Abstract
Biphasic lesions composed of melanocytic and epithelial components are not uncommon. Combined trichoepithelioma and cellular blue naevus is a rare lesion that may mimic melanoma clinically. The authors describe what they believe to be the fourth case of this rare tumor and review the literature. Recent descriptions of malignant basomelanocytic tumors have raised interesting questions surrounding the pathogenesis of lesions composed of these cell lineages.
The authors describe a combined tumor composed of a cellular blue nevus and a trichoepithelioma. This highly distinctive tumor may not be as uncommon as is reflected in the literature. To the best of the authors’ knowledge, there have only been three previous cases reported in the literature (Table 1).1,2
TABLE 1.
Previously reported cases of combined cellular blue nevus and trichoepithelioma
CASE REPORT
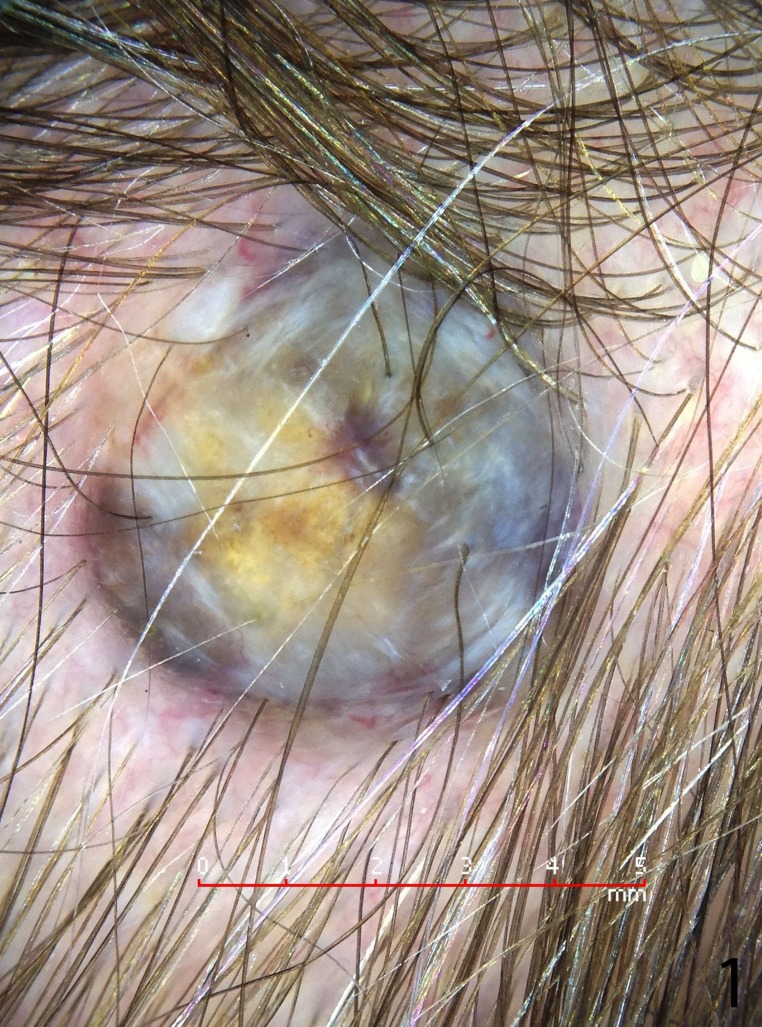
A 56-year-old woman presented with a darkly pigmented lesion on her scalp that her hairdresser had noticed and was concerned it had increased in size. The patient reported the lesion had been present for years. She had no previous skin cancers and there was no family history of melanoma. Clinical examination revealed Fitzpatrick skin type II with a raised, nodular, variably pigmented lesion on the right posterior scalp measuring approximately 6x5mm. Dermoscopic (DermLite, DL-1) examination with cross polarized light (Figure 1) showed an irregularly colored lesion with focal homogeneous blue black pigmentation and yellow areas. An excision biopsy was performed with 2mm margins as per guidelines.
Figure 1.
Dermatoscopic image shows a heavily pigmented lesion with yellow areas. This was presented on a dermoscopy blog (International Society of Dermoscopy) and was thought to be either a blue nevus, a melanoma, or a reactive change to a ruptured cyst.
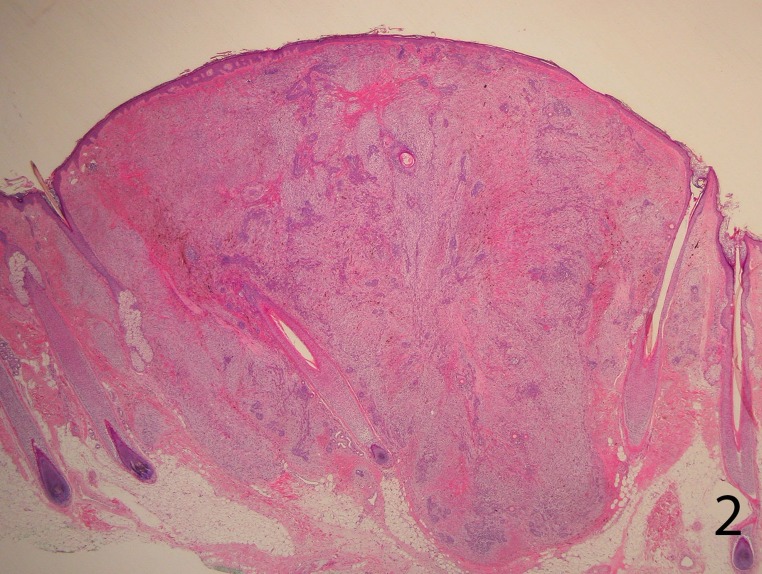
Histopathological examination revealed a fairly well-circumscribed biphasic dermal proliferation involving the full thickness of the dermis and shows tongues of rounded tumor fronts, which encroached on subcutaneous fat. The overlying epidermis was uninvolved (Figure 2).
Figure 2.
A well-circumscribed, biphasic, dermal proliferation involving the full thickness of the dermis and shows tongues of rounded tumor fronts in the deep dermis. The overlying epidermis was uninvolved (H&E stain, 4X)
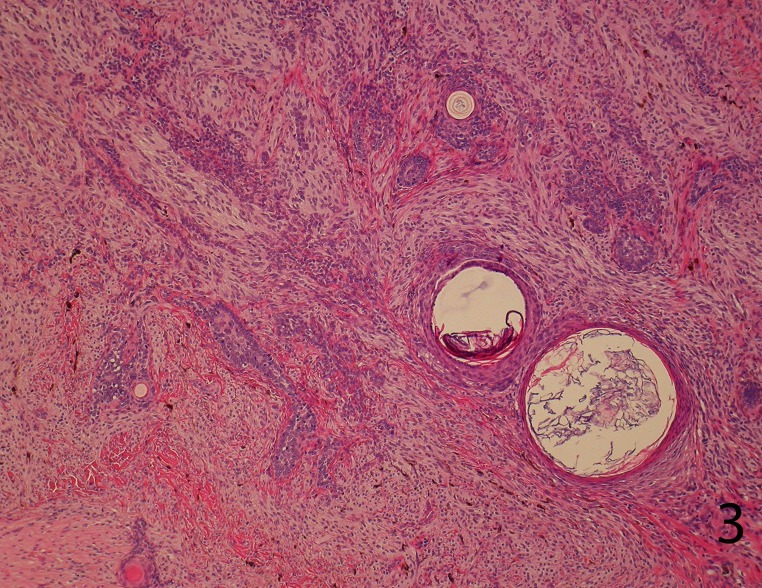
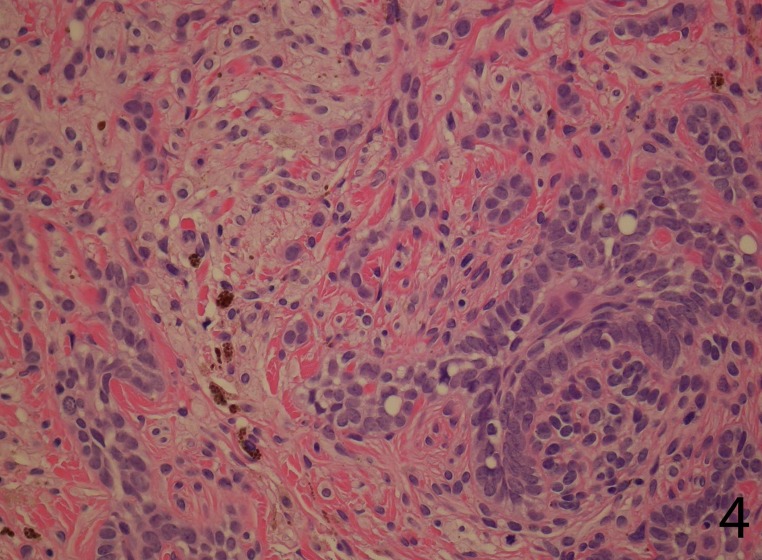
The tumor comprised intimately mixed populations of melanocytes and basaloid cells. The melanocytic component consisted of islands of plump spindled cells with an abundant pale cytoplasm and minimal pigment and rarer slender dendritic melanocytes with heavy melanin pigmentation. Regularly intermixed with this, there were nests of basaloid cells anastomosed in a lacelike pattern and forming horn cysts focally (Figure 3). The follicular component was surrounded by a prominent stroma, which condensed around the basaloid islands as hair papilla-like structures (Figure 4).
Figure 3.
Sheets of plump spindled melanocytes with an abundant pale cytoplasm and rarer collections of dendritic melanocytes with heavy melanin pigmentation are intimately intermixed with nests of basaloid cells anastomosed in a lace-like. Horn cysts are seen focally (H&E stain, 20X).
Figure 4.
The follicular component is surrounded by the cellular blue nevus and focally the basaloid islands as hair papilla-like structures (H&E stain, 40X)
The follicular and melanocytic components stained strongly with p63 and S100, respectively, and there was no cross-over of staining.
The combination of common melanocytic nevi with other tumors of adnexal origin has often been described.3-5 Melanocytic nevi may be closely associated with eccrine sweat ducts,6 cystic dilatation of hair follicles, or cysts.7 The most common association is a dermal nevus with epidermoid cyst. This is so common that in French-speaking countries it has an eponym: Duperrat nevus. Combinations of pigmented nevi and various adnexal proliferations have also been reported.8 About 10 percent of cases of desmoplastic trichoepitheliomas are associated with a coexisting intradermal melanocytic nevus.5
There are several hypotheses to explain these distinct cell types within the same lesion. It has been proposed that pigmented nevi include not only benign neoplasms but also hamartomas in which nevus cells occur together with several different structures of ectodermal origin.5 Another hypothesis is that the melanocytic lesion elicits an inductive effect on epithelia. Perhaps the most compelling example of this can be seen in an example of a melanocytic nevus stimulating hair follicle formation on the soles.9 Similarly, epidermal hyperplasia and follicular induction is well known to occur in conjunction with dermal nevi.10 Keen11 suggested that the local paracrine effect of cytokines or growth factors secreted by the nevus cells accounts for epithelial proliferation.11,12 It seems likely that at least some of the benign cystic proliferations associated with nevi may be due to occlusion of follicles rather than some type of chemical paracrine effect.13
Malignant tumors with dual follicular and melanocytic differentiation (basomelanocytic tumors) have received some attention in the literature recently. It remains unclear whether these represent tumor collision, or melanocytic colonization, or whether they illustrate a previously undelineated phenomenon by which pleuropotential stem cell populations can exhibit dual differentiation into epithelial and melanocytic cell lines.14-16 This later theory is particularly troublesome as the evidence for the neural crest origin of melanocytes is compelling and has been widely accepted.17 Rodriguez et al14 advanced an interesting analogy with the neuroendocrine cells of the gastrointestinal tract. Those cells were long believed to be of neural crest derivation, but the occurrence of mixed adenocarcinomas-neuroendocrine carcinomas provided one of the first clues that eventually debunked that theory and led to the currently held belief that those cells are of the same endodermal derivation than the other epithelial cells of the gastrointestinal mucosa.14
Though there is no evidence from the current case that there is a single pluripotent cell line, it is interesting to consider that trichoepithelioma is generally regarded as a bona fide tumor rather than a hamartoma or hyperplasia, complete with an oncogene that is convincingly involved in its pathogenesis (CYLD). On the other hand, dendritic melanocytes are thought to be derived from precursor cells which do not complete migration from the neural crest to the epidermis during embryogenesis.18 How these two populations are interrelated is unclear at this time.
CONCLUSION
Combined cellular blue nevus and trichoepithelioma is a rarely reported but distinctive biphasic tumor. It can have a striking clinical presentation that mimics melanoma. The pathogenesis of such tumors is unclear.
ACKNOWLEDGMENT
The authors are grateful to David Weedon for kindly providing consultation for this case.
Footnotes
DISCLOSURE:The authors report no relevant conflicts of interest.
REFERENCES
- 1.Newton JA, McGibbon DH. Blue naevus associated with trichoepithelioma: a report of two cases. J Cutan Pathol. 1984;11(6):549. doi: 10.1111/j.1600-0560.1984.tb00417.x. [DOI] [PubMed] [Google Scholar]
- 2.Al-Ghamdi AM, Trotter MJ. Trichoepithelioma associated with cellular blue nevus. J Cutan Med Surg. 1999;3(6):317. doi: 10.1177/120347549900300609. [DOI] [PubMed] [Google Scholar]
- 3.Rahbari H, Mehregan AH. Trichoepithelioma and pigmented nevus. A combined malformation. J Cutan Pathol. 1975;2(5):225. doi: 10.1111/j.1600-0560.1975.tb00170.x. [DOI] [PubMed] [Google Scholar]
- 4.Brownstein MH, Starink TM. Desmoplastic trichoepithelioma and intradermal nevus: a combined malformation. J Am Acad Dermatol. 1987;17(3):489. doi: 10.1016/s0190-9622(87)70234-5. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Vela MC, Val-Bernal JF, Garcia-Alberdi E, et al. Trichoadenoma associated with an intradermal melanocytic nevus: a combined malformation. Am J Dermatopathol. 2007;29(1):92. doi: 10.1097/DAD.0b013e31802e462f. [DOI] [PubMed] [Google Scholar]
- 6.Mishima Y. Eccrine-centered nevus. Arch Dermatol. 1973;107(1):59. [PubMed] [Google Scholar]
- 7.Tsuruta D, Nakagawa K, Taniguchi S, et al. Combined cutaneous hamartoma encompassing benign melanocytic naevus, vellus hair cyst and epidermoid cyst. Clin Exp Dermatol. 2000;25(1):38. doi: 10.1046/j.1365-2230.2000.00569.x. [DOI] [PubMed] [Google Scholar]
- 8.Malhotra R, Bhawan J, Stadecker M. Association of syringoma and intradermal nevus. Int J Dermatol. 1986;25(6):397. doi: 10.1111/j.1365-4362.1986.tb03434.x. [DOI] [PubMed] [Google Scholar]
- 9.Mehregan AH, Coskey RJ. Pigmented nevi of sole. A report of two cases with histologic evidence of hair follicle formation. Arch Dermatol. 1972;106(6):886. doi: 10.1001/archderm.106.6.886. [DOI] [PubMed] [Google Scholar]
- 10.Horenstein MG, Prieto VG, Burchette JL, Jr, Shea CR. Keratotic melanocytic nevus: a clinicopathologic and immunohistochemical study. J Cutan Pathol. 2000;27(7):344. doi: 10.1034/j.1600-0560.2000.027007344.x. [DOI] [PubMed] [Google Scholar]
- 11.Keen CE. Combined skin lesions. Am J Dermatopathol. 1996;18(5):527. doi: 10.1097/00000372-199610000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Requena L, Yus ES, Simon P, del Rio E. Induction of cutaneous hyperplasias by altered stroma. Am J Dermatopathol. 1996;18(3):248. doi: 10.1097/00000372-199606000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Erkek E, Bozdogan O. Pigmented terminal hair cysts within an intradermal melanocytic naevus. J Eur Acad Dermatol Venereol. 2003;17(1):94. doi: 10.1046/j.1468-3083.2003.00638.x. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez J, Nonaka D, Kuhn E, et al. Combined high-grade basal cell carcinoma and malignant melanoma of the skin (“malignant basomelanocytic tumor”): report of two cases and review of the literature. Am J Dermatopathol. 2005;27(4):314. doi: 10.1097/01.dad.0000171600.17692.65. [DOI] [PubMed] [Google Scholar]
- 15.Erickson LA, Myers JL, Mihm MC, et al. Malignant basomelanocytic tumor manifesting as metastatic melanoma. Am J Surg Pathol. 2004;28(10):1393. doi: 10.1097/01.pas.0000135526.19189.31. [DOI] [PubMed] [Google Scholar]
- 16.Riccioni L, Cenacchi G, Ragazzini T, Collina G. Follicular baso-squamous melanocytic tumour of the skin. Histopathology. 2002;41(4):337. doi: 10.1046/j.1365-2559.2002.01508.x. [DOI] [PubMed] [Google Scholar]
- 17.Le Douarin N, Kalcheim C. The neural crest. 2nd ed. Cambridge, New York: Cambridge University Press; 1999. [Google Scholar]
- 18.Zembowicz A, Mihm MC. Dermal dendritic melanocytic proliferations: an update. Histopathology. 2004;45(5):433. doi: 10.1111/j.1365-2559.2004.01975.x. [DOI] [PubMed] [Google Scholar]