Abstract
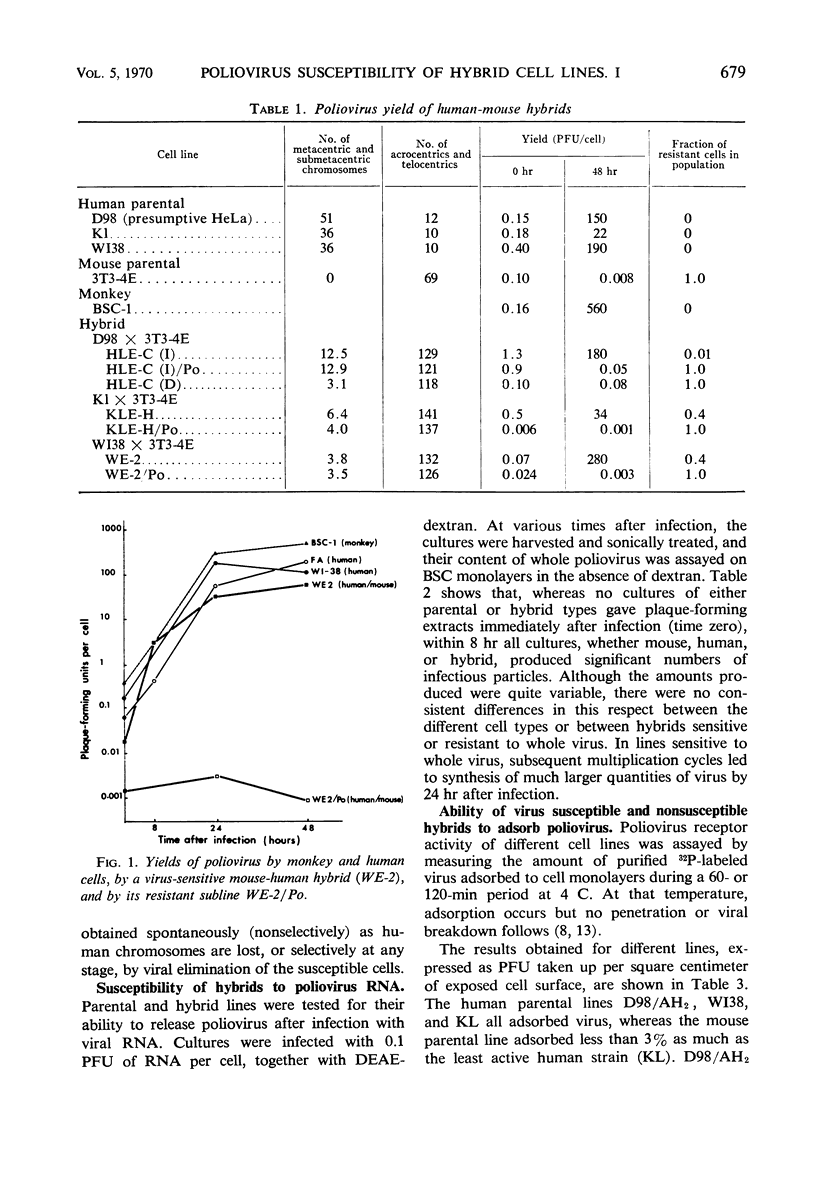
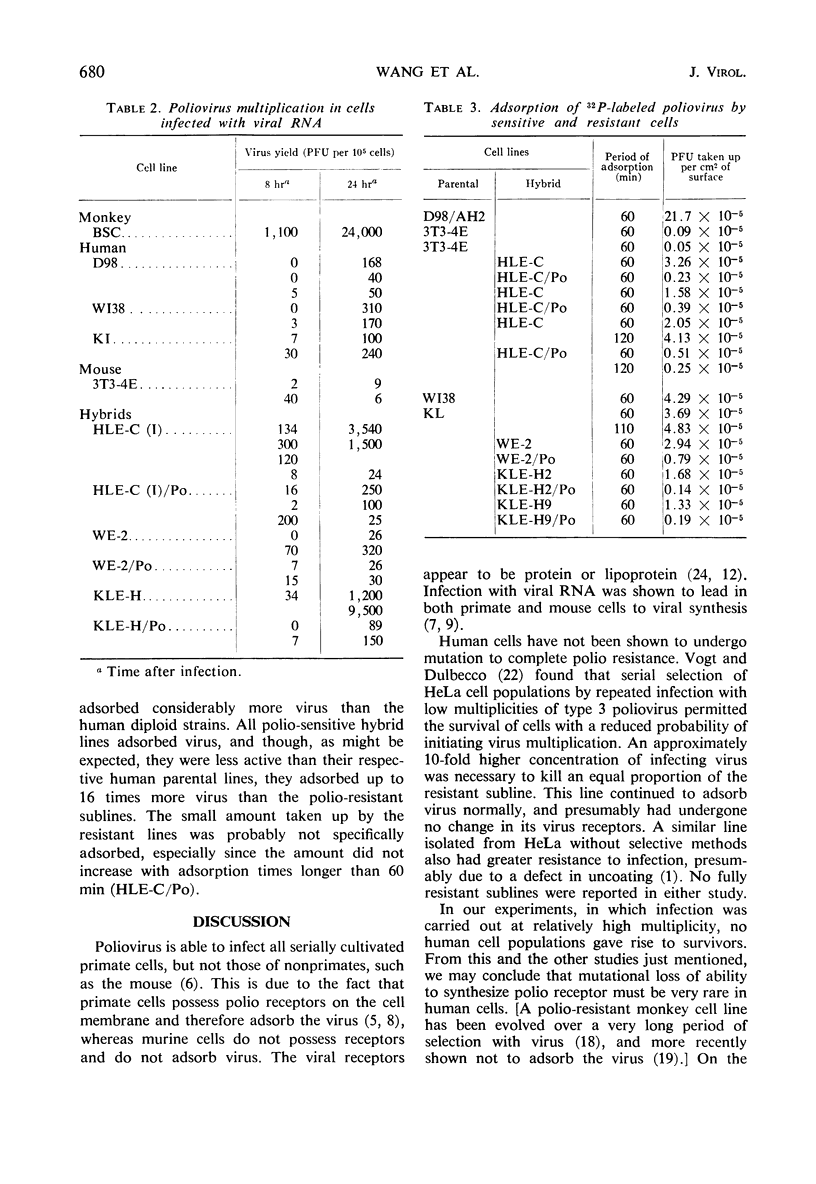
A number of human-mouse somatic hybrid cell lines have been prepared, containing from 3 to 12 human biarmed chromosomes. These lines were susceptible to poliovirus type 1, producing viral yields comparable to those of the human parental cells. A small proportion of the cells of these lines survived the polio infection, and their progeny were solidly resistant to reinfection with the virus. Both sensitive and resistant hybrids produced virus following infection with viral ribonucleic acid, indicating that the cytoplasm of the resistant hybrids was able to support viral multiplication. Viral adsorption studies carried out at 4 C showed that the resistant sublines had negligible ability to adsorb the virus. It was concluded that the hybrid cells became resistant to polio through loss of the human chromosome bearing the gene for the receptor substance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DARNELL J. E., Jr, SAWYER T. K. The basis for variation in susceptibility to poliovirus in HeLa cells. Virology. 1960 Aug;11:665–675. doi: 10.1016/0042-6822(60)90113-6. [DOI] [PubMed] [Google Scholar]
- Gartler S. M. Genetic markers as tracers in cell culture. Natl Cancer Inst Monogr. 1967 Sep;26:167–195. [PubMed] [Google Scholar]
- HAYFLICK L., MOORHEAD P. S. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961 Dec;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J. ENTEROVIRUS ENTRANCE INTO SPECIFIC HOST CELLS, AND SUBSEQUENT ALTERATIONS OF CELL PROTEIN AND NUCLEIC ACID SYNTHESIS. Bacteriol Rev. 1964 Mar;28:2–13. doi: 10.1128/br.28.1.2-13.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J., HOYER B. H., McLAREN L. C., SYVERTON J. T. Enteroviral ribonucleic acid. I. Recovery from virus and assimilation by cells. J Exp Med. 1960 Nov 1;112:821–839. doi: 10.1084/jem.112.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C., SYVERTON J. T. The mammalian cell-virus relationship. IV. Infection of naturally insusceptible cells with enterovirus ribonucleic acid. J Exp Med. 1959 Jul 1;110(1):65–80. doi: 10.1084/jem.110.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C. The mammalian cell-virus relationship. II. Adsorption, reception, and eclipse of poliovirus by HeLa cells. J Exp Med. 1959 May 1;109(5):487–504. doi: 10.1084/jem.109.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J. Receptor affinities as major determinants of enterovirus tissue tropisms in humans. Virology. 1961 Nov;15:312–326. doi: 10.1016/0042-6822(61)90363-4. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Mandel B. The interaction of neutralized poliovirus with HeLa cells. I. Adsorption. Virology. 1967 Feb;31(2):238–247. doi: 10.1016/0042-6822(67)90167-5. [DOI] [PubMed] [Google Scholar]
- Matsuya Y., Green H. Somatic cell hybrid between the established human line D98 (presumptive HeLa) and 3T3. Science. 1969 Feb 14;163(3868):697–698. doi: 10.1126/science.163.3868.697. [DOI] [PubMed] [Google Scholar]
- McLAREN L. C., HOLLAND J. J., SYVERTON J. T. The mammalian cell-virus relationship. I. Attachment of poliovirus to cultivated cells of primate and non-primate origin. J Exp Med. 1959 May 1;109(5):475–485. doi: 10.1084/jem.109.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren L. C., Scaletti J. V., James C. G. Isolation and properties of enterovirus receptors. Wistar Inst Symp Monogr. 1968;8:123–135. [PubMed] [Google Scholar]
- Pagano J. S., McCutchan J. H., Vaheri A. Factors influencing the enhancement of the infectivity of poliovirus ribonucleic acid by diethylaminoethyl-dextran. J Virol. 1967 Oct;1(5):891–897. doi: 10.1128/jvi.1.5.891-897.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLOVEV V. D., GULEVICH N. E., VARSHAVER N. B. VIRUSOLOGICHESKOE I KARIOLOGICHESKOE IZUCHENIE REZISTENTNO I K VIRUSU POLIOMIELITA KLETOCHNO I LINII. Vopr Virusol. 1963 Sep-Oct;67:580–583. [PubMed] [Google Scholar]
- Seegmiller J. E., Rosenbloom F. M., Kelley W. N. Enzyme defect associated with a sex-linked human neurological disorder and excessive purine synthesis. Science. 1967 Mar 31;155(3770):1682–1684. doi: 10.1126/science.155.3770.1682. [DOI] [PubMed] [Google Scholar]
- Soloviev V. D., Krispin T. I., Zaslavsky V. G., Agol V. I. Mechanism of resistance to enteroviruses of some primate cells in tissue culture. J Virol. 1968 Jun;2(6):553–557. doi: 10.1128/jvi.2.6.553-557.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGT M., DULBECCO R. Properties of a HeLa cell culture with increased resistance to poliomyelitis virus. Virology. 1958 Jun;5(3):425–434. doi: 10.1016/0042-6822(58)90037-0. [DOI] [PubMed] [Google Scholar]
- Weiss M. C., Green H. Human-mouse hybrid cell lines containing partial complements of human chromosomes and functioning human genes. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1104–1111. doi: 10.1073/pnas.58.3.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAJAC I., CROWELL R. L. EFFECT OF ENZYMES ON THE INTERACTION OF ENTEROVIRUSES WITH LIVING HELA CELLS. J Bacteriol. 1965 Mar;89:574–582. doi: 10.1128/jb.89.3.574-582.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]


