associated with significantly older age, female sex, and a higher prevalence of each of the comorbidities measured except for smoking status (Table 2). However, these trends did not hold for the severe anaemia group, which had a lower prevalence of most comorbidities (aside from CHF and atrial fibrillation/flutter) compared to the trend noted in the mildly and moderately anaemic groups.
Figure 2.
Distribution of initial haemoglobin values in the STEMI population.
Overall, 83.1% (77,035) of patients were non-anaemic (>13.0 g/dl for men and >12.0 g/dl for women), 11.6% (10,710) mildly anaemic (11.1−13.0 g/dl for men and 11.1−12.0 g/dl for women), 4.4% (4059) moderately anaemic (9.1−11.0 g/dl), and 1.0% (882) severely anaemic (<9.0 g/dl).
Table 2.
Study population baseline characteristics and hospitalization variables, stratified by initial haemoglobin category.
| Baseline characteristic | Non-anaemic (n=77,035) | Mild anaemia (n=10,710) | Moderate anaemia (n=4,059) | Severe anaemia (n=882) | p-value |
|---|---|---|---|---|---|
| Comorbidities | |||||
| Age (years) | 59.0 (51.0−68.0) | 68.0 (58.0−78.0) | 72.0 (61.0−82.0) | 70.0 (58.0−80.0) | <0.01 |
| Male gender | 55,626 (72.2) | 7,377 (68.9) | 1,636 (40.3) | 388 (44.0) | <0.01 |
| Race | <0.01 | ||||
| Caucasian | 65,769 (85.4) | 8,392 (78.4) | 3,119 (76.8) | 638 (72.3) | |
| Black | 5,453 (7.1) | 1,451 (13.6) | 608 (15.0) | 165 (18.7) | |
| Other | 5,813 (7.5) | 867 (8.0) | 332 (8.2) | 79 (9.0) | |
| Current or recent (<1 year) smoker | 36,288 (47.1) | 3,244 (30.3) | 1,030 (25.4) | 250 (28.3) | <0.01 |
| Hypertension | 46,109 (59.9) | 7,798 (72.8) | 3,214 (79.2) | 661 (74.9) | <0.01 |
| Dyslipidaemia | 38,994 (50.6) | 6,026 (56.3) | 2,292 (56.5) | 457 (51.8) | <0.01 |
| Chronic lung disease | 4,900 (8.7) | 1,029 (13.9) | 541 (18.2) | 109 (16.2) | <0.01 |
| Diabetes mellitus | 15,942 (20.7) | 3,434 (32.1) | 1,581 (39.0) | 311 (35.3) | <0.01 |
| Prior myocardial infarction | 13,437 (17.4) | 2,582 (24.1) | 1,124 (27.7) | 204 (23.1) | <0.01 |
| History of CHF | 2,670 (3.5) | 994 (9.3) | 615 (15.2) | 137 (15.5) | <0.01 |
| Prior revascularization | 16,482 (21.4) | 3,250 (30.4) | 1,283 (31.6) | 231 (26.2) | <0.01 |
| History of atrial fibrillation or flutter | 1,507 (2.7) | 381 (5.2) | 201 (6.7) | 53 (7.9) | <0.01 |
| Prior stroke | 2,913 (3.8) | 856 (8.0) | 487 (12.0) | 92 (10.4) | <0.01 |
| Peripheral vascular disease | 3,476 (4.5) | 1,005 (9.4) | 522 (12.9) | 104 (11.8) | <0.01 |
| Hospitalization variables | |||||
| Signs of CHF | 6,377 (8.3) | 1,665 (15.6) | 939 (23.1) | 205 (23.2) | <0.01 |
| Cardiogenic shock | 4,635 (6.0) | 1,144 (10.7) | 650 (16.0) | 171 (19.4) | <0.01 |
| Signs of CHF and shock | 1,987 (2.6) | 508 (4.7) | 300 (7.4) | 74 (8.4) | <0.01 |
| Initial creatinine (mg/dl) | 1.0 (0.9−1.2) | 1.1 (0.9−1.4) | 1.2 (0.9−1.7) | 1.2 (0.9−1.8) | <0.01 |
| Initial BNP (pg/ml) | 101.0 (28.0−336.0) | 273.0 (82.0−814.0) | 530.0 (178.0−1264.0) | 499.5 (181.0−1306.0) | <0.01 |
| Initial troponin ratio | 1.0 (0.2−8.3) | 1.5 (0.3−19.2) | 2.8 (0.4−27.3) | 3.0 (0.5−23.9) | <0.01 |
| Peak troponin ratio | 145.6 (33.3−560.0) | 123.8 (27.9−500.0) | 118.2 (25.5−456.4) | 110.5 (24.0−500.0) | <0.01 |
| Lowest haemoglobin value (g/dl) | 12.7 (11.3−13.8) | 10.5 (9.3−11.4) | 8.9 (8.2−9.7) | 7.6 (6.9−8.3) | <0.01 |
| LDL value (mg/dl) | 104.0 (80.0−131.0) | 88.0 (65.0−114.0) | 79.0 (58.0−104.0) | 76.0 (55.0−100.0) | <0.01 |
| Required in-hospital red blood cell transfusion | 4,914 (6.6) | 1,585 (15.3) | 1,286 (32.9) | 536 (63.1) | <0.01 |
| Length of stay (days) | 3.0 (2.0−4.0) | 3.0 (2.0−6.0) | 4.0 (3.0−7.0) | 5.0 (3.0−8.0 | <0.01 |
| Unadjusted in-hospital mortality | 3,057 (4.0) | 1,098 (10.6) | 679 (17.4) | 178 (20.2) | <0.01 |
| Time from hospital arrival to death (hours) | 52.6 (14.5−131.9) | 53.4 (15.6−127.7) | 40.7 (13.0−110.7) | 43.1 (8.6−126.5) | 0.03 |
Values are n (%) or median (IQR). p-values test for linear trend across anaemia categories from non-anaemic to severe anaemia groups.
BNP, brain natriuretic peptide; CHF, congestive heart failure; LDL, low-density lipoprotein.
Patient presentations differed across Hgb groups (Table 2) as well. Increasing levels of anaemia were associated with greater prevalence of CHF and cardiogenic shock on presentation. Lower Hgb levels were also associated with higher initial brain natriuretic peptide values and initial troponin ratios, but lower peak troponin ratios. Lower admission Hgb values were associated with longer lengths of hospital stay as well.
Use of class I treatments
Increasing degrees of anaemia were associated with lower odds of receiving anticoagulation (heparin, fondaparinux, or bivalirudin), glycoprotein IIb-IIIa inhibitors, beta-blockers, and ACEI/ARBs (in patients with measured left ventricular ejection fraction <40%), even after adjustment for patient demographics, comorbidities, and presentation variables (Table 3). Increasing levels of anaemia were also associated with decreased adjusted odds of having left ventricular ejection fraction evaluated and undergoing reperfusion therapy (fibrinolytic therapy or primary PCI). Notably, anaemic patients were found to be more likely to undergo primary PCI compared to non-anaemic patients, though they were less likely to receive coronary stents. Among those receiving stents, anaemic patients were less likely to receive a drug-eluting stent compared to non-anaemic patients (47.1 vs. 55.4%, p<0.01).
Table 3.
Adjusted odds ratios for receipt of guidelines-based treatment for STEMI stratified by admission haemoglobin level.
| Mild anaemia (n=10,710) | Moderate anaemia (n=4059) | Severe anaemia (n=882) | p-value | |
|---|---|---|---|---|
| Aspirin | 0.76 (0.63−0.91) | 1.27 (0.85−1.90) | 0.53 (0.32−0.87) | 0.08 |
| Clopidogrel or prasugrel for PCI patients | 0.99 (0.90−1.09) | 0.94 (0.80−1.10) | 0.71 (0.53−0.96) | 0.09 |
| Heparin, fondaparinux, or bivalirudin | 0.82 (0.76−0.88) | 0.78 (0.69−0.87) | 0.54 (0.44−0.67) | <0.01 |
| GP IIb-IIIa inhibitor | 0.93 (0.90−0.97) | 0.86 (0.81−0.92) | 0.69 (0.61−0.79) | <0.01 |
| Beta-blocker | 0.89 (0.83−0.96) | 0.82 (0.74−0.90) | 0.73 (0.59−0.90) | <0.01 |
| ACEI or ARB in patients with LVEF <40% | 0.85 (0.77−0.92) | 0.79 (0.68−0.92) | 0.68 (0.50−0.94) | <0.01 |
| Evaluation of LVEF | 0.93 (0.85−1.01) | 0.74 (0.65−0.83) | 0.78 (0.60−1.02) | <0.01 |
| Thrombolytic therapy | 0.79 (0.71−0.88) | 0.63 (0.53−0.74) | 0.54 (0.38−0.77) | <0.01 |
| Door-to-needle time ≤30 minutes | 0.87 (0.40−1.89) | 1.44 (0.44−4.68) | 0.39 (0.00−488.48) | 0.92 |
| Primary PCI | 1.10 (1.03−1.18) | 1.18 (1.04−1.33) | 1.07 (0.87−1.32) | <0.01 |
| Stented | 0.79 (0.72−0.87) | 0.79 (0.69−0.92) | 0.68 (0.52−0.88) | <0.01 |
| Door-to-balloon ≤90 minutes for primary PCI | 1.00 (0.93−1.08) | 1.02 (0.90−1.14) | 0.93 (0.73−1.20) | 0.96 |
| Reperfusion therapy | 0.93 (0.86−1.00) | 0.87 (0.77−0.98) | 0.72 (0.58−0.90) | <0.01 |
Values are odds ratio (95% confidence interval). Using the non-anaemic group for comparison and adjusted for: demographics: age, race, gender, and insurance status; past medical history: prior stroke, current/recent smoker, weight, diabetes, peripheral artery disease, dyslipidaemia, prior PCI or coronary artery bypass graft, prior myocardial infarction, congestive heart failure, and hypertension; and presentation variables: initial serum creatinine, systolic blood pressure, baseline troponin ratio, heart failure and/or shock, and heart rate.
p-values test for linear trend across anaemia categories from non-anaemic to severe anaemia groups using the non-anaemic group for comparison.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin-II receptor blocker; GP, glycoprotein; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention.
Mortality
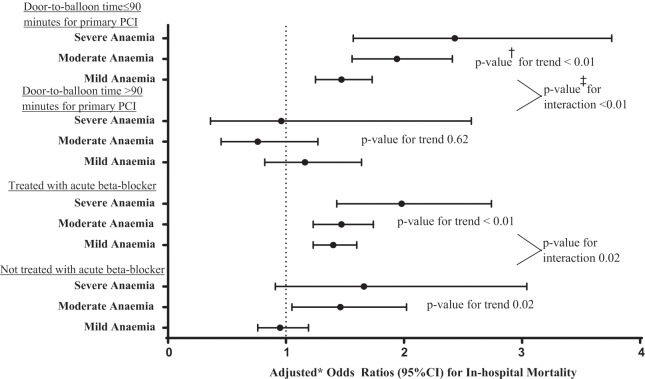
Unadjusted mortality ranged from 4.0% (3057 out of 77,035) in the non-anaemic group to 20.2% (178 out of 882) in the severely anaemic group (Table 2). After adjustments for baseline comorbidities and presentation variables, a statistically significant association between anaemia and mortality persisted. Anaemia remained significantly associated with in-hospital mortality after adjustment for use of each of the class I treatments as well (Table 4). Significant interactions between admission Hgb categories and use of beta-blockers within 24 hours of admission (p=0.02) and door-to-balloon time ≤90 minutes (p<0.01) were identified with respect to in-hospital mortality. After adding interaction terms to the adjusted models, beta-blocker administration within 24 hours of admission was associated with a trend of increased in-hospital mortality among increasingly anaemic STEMI patients compared with non-anaemic patients (Figure 3).
Table 4.
Adjusted odds ratios for in-hospital mortality among STEMI patients, controlling for individual treatments, stratified by admission haemoglobin level.
| Treatment added to adjusted model | Mild anaemia | Moderate anaemia | Severe anaemia | p-value |
|---|---|---|---|---|
| No treatment added to adjusted model | 1.36 (1.24−1.49) | 1.50 (1.35−1.67) | 1.73 (1.39−2.16) | <0.01 |
| Aspirin | 1.37 (1.17−1.61) | 1.50 (1.25−1.80) | 1.50 (1.02−2.21) | <0.01 |
| Clopidogrel or prasugrel (among primary PCI patients) | 1.44 (1.27−1.63) | 1.68 (1.43−1.97) | 2.25 (1.62−3.11) | <0.01 |
| Unfractionated heparin, LMWH, bivalirudin, or fondaparinux | 1.34 (1.22−1.47) | 1.50 (1.35−1.68) | 1.57 (1.26−1.97) | <0.01 |
| Beta-blocker | 1.28 (1.14−1.44) | 1.47 (1.26−1.71) | 1.90 (1.44−2.51) | <0.01 |
| ACEI or ARB in patients with LVEF <40% | 1.22 (1.04−1.43) | 1.28 (1.01−1.63) | 1.30 (0.86−1.97) | <0.01 |
| Door-to-balloon time ≤ 90 minutes | 1.40 (1.22−1.62) | 1.64 (1.34−2.00) | 2.02 (1.36−2.98) | <0.01 |
Using non-anaemic group as comparison group and adjusted for: demographics: age, race, gender, and insurance status; past medical history: prior stroke, current/recent smoker, weight, diabetes, peripheral artery disease, dyslipidaemia, prior PCI or CABG, prior MI, CHF, and hypertension; and presentation variables: initial serum creatinine, heart rate, systolic blood pressure, baseline troponin ratio, heart failure, and shock.
p-values test for linear trend across anaemia categories from non-anaemic to severe anaemia groups using the non-anaemic group for comparison.
ACE, angiotensin-converting enzyme inhibitor; ARB, angiotensin-II receptor blocker; LMWH, low-molecular-weight heparin; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention.
Figure 3.
Forest plot of treatment interactions with admission haemoglobin level in adjusted model for in-hospital mortality for STEMI patients.
Statistically significant interactions between admission haemoglobin categories and the use of beta-blockers and door-to-balloon times ≤90 minutes were identified with respect to in-hospital mortality.
*Using the non-anaemic group for comparison and adjusted for: demographics: age, race, gender, and insurance status; past medical history: prior stroke, current/recent smoker, weight, diabetes, peripheral artery disease, dyslipidaemia, prior percutaneous coronary intervention or coronary artery bypass graft, prior myocardial infarction, congestive heart failure, and hypertension; and presentation variables: initial serum creatinine, heart rate, systolic blood pressure, baseline troponin ratio, heart failure, and shock.
†These p-values test for linear trend across anaemia categories from non-anaemic to severe anaemia group using the non-anaemic group for comparison.
‡These p-values test whether there is a significant interaction between anaemia categories and each treatment in predicting in-hospital mortality.
Discussion
Using data from the ACTION-GWTG registry, we found that 16.9% of STEMI patients had some degree of anaemia at presentation. Anaemia was associated with a significantly increased prevalence of baseline comorbidities and lower use of several ACC/AHA guidelines-based therapies. Further, increasing degrees of anaemia were associated with increasing odds of in-hospital mortality. However, the lower adherence rates to guidelines-based care in anaemic STEMI patients did not entirely explain their increased risk for in-hospital mortality.
Anaemia and myocardial ischaemia
Healthy patients may tolerate isovolaemic Hgb values as low as 5.0 g/dl without developing myocardial ischaemia.14 However, the presence of an obstructive coronary stenosis raises the Hgb threshold for ischaemia and ischaemic myocardial dysfunction has been shown to occur at Hgb values ≤10 g/dl in this setting.15 The association between anaemia, myocardial ischaemia, and unfavourable clinical outcomes has been demonstrated in patients undergoing PCI or CABG surgery.2 In these settings, the risk was not attenuated by blood transfusion. Further, the converse has been observed in patients with ACS among whom transfusion has been associated with greater risk for short-term mortality.3 Several physiological mechanisms have been proposed to explain this association between anaemia and adverse outcomes, including decreased oxygen delivery, increased myocardial oxygen demand, and decreased ability of a fixed coronary lesion to compensate for hypoxaemia during an ischaemic insult.3
Anaemia may also be a marker as opposed to a mediator of increased risk in patients with ACS. Our data supports this hypothesis, showing that none of the treatments investigated significantly modified the odds of in-hospital mortality in anaemic compared with non-anaemic STEMI patients (Table 4). Anaemia is considered to be a marker for generally ‘sicker’ patients with more comorbidities, both chronically and in the acute setting, which was observed in our study population as well (Table 2) and could be the primary driver of the increased in-hospital mortality seen in increasingly anaemic STEMII patients. Interestingly, the severely anaemic group in our study had a lower prevalence of most of the comorbidities that were increased in the mildly and moderately anaemic groups compared to the non-anaemic group. However, the smaller number of patients in the severely anaemic group may indicate that (1) the prevalence of these markers of clinical instability were sufficiently high enough in this group to prevent many of them from surviving to reach a treating facility or (2) those that were captured in this database were the ‘healthier’ patients in this subgroup.
Treatment of anaemic STEMI patients
Selection of treatment strategies for AMI should be balanced between risk for poor outcomes related to ischaemia and potential complications from the treatment, such as bleeding.16 Thus, it was not surprising that many of the anticoagulant and antithrombotic strategies were less often administered to anaemic STEMI patients in our study group, which is similar to findings published by Bassand et al.6 Current ACC/AHA STEMI treatment guidelines do not address treatment of this patient group. The 2008 ESC guidelines for the treatment of STEMI patients do not specifically address the treatment of anaemic STEMI patients either, though the 2012 update of these guidelines mention anaemia as a risk factor for worse outcomes in this patient group and that dual antiplatelet therapy should be undertaken with caution in these patients.11,17 The 2011 ESC Guidelines for the management of ACS in patients presenting without persistent ST-segment elevation have a section dedicated to anaemic patients, though they note that the management of this patient group is based on empirical data.18
However, lower adjusted adherence rates for certain therapies (e.g. beta-blockers) cannot be explained by the presence of anaemia alone. These findings suggest that physicians may be electing to avoid treatment risk and adopt a ‘do no harm’ strategy in the treatment of anaemic STEMI patients in whom Hgb is measured prior to the administration of treatment. This ‘treatment-risk paradox’ has been shown in other studies investigating differences in treatment of ACS patients related to baseline comorbidities.4,5
Despite concerns over administering many of the recommended anticoagulant therapies in anaemic STEMI patients, no statistically significant interaction was noted between the degree of anaemia on presentation and use of aspirin, thienopyridines for patients undergoing primary PCI, heparin, bivalirudin, or fondaparinux. These data likely suggests that physicians correctly identified the appropriate candidates for receipt of these therapies, though they could also suggest that these medications could be administered to anaemic STEMI patients without significantly increasing their risk of in-hospital mortality. However, this did not hold true for acute beta-blocker administration. The increased risk for in-hospital mortality shown in our study for anaemic patients who received beta-blocker therapy adds to the concerns about general use of beta-blockers in ACS patients at increased risk for haemodynamic instability.19
Anaemic patients also appeared to be as likely to undergo primary PCI and have door-to-needle times within 90 minutes, but much less likely to receive adjunctive anticoagulation or antiplatelet therapy compared to non-anaemic STEMI patients. This is an important observation since it is known that patients who undergo PCI with stents do poorly if they are not treated with concomitant pharmacological therapy.20 Thus, our data may suggest inconsistent priorities in STEMI care. Perhaps the emphasis on door-to-balloon times has somewhat skewed the care of STEMI patients to prioritize the mechanical aspect of revascularization over necessary concurrent pharmacological interventions.
Limitations
Data from this type of registry introduces selection bias as hospitals that self-select for this type of registry tend to be larger tertiary referral centres and are more likely to follow guidelines-based care recommendations. We had to exclude 4108 patients in the registry from our analyses due to measurement of an initial Hgb value ≥6 hours from initial presentation, which could introduce a selection bias as well. Additionally, outcomes in the registry are limited to in-hospital events and causes of death are not differentiated. Similarly, history or type of prior anaemia, chronic kidney disease, cancer, and bleeding disorders are not covariates collected in the registry, which could affect presenting haemoglobin values, provider treatment decisions, and outcomes. Thus, there could be other inherent differences between anaemic and non-anaemic STEMI patients that could not be accounted for in our analysis, even with further analyses such as a propensity analysis. Systemic anticoagulation is also a concern in the management of patients with STEMI, especially since there is an increasing prevalence of home warfarin use in patients presenting with AMI.21,22 We excluded patients on warfarin from this study, which limits its generalizability to this group. Despite these limitations, the registry reflects a broad, national experience in actual clinical care of STEMI patients.
In conclusion, anaemia was an independent risk factor associated with in-hospital mortality for STEMI patients. While evidence of the ‘treatment-risk paradox’ was present in anaemic compared with non-anaemic patients, the lower adherence rates to ACC/AHA guidelines-based care in anaemic STEMI patients did not entirely explain their increased risk for in-hospital mortality, which could be due to increased comorbidities, low Hgb levels in the setting of myocardial ischaemia, or other factors not captured in the database.
Acknowledgments
We would like to thank Anita Y. Chen for her contributions to this manuscript.
Footnotes
Funding: This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (grant number T32 HL076132] and the American College of Cardiology Foundation’s National Cardiovascular Data Registry (NCDR). The ACTION Registry-GWTG is an initiative of the American College of Cardiology Foundation and the American Heart Association with partnering support from the Society of Chest Pain Centers, the American College of Emergency Physicians, and the Society of Hospital Medicine. The ACTION Registry-GWTG is sponsored in part by the Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership.
Conflict of interest: None declared.
References
- 1. Sabatine MS, Morrow DA, Giugliano RP, et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation 2005; 111: 2042–2049 [DOI] [PubMed] [Google Scholar]
- 2. Maluenda G, Lemesle G, Collins SD, et al. The clinical significance of hematocrit values before and after percutaneous coronary intervention. Am Heart J 2009; 158: 1024–1030 [DOI] [PubMed] [Google Scholar]
- 3. Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA 2004; 292: 1555–1562 [DOI] [PubMed] [Google Scholar]
- 4. McKechnie RS, Smith D, Montoye C, et al. Prognostic implication of anemia on in-hospital outcomes after percutaneous coronary intervention. Circulation 2004; 110: 271–277 [DOI] [PubMed] [Google Scholar]
- 5. van Straten AH, Bekker MW, Soliman Hamad MA, et al. Transfusion of red blood cells: the impact on short-term and long-term survival after coronary artery bypass grafting, a ten-year follow-up. Interact Cardiovasc Thorac Surg 2010; 10: 37–42 [DOI] [PubMed] [Google Scholar]
- 6. Bassand J-P, Afzal R, Eikelboom J, et al. ; on behalf of the OASIS 5 and OASIS 6 Investigators. Relationship between baseline haemoglobin and major bleeding complications in acute coronary syndromes. Eur Heart J 2010; 31: 50–58 [DOI] [PubMed] [Google Scholar]
- 7. Peterson ED, Roe MT, Rumsfeld JS, et al. A call to ACTION (Acute Coronary Treatment and Intervention Outcomes Network): a national effort to promote timely clinical feedback and support continuous quality improvement for acute myocardial infarction. Circ Cardiovasc Qual Outcomes 2009; 2: 491–499 [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization Nutritional anemias: report of a WHO Scientific Group. Geneva: World Health Organization, 1968 [PubMed] [Google Scholar]
- 9. Kushner FG, Hand M, Smith SC, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol 2009; 54: 2205–2241 [DOI] [PubMed] [Google Scholar]
- 10. Krumholz HM, Anderson JL, Bachelder BL, et al. ACC/AHA 2008 performance measures for adults with ST-elevation and non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association task force on performance measures (writing committee to develop performance measures for ST-elevation and non-ST-elevation myocardial infarction). Circulation 2008; 118: 2596–2648 [DOI] [PubMed] [Google Scholar]
- 11. Van de Werf F, Bax J, Betriu A, et al. ; for the Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation. Eur Heart J 2008; 29: 2909–2945 [DOI] [PubMed] [Google Scholar]
- 12. Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986; 42: 121–130 [PubMed] [Google Scholar]
- 13. Chin CT, Chen AY, Wang TY, et al. Risk adjustment for in-hospital mortality of contemporary patients with acute myocardial infarction: the Acute Coronary Treatment and Intervention Outcomes Network (ACTION) registry − get with the guidelines (GWTG) acute myocardial infarction mortality model and risk score. Am Heart J 2011; 161: 113–122 [DOI] [PubMed] [Google Scholar]
- 14. Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA 1998; 279: 217–221 [DOI] [PubMed] [Google Scholar]
- 15. Hagl S, Heimisch W, Meisner H, et al. The effect of hemodilution on regional myocardial function in the presence of coronary stenosis. Basic Res Cardiol 1977; 72: 344–364 [DOI] [PubMed] [Google Scholar]
- 16. Moscucci M, Fox KA, Cannon CP, et al. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J 2003; 24: 1815–1823 [DOI] [PubMed] [Google Scholar]
- 17. Steg PG, James SK, Atar D, et al. ; for The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012; 33: 2569–2619 [DOI] [PubMed] [Google Scholar]
- 18. Hamm CW, Bassand J-P, Agewall S, et al. ; for The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2011; 32: 2999–3054 [DOI] [PubMed] [Google Scholar]
- 19. Chen ZM, Pan HC, Chen YP, et al. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366: 1622–1632 [DOI] [PubMed] [Google Scholar]
- 20. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011; 124: e574–651 [DOI] [PubMed] [Google Scholar]
- 21. Wang TY, Chen AY, Peterson ED, et al. Impact of home warfarin use on treatment patterns and bleeding complications for patients with non-ST-segment elevation acute coronary syndromes: observations from the CRUSADE quality improvement initiative. Eur Heart J 2008; 29: 1103–1109 [DOI] [PubMed] [Google Scholar]
- 22. Mathews R, Peterson ED, Chen AY, et al. In-hospital major bleeding during ST-elevation and non-ST-elevation myocardial infarction care: derivation and validation of a model from the Action Registry®-GWTG™. Am J Cardiol 2011; 107: 1136–1143 [DOI] [PubMed] [Google Scholar]