Abstract
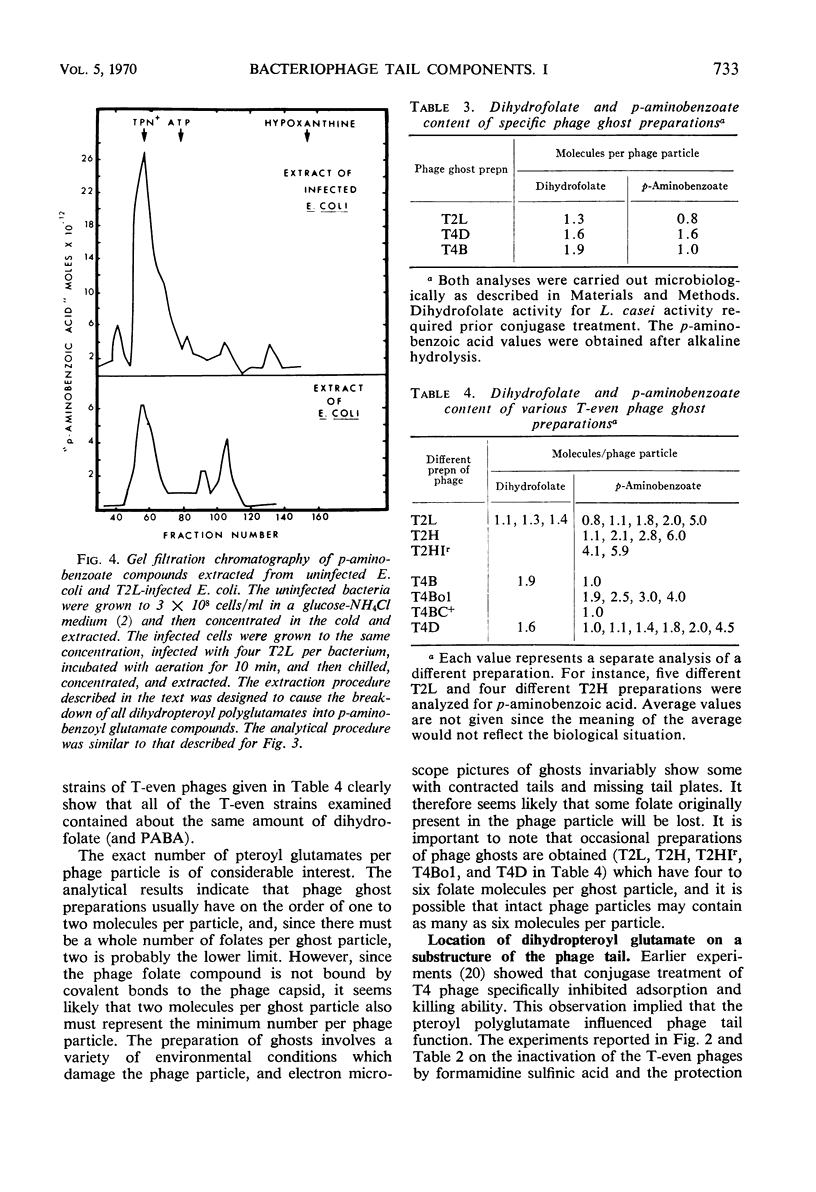
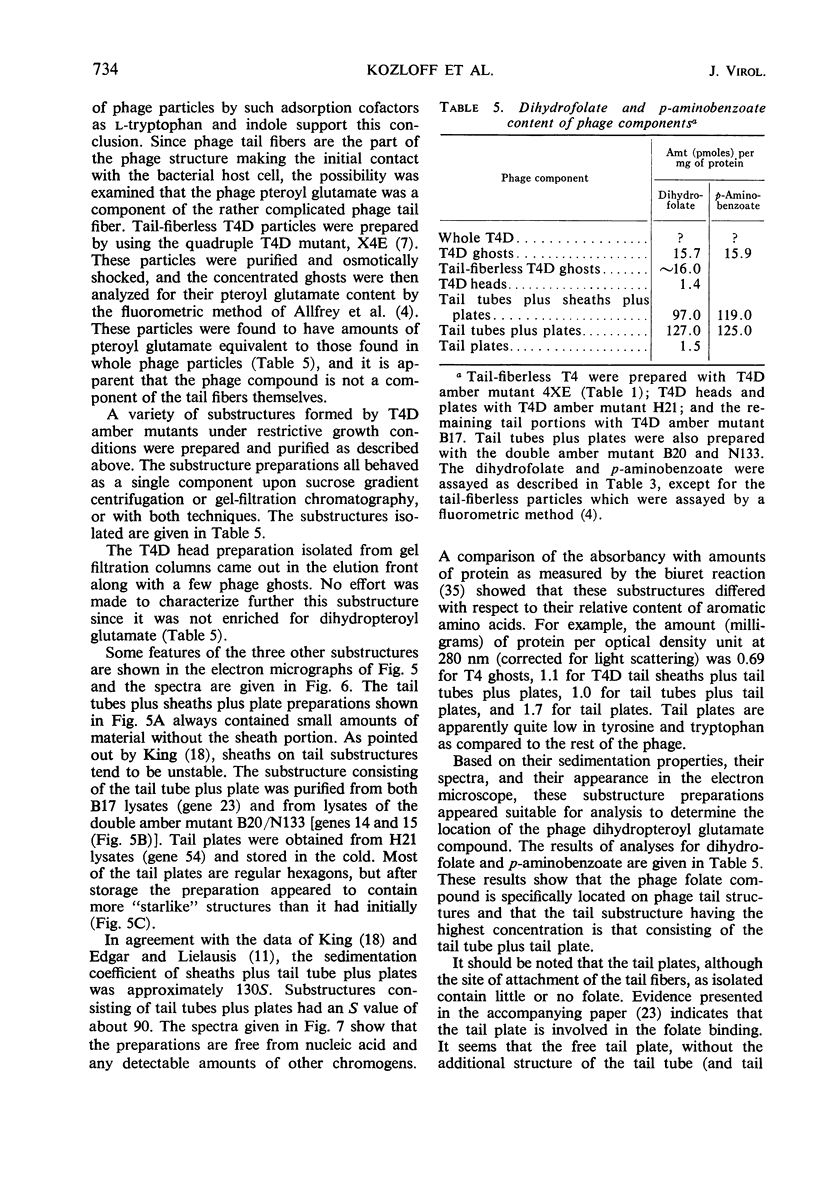
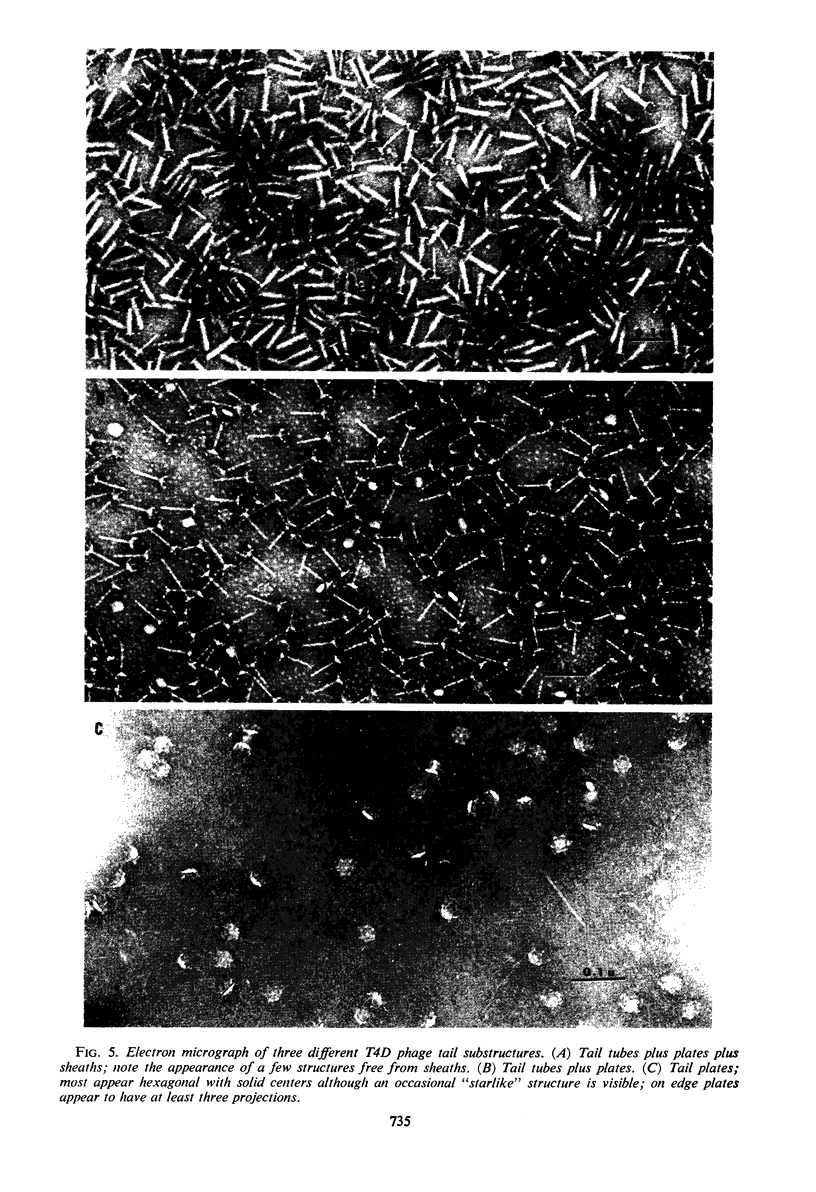
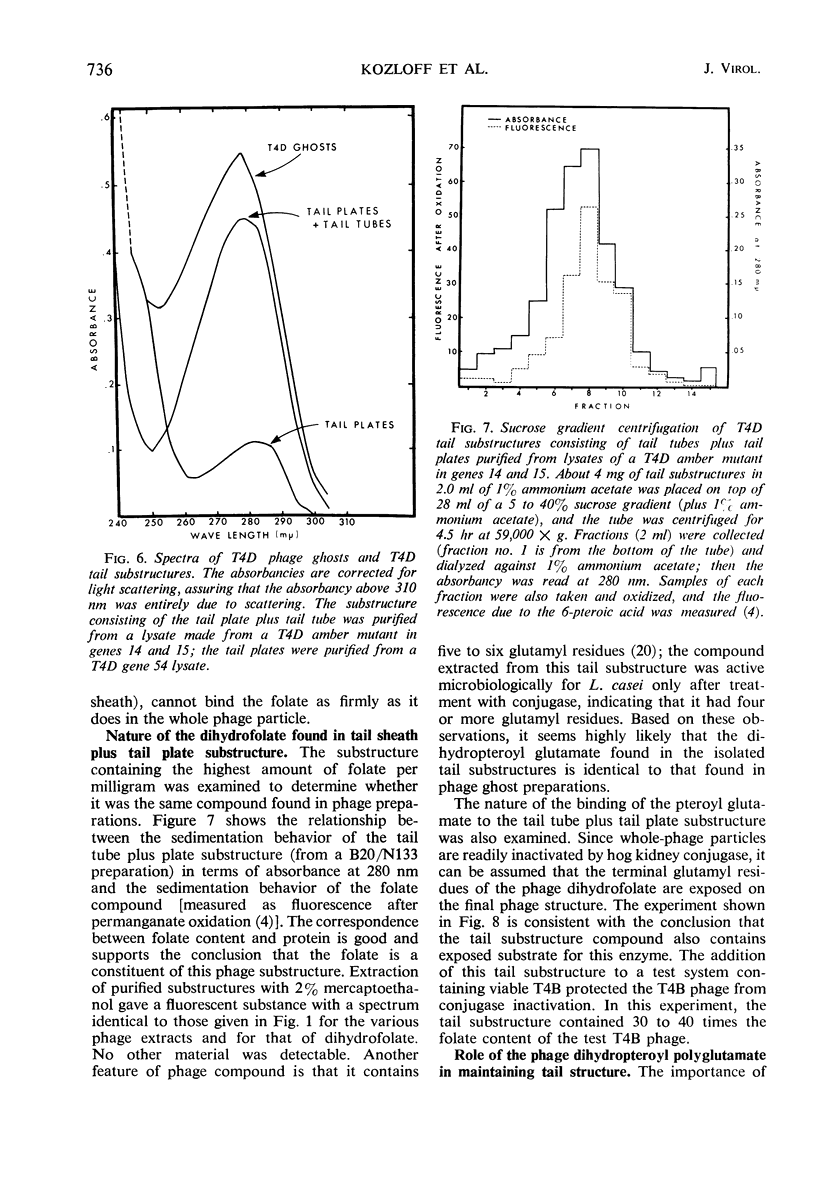
A pteroylpolyglutamate has been found to be a constituent of all Escherichia coli T-even bacteriophages and has been characterized with regard to its oxidation state, molecular weight, origin, and location on the phage particle. The phage compound has been shown to be a dihydropteroyl penta- or hexaglutamate on the basis of its chemical and physical properties. Analyses of extracts of uninfected and T2L-infected E. coli have indicated that the phage dihydropteroyl polyglutamate was present only in infected cells. Its synthesis was sensitive to the addition of chloramphenicol before infection, and the compound appeared to be specifically induced by phage infection. Analyses of isolated phage ghosts and tail substructures have shown that each phage particle contains between two and six phage-specific pteroyl derivatives and that the juncture of the phage tail plate with the tail tube is the most likely site of binding of the phage-induced pteroyl compound.
Full text
PDF



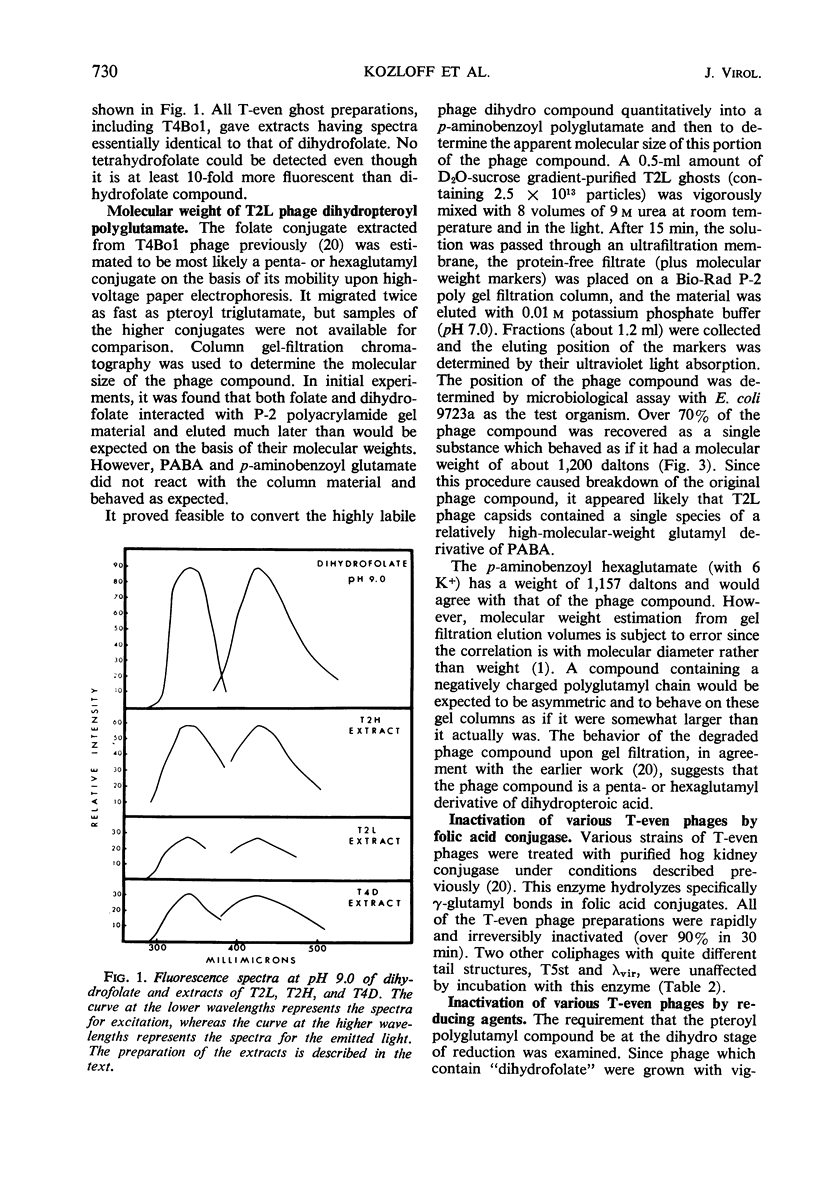
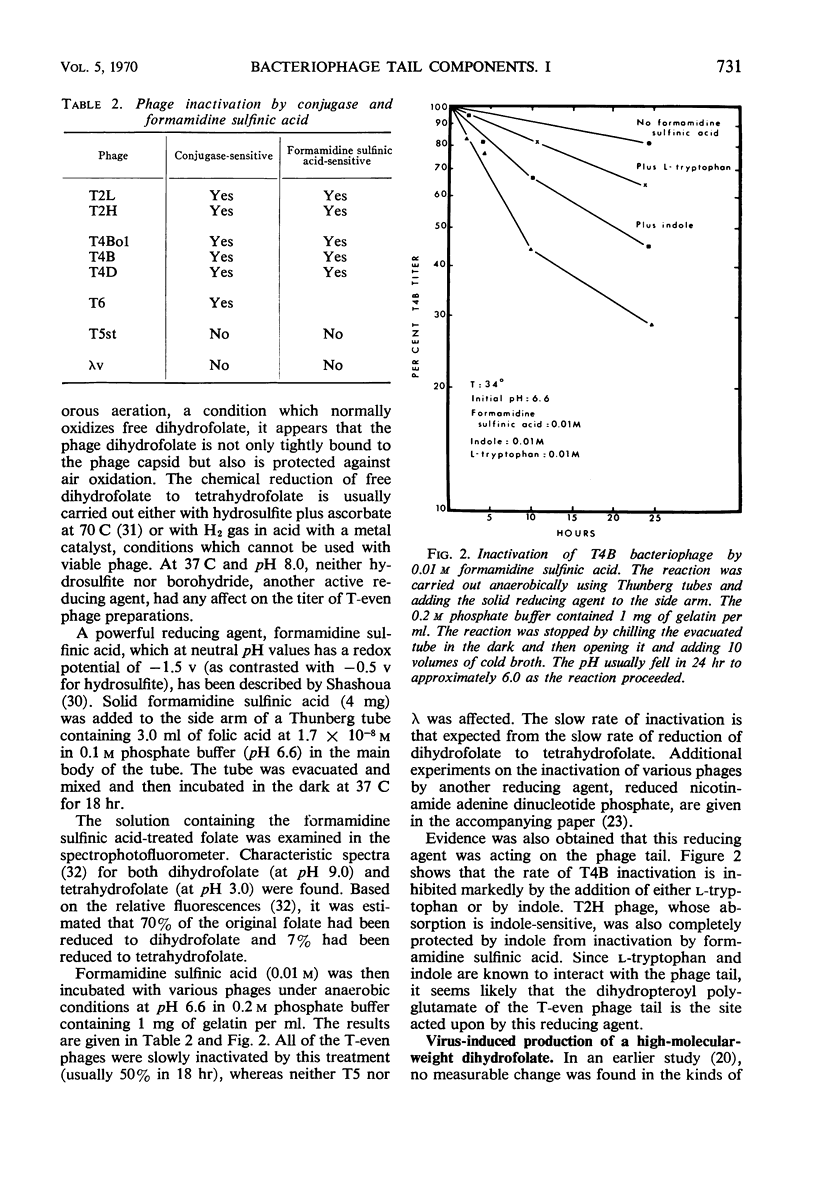
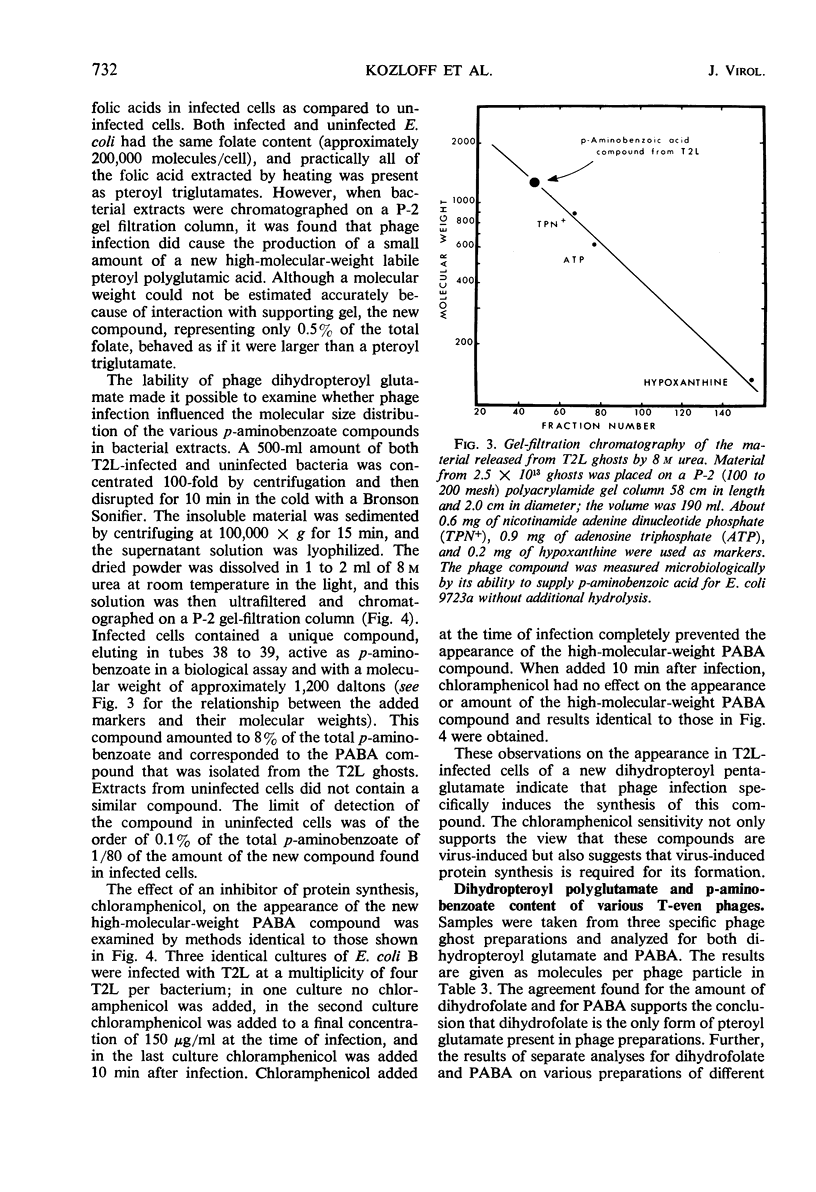


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERTSSON P. A., FRICK G. Partition of virus particles in a liquid two-phase system. Biochim Biophys Acta. 1960 Jan 15;37:230–237. doi: 10.1016/0006-3002(60)90228-6. [DOI] [PubMed] [Google Scholar]
- BAKERMAN H. A. A method for measuring the microbiological activity of tetrahydrofolic acid and other labile reduced folic acid derivatives. Anal Biochem. 1961 Dec;2:558–567. doi: 10.1016/0003-2697(61)90023-9. [DOI] [PubMed] [Google Scholar]
- CUMMINGS D. J. SEDIMENTATION AND BIOLOGICAL PROPERTIES OF T-PHAGES OF ESCHERICHIA COLI. Virology. 1964 Jul;23:408–418. doi: 10.1016/0042-6822(64)90264-8. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Chapman V. A., DeLong S. S. Disruption of T-even bacteriophages by dimethyl sulfoxide. J Virol. 1968 Jun;2(6):610–620. doi: 10.1128/jvi.2.6.610-620.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., Chapman V. A., DeLong S. S. The sedimentation and conformational variance among T-even bacteriophages. Virology. 1969 Jan;37(1):94–108. doi: 10.1016/0042-6822(69)90310-9. [DOI] [PubMed] [Google Scholar]
- DE MARS R. I. The production of phage-related materials when bacteriophage development in interrupted by proflavine. Virology. 1955 May;1(1):83–99. doi: 10.1016/0042-6822(55)90007-6. [DOI] [PubMed] [Google Scholar]
- Edgar R. S., Lielausis I. Some steps in the assembly of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):263–276. doi: 10.1016/0022-2836(68)90008-9. [DOI] [PubMed] [Google Scholar]
- Edgar R. S., Wood W. B. Morphogenesis of bacteriophage T4 in extracts of mutant-infected cells. Proc Natl Acad Sci U S A. 1966 Mar;55(3):498–505. doi: 10.1073/pnas.55.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERRIOTT R. M., BARLOW J. L. The protein coats or ghosts of coliphage T2. I. Preparation, assay, and some chemical properties. J Gen Physiol. 1957 May 20;40(5):809–825. doi: 10.1085/jgp.40.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. H. Mutants of bacteriophage T4 unable to induce dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1967 Aug;58(2):584–591. doi: 10.1073/pnas.58.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. H., Tessman I., Karlström O. Linkage of T4 genes controlling a series of steps in pyrimidine biosynthesis. Virology. 1967 Mar;31(3):442–448. doi: 10.1016/0042-6822(67)90224-3. [DOI] [PubMed] [Google Scholar]
- KANNER L. C., KOZLOFF L. M. THE REACTION OF INDOLE AND T2 BACTERIOPHAGE. Biochemistry. 1964 Feb;3:215–223. doi: 10.1021/bi00890a013. [DOI] [PubMed] [Google Scholar]
- KOZLOFF L. M., LUTE M., HENDERSON K. Viral invasion. I. Rupture of thiol ester bonds in the bacteriophage tail. J Biol Chem. 1957 Sep;228(1):511–528. [PubMed] [Google Scholar]
- King J. Assembly of the tail of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):231–262. doi: 10.1016/0022-2836(68)90007-7. [DOI] [PubMed] [Google Scholar]
- Kozloff L. M., Lute M., Crosby L. K., Wong R., Stern B. Critical arginine residue for maintaining the bacteriophage tail structure. J Virol. 1969 Feb;3(2):217–227. doi: 10.1128/jvi.3.2.217-227.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozloff L. M., Lute M. Folic acid, a structural component of T4 bacteriophage. J Mol Biol. 1965 Jul;12(3):780–792. doi: 10.1016/s0022-2836(65)80327-8. [DOI] [PubMed] [Google Scholar]
- Kozloff L. M., Verses C., Lute M., Crosby L. K. Bacteriophage tail components. II. Dihydrofolate reductase in T4D bacteriophage. J Virol. 1970 Jun;5(6):740–753. doi: 10.1128/jvi.5.6.740-753.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILIPSON L., ALBERTSSON P. A., FRICK G. The purification and concentration of viruses by aqueous polymerphase systems. Virology. 1960 Jul;11:553–571. doi: 10.1016/0042-6822(60)90100-8. [DOI] [PubMed] [Google Scholar]
- SARKAR N., SARKAR S., KOZLOFF L. M. TAIL COMPONENTS OF T2 BACTERIOPHAGE. I. PROPERTIES OF THE ISOLATED CONTRACTILE TAIL SHEATH. Biochemistry. 1964 Apr;3:511–517. doi: 10.1021/bi00892a008. [DOI] [PubMed] [Google Scholar]
- SARKAR S., SARKAR N., KOZLOFF L. M. TAIL COMPONENTS OF T2 BACTERIOPHAGE. II. PROPERTIES OF THE ISOLATED TAIL CORES. Biochemistry. 1964 Apr;3:517–521. doi: 10.1021/bi00892a009. [DOI] [PubMed] [Google Scholar]
- SHASHOUA V. E. FORMAMIDINE SULFINIC ACID AS A BIOCHEMICAL REDUCING AGENT. Biochemistry. 1964 Nov;3:1719–1720. doi: 10.1021/bi00899a021. [DOI] [PubMed] [Google Scholar]
- UYEDA K., RABINOWITZ J. C. Fluorescence properties of tetrahydrofolate and related compounds. Anal Biochem. 1963 Jul;6:100–108. doi: 10.1016/0003-2697(63)90012-5. [DOI] [PubMed] [Google Scholar]
- WESTLEY J., LAMBETH J. Protein determination on the basis of copper-binding capacity. Biochim Biophys Acta. 1960 May 20;40:364–366. doi: 10.1016/0006-3002(60)91368-8. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R. C., FRASER D. Structural and functional differentiation in T2 bacteriophage. Virology. 1956 Jun;2(3):289–307. doi: 10.1016/0042-6822(56)90024-1. [DOI] [PubMed] [Google Scholar]
- Wood W. B., Edgar R. S., King J., Lielausis I., Henninger M. Bacteriophage assembly. Fed Proc. 1968 Sep-Oct;27(5):1160–1166. [PubMed] [Google Scholar]
- ZAKRZEWSKI S. F. Studies on the substrate specificity of folic acid reductase. J Biol Chem. 1960 Jun;235:1780–1784. [PubMed] [Google Scholar]