Abstract
Variations in the expression levels of the dopamine transporter (DAT) can influence responsiveness to psychostimulant drugs like cocaine. To better understand this relationship, we studied a new DAT-low expresser (DAT-LE) mouse model and performed behavioral and biochemical studies with it. Immunoblotting and [3H]WIN 35,428 binding analyses revealed that these mice express ~35% of wildtype (WT) mouse striatal DAT levels. Compared to WT mice, DAT-LE mice were hyperactive in a novel open-field environment. Despite their higher basal locomotor activity, cocaine (10 or 20 mg/kg, i.p.) induced greater locomotor activation in DAT-LE mice than in WT mice. The maximal velocity (Vmax) of DAT-mediated [3H]DA uptake into striatal synaptosomes was reduced by 46% in DAT-LE mice, as compared to WT. Overall, considering the reduced number of DAT binding sites (Bmax) along with the reduced Vmax in DAT-LE mice, a 2-fold increase in DA uptake turnover rate (Vmax/Bmax) was found, relative to WT mice. This suggests that neuroadaptive changes have occurred in the DAT-LE mice that would help to compensate for their low DAT numbers. Interestingly, these changes do not include a reduction in tyrosine hydroxylase levels, as was previously reported in DAT knockout homozygous and heterozygous animals. Further, these changes are not sufficient to prevent elevated novelty- and cocaine-induced locomotor activity. Hence, these mice represent a unique model for studying changes of in vivo DAT function and regulation that result from markedly reduced levels of DAT expression.
Keywords: SLC6; neurotransmitter transporter; low-expresser; mouse model; psychostimulant; [3H]WIN 35,428
Introduction
The dopamine (DA) transporter (DAT) is essential for clearance of extracellular DA, resulting in termination of DA neurotransmission, and is a primary target for psychostimulant drugs like cocaine (Giros et al., 1996). Thus, knock-in mice that express a cocaine-insensitive DAT with ~50% of normal DA uptake activity exhibit no acute cocaine-induced increases in extracellular DA in nucleus accumbens or locomotor activation (Chen et al., 2005 & 2006). Likewise, in these mice, repeated cocaine exposure does not result in cocaine-induced locomotor sensitization, cocaine reward as measured by conditioned place preference (CPP), cocaine reinforcement as measured by self-administration, or increases in dendritic spine density in nucleus accumbens (Chen et al., 2006; Martin et al., 2011; Thomsen et al., 2009). It is known that cocaine-induced locomotion and reward, as well as other functions of DA-mediated neurotransmission, are influenced by variations in the number of DATs (Uhl et al., 2002). Studies have helped to identify the prevalence of individual differences in levels of DAT in humans (Drgon et al., 2006; Kurian et al., 2011) and strain-related differences in mice (Janowsky et al., 2001). At least 47% of DATs need to be occupied by cocaine before addicts report subjective effects of the drug, suggesting the existence of a ‘spare’ pool of DATs (Volkow et al., 1997). Additionally, abuse of cocaine has been shown to alter DAT numbers in humans (Little et al., 1993; Mash et al., 2002).
Several studies have shown that variations in DAT expression levels are associated with alterations in psychostimulant drug effects. It was found that in human embryonic kidney (HEK293) cells, over-expression of DAT resulted in reduced potency of cocaine, as well as other DAT inhibitors (Chen and Reith, 2007). On the other hand, in DAT knockout mice, serotonin transporter (SERT) and norepinephrine transporter (NET) inhibitors are capable of eliciting reward, unlike in wild type (WT) mice (Hall et al., 2002). Since a complete null mutation of DAT leads to extensive developmental and neurochemical changes (Bosse et al., 1997; Jones et al., 1998), studying mouse models with altered DAT expression levels may offer several advantages for understanding the function of the transporter and how it affects the actions of psychostimulants like cocaine. Previously, mouse models with DAT levels reduced to ~ 10% and ~ 50% of WT have been generated and were shown to have elevated levels of extracellular DA due to slower clearance rates, along with retention of cocaine-induced hyperlocomotion and cocaine reward (Giros et al., 1996; Tilley et al., 2007; Zhuang et al., 2001). On the other hand, transgenic mice over-expressing DAT by 20 – 30 %, compared to WT, exhibit increased cocaine reward (Donovan et al., 1999).
Our past research using outbred Sprague-Dawley rats showed that these animals exhibit individual differences in their maximal number of striatal DAT binding sites (Bmax; Nelson et al., 2009). Rats with an average of 33% lower DAT Bmax than the group mean are more responsive to the DAT inhibiting and locomotor activating effects of acute low dose cocaine but also more rapidly up-regulate their DAT function (Mandt and Zahniser, 2010; Nelson et al., 2009; Sabeti et al., 2002). In response to repeated cocaine administration, we found that the rats that initially had fewer striatal DATs are less likely to develop locomotor sensitization, exhibit lower cocaine CPP and appear less motivated to self-administer cocaine (Allen et al., 2007; Mandt et al., 2008). However, given the variability in DAT numbers and behavioral responsiveness among individual animals, it is difficult to understand the exact relationship. Here we report results obtained from behavioral and biochemical studies using a novel genetically altered DAT low-expresser (DAT-LE) mouse model that has ~30% of WT striatal DAT protein levels (Rao et al., 2012).
Materials and Methods
Animals
Details regarding the generation and maintenance of DAT-LE mice have been previously published (Rao et al., 2012). DAT-LE heterozygous (DAT-LE HET) mice were back-crossed for 5 generations with C57/Bl6 mice obtained from Jackson Laboratories (Bar Harbor, ME). These DAT-LE HET mice were then crossed to obtain homozygous mice (referred to as DAT-LE). Their WT littermates were used for all control experiments. Mice were housed in groups of 5 on a 14:10 lights on:off cycle with food and water available ad libitum. All male and female mice were 2-4 months old at the time of the experiments. Prior to brain dissections, the mice were quickly euthanized by cervical dislocation. All experimental procedures involving the generation and use of the mice were conducted in accordance with National Institutes of Health guidelines as approved by the Institutional Animal Care and Use Committee at the University of Colorado -Anschutz Medical Campus.
Immunoblotting
For immunoblotting, tissue samples were prepared from mice of all 3 genotypes (WT, DAT-LE HET, and DAT-LE). To examine striatal DAT, striatal tissue was dissected out and solubilized in Triton X-100/glycerol/HEPES lysis buffer, and subsequently denatured in sample buffer at 75°C for 10 min. Lysates were then electrophoresed and blotted with the following antibodies: HA.11 (1:1000 dilution; Covance, Princeton, NJ), rat monoclonal N-terminal DAT (1:1000 dilution; Chemicon, Temecula, CA), C-terminal DAT (1:1000 dilution; Santa Cruz Biotechnology Inc, Santa Cruz, CA.), tyrosine hydroxylase (TH; 1:5000 dilution; Chemicon, Temecula, CA) or synaptophysin (1:10,000 dilution; Santa Cruz Biotechnology Inc.). Quantitative analysis of band intensity was performed using densitometry and Image J software (National Institutes of Health, Bethesda, MD).
To investigate the less abundant mesencephalic DAT protein levels, midbrain was dissected out and the transporter was concentrated from the mesencephalic lysates via immunoprecipitation. Briefly, mesencephalic lysates were incubated with polyclonal goat DAT antibody (1:1000; Santa Cruz Biotechnology Inc.) at 4°C overnight. The resulting supernatant was incubated with Protein G beads (ThermoFisher Scientific, Rockford, IL) for 1 hr at 4°C. Subsequently, the beads were washed and eluted using 200 mM glycine. The eluted mesencephalic DAT was then prepared for immunoblotting as described above.
Quantitative Real time PCR
To investigate the total mRNA levels of DAT in mesencephalon, the ventral tegmental area and substantia nigra of the DAT-LE and WT mice were dissected out in cold RNA later buffer (Qiagen), and tissue was frozen on dry ice. Total RNA extraction and subsequent DAT mRNA measurements by real-time quantitative RT-PCR were done in the Endocrinology PCR Core, University of Colorado Denver, using an ABI Prism 7900 Sequence detector and TaqMan gene expression assay (Mm00438396_m1; Slc6a3) purchased from Applied Biosystems (Foster City, CA). DAT mRNA quantities in all samples were normalized to the corresponding 18S ribosomal RNA levels.
[3H]WIN 35,428 Binding assays
Indirect saturation binding assays were performed using a previously described protocol (Hanania et al., 2004). Briefly, striata were dissected out from the DAT-LE mice and their WT littermates, homogenized in ice-cold assay buffer (30 mM NaH2PO4, 15 mM Na2HPO4, and 0.32 M sucrose; pH 7.4) using a sonicator, and centrifuged at 20,000g for 20 min at 4°C. The pellet was re-suspended in assay buffer and subsequently incubated with [3H]WIN 35,428 (~ 4.2 nM; Perkin-Elmer Life Sciences, Boston, MA) and 10 concentrations of unlabeled WIN 35,428 (1 × 10−5 – 3.16 ×10−10 M) on ice for 60 min. Incubation with 30 μM benztropine (Sigma-Aldrich, St. Louis, MO) was used to obtain the non-specific binding. Vacuum filtration with 2 washes was performed using Whatman GF/C glass microfiber filters (Brandel Inc., Gaithersburg, MD), and the resulting radioactivity on the filters was measured by liquid scintillation spectrometry. The amount of protein in each sample was determined (Bradford, 1976). Nonlinear curve fitting of the indirect saturation curves with Prism 5 (GraphPad Software, La Jolla, CA) was used to determine the values for the apparent binding affinity (IC50, Kd) and the total number of binding sites (Bmax; DeBlasi et al., 1989).
Behavioral testing
Locomotor activity was tested in open-field chambers (16″ ×16″ ×15″) surrounded by an 8 × 8 photobeam frames (San Diego Instruments, San Diego, CA). The mice were divided into 3 groups based on the cocaine doses to be administered on day 2. Each group consisted of 6 DAT-LE and 6 WT littermates. Both males and females were used, and the genders were equally distributed in all of the groups. All testing was performed between 9 AM-12 PM. On day 1, mice were transferred to the testing room and kept in their home cages for a 30-min habituation period. Subsequently, mice were placed in the novel open-field chambers, and their locomotor activity was recorded for 90 min. Locomotor activity was determined from the number of consecutive horizontal photobeam breaks per 10 min. Thereafter, all mice were injected with saline (1 ml/kg; i.p.) and re-placed in the chamber. Their activities were recorded for another 60 min, after which all mice were returned to their home cages and the colony room. On day 2, the same set of mice was subjected to the same protocol but were injected with a single dose of cocaine (5, 10, or 20 mg/kg; i,p,). These doses of cocaine were chosen because they have been previously shown to elicit a linearly increasing locomotor response in C57/Bl6 mice (Tilley et al., 2007). At the end of the day 2 session, all mice were euthanized 150 min after the cocaine injection; and their striatal tissue was used immediately for [3H]DA uptake measurements. This time period was chosen because it is 5 times the half-life of cocaine; thus, tissue cocaine levels should be negligible (Maisonneuve and Kreek, 1994).
[3H]DA uptake assays
Striatal synaptosomes were prepared according to Rao et al. (2012) from mice on day 2 after the behavioral testing (see above) and in another control group of WT and DAT-LE mice (N = 4/genotype) that had received saline injections on days 1 and 2, but no behavioral testing. Briefly, striata were homogenized with a Teflon glass homogenizer in ice-cold phosphate buffer (3.3 mM NaH2PO4, 12.7 mM Na2HPO4, 0.32 M sucrose; pH 7.4) and subsequently centrifuged at 1,000g for 12 min at 4°C. The resulting supernatant was centrifuged at 12,500g for 15 min to obtain the synaptosomal fraction. Synaptosomes were re-suspended at 40 mg/ml (wet weight of tissue) in assay buffer (134 mM NaCl, 4.8 mM KCl, 1.3 mM CaCl2, 1.4 mM MgSO4, 3.3 mM NaH2PO4, 12.7 mM Na2HPO4, 11 mM glucose, and 1 mM ascorbic acid; pH 7.4). 1 μM pargyline (Sigma-Aldrich) was added to inhibit monoamine oxidase before pre-incubation of the synaptosomes with assay buffer for 10 min at 37°C. Next, 0.5 nM [3H]DA (Perkin Elmer Life Sciences; Boston, MA) and one of eight concentrations of unlabeled DA (0, 10, 50, 75, 100, 175, 250, or 500 nM) was added before incubation for 3 min at 37°C. Non-specific uptake was determined in the presence of 100 μM cocaine (gift from the National Institute on Drug Abuse, Research Triangle Institute International, Research Triangle Park, NC). The samples were filtered through Whatman GF/C glass microfiber filters with a cell harvester and washed 3 times with ice-cold 0.32 M sucrose solution. Radioactivity and protein concentrations were determined as described above. Nonlinear curve fitting using GraphPad Prism 5 software was performed to obtain values for apparent affinity (Km) and maximal velocity (Vmax).
Statistical analysis
Data are expressed as mean values ± SEM. Prism 5 (GraphPad Software) was used to determine the independent sample t-test, as well as the radioligand binding and uptake kinetic parameters. SPSS, version 16.0 (SPSS Inc., Chicago, IL) was used to perform the repeated measures analysis of variance (ANOVA) of the behavioral experiments. Post-hoc pair wise comparison was carried out with either Bonferroni or Tukey HSD test. The level of significance was set at p<0.05.
Results
Characterization of the DAT-LE mouse model
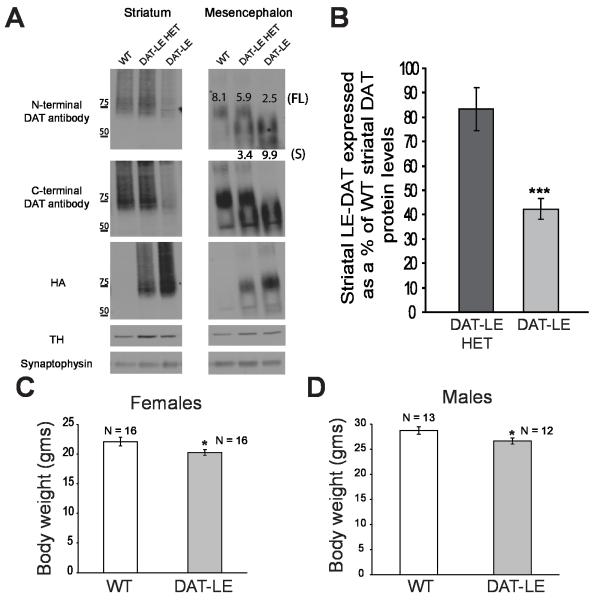
We generated a knock-in mouse model, DAT-LE, with a modified endogenous DAT locus (Rao et al., 2012). These mice contain an altered DAT allele with a floxed neomycin cassette in intron 3 and an HA11 (haemagglutinin) epitope sequence cloned into exon 4 of the DAT gene. Western blotting revealed that there is a marked reduction of DAT protein levels in the striatum of DAT-LE and DAT-LE HET mice (Fig. 1A). We have previously demonstrated that crossing DAT-LE mice with β-actin driven cre mice results in the elimination of the floxed neomycin cassette from the DAT locus and a subsequent rescue of the DAT low expression phenotype (Rao et al., 2012). These results suggest that the HA11 epitope in exon 4 does not interfere with DAT expression levels and that the insertion of the floxed neomycin cassette in intron 3 is the likely cause of this aberrant DAT expression..
Fig. 1. Characterization of DAT protein levels and body weights of DAT-LE mice.
(A) Representative western blots of striatal (left panel) and mesencephalic tissue (right panel) from mice of all three genotypes (WT, DAT-LE HET and DAT-LE). Striatal lysates or mesencephalic immunoprecipitates using DAT-specific antibodies were electrophoresed and probed by blotting with antibodies to DAT (both against the N- and C-termini), HA, TH and synaptophysin (loading control). The molecular weight markers in kilodaltons are indicated to the left of the striatal blot. The values shown on the mesencephalic blots are the calculated signal intensity (in AU) of mesencephalic DAT protein relative to TH protein levels. The values at the top correspond to the higher molecular weight full-length (FL) DAT (~75 KDa) while the values at the bottom correspond to the short (S) form of DAT (~60 KDa), denoted as FL and S, respectively, on the right side of the blot. (B) Relative intensity of striatal tissue DAT western blot signals, as detected by the N-terminal DAT antibody, in the DAT-LE HET and DAT-LE animals expressed as a percentage of the WT animals. The DAT signal intensity was normalized to that of the TH levels. Mean values ± SEM (N = 4; *** p<0.001 DAT-LE vs. DAT-LE HET). (C & D) Body weights of adult (2-4 mo) female (C) and male (D) DAT-LE mice compared to their WT littermates. Mean values ± SEM (N = 12-16; * p<0.05, DAT-LE vs WT).
Densitometery was used to quantify levels of striatal and mesencephalic DAT protein (~75 KDa) that were then normalized to TH levels (TH levels were not altered in these mutant mice; Fig. 1A). We found that DAT-LE mice expressed 42.3 ± 8.7 % (N = 4) of the WT striatal DAT protein levels (Fig. 1B). In contrast, DAT-LE HET mice expressed 83.4 ± 17.7 % (N = 4) of WT striatal DAT protein levels (Fig. 1B). Similar results were obtained using N- and C-terminal DAT antibodies (data not shown). In the mesencephalic tissue, immunoprecipitation of DAT (see Methods) revealed a truncated form of the DAT protein (~60 KDa) that was not delivered to the DA axons in striatum (Fig. 1A). The signal intensity of this DAT short form (S; 9.9 arbitrary units (AU)) was similar to that of the full-length (FL) WT DAT (8.1 AU) in mesencephalon, suggesting that it is not degraded in the soma and is relatively stable in the endoplasmic reticulum (ER) or post-ER compartments. Additionally, total mesencephalic DAT mRNA levels were not significantly different in DAT-LE and WT mice (DAT-LE: 55.2 ± 12.1 fg/ng 18S, N = 3; WT: 137.3 ± 62.9 fg/ng 18S, N = 4; p value = 0.2848).
DAT is known to be important for development (Bosse et al., 1997; Jones et al., 1998). Thus, we examined the total body weight of the DAT-LE mice. We found that there was a significant 10% reduction in body weight of both adult female and male DAT-LE mice, as compared to their WT littermates (Figs. 1C & D).
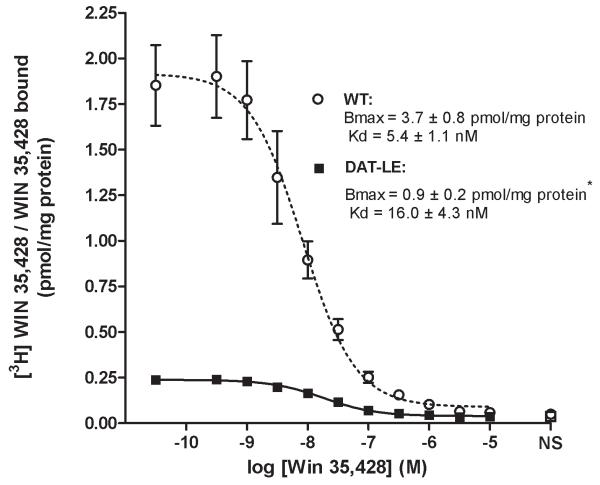
We next determined the affinity and number of DATs in striatal membranes by indirect [3H]WIN 35,428 saturation binding analysis (Fig. 2). There were no statistical differences in the affinities for the radioligand in the mutant DAT and WT DAT mice (Kd values, DAT-LE: 16.0 ± 4.3 and WT: 5.4 ± 1.1 nM). In contrast, DAT-LE mice had a significantly lower number of striatal DATs: 25.6 % of WT mice (Bmax values, DAT-LE: 0.9 ± 0.2 and WT: 3.7 ± 0.8 pmol [3H]WIN 35,428/WIN 35,428 specifically bound/mg protein). Together, the immunoblotting and binding results indicate that striatal DATs in the DAT-LE mice were reduced to ~35% of those in WT mice.
Fig. 2. [3H]WIN 35,428/WIN 35,428 indirect saturation binding isotherms to DATs in striatal membranes of DAT-LE and WT mice.
Kd and Bmax values for both genotypes derived from this analysis are indicated. NS = nonspecific binding. Mean values ± SEM (N = 4 mice/genotype; *p<0.05, DAT-LE vs WT mice).
DAT-LE mice display increased locomotor activity
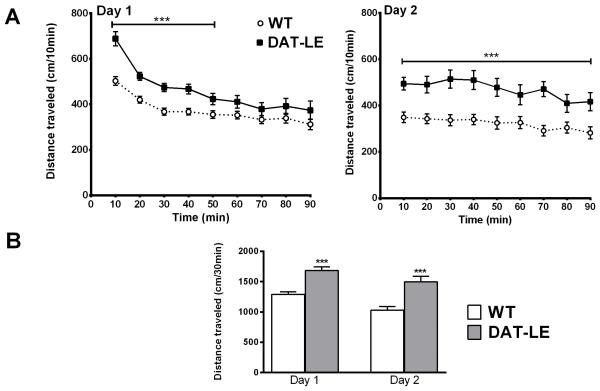
We next analyzed basal locomotor activity of age- and sex-matched naïve DAT-LE and WT mice when exposed to a novel open-field environment (Fig. 3). On day 1, locomotor activity differed significantly between the two genotypes [F(1, 34) = 14.90, p < 0.001]. Post-hoc analysis revealed that during the initial 50 min in the novel environment DAT-LE mice displayed significantly higher locomotor activity (p < 0.001; Fig. 3A). However, towards the end of the initial 90 min-session, mice of both genotypes exhibited similar lower levels of locomotor activation. On day 2, differences between the two genotypes were maintained [F(1, 34) =14.75, p < 0.01]. In this case, DAT-LE mice displayed higher locomotor activity for the entire 90 min, as compared to the WT mice (p < 0.001). Furthermore, on both days, DAT-LE mice traveled ~70% longer distances during the first 30 min in the open-field (p < 0.001), indicating hyperactivity in the DAT-LE mice (Fig. 3B).
Fig. 3. Effects of novelty on locomotor activity in DAT-LE and WT mice.
Naïve animals were placed in open-field chambers on day 1, and their locomotor activity in the novel environment was recorded for 90 min (mean values ± SEM; N = 18/genotype). Subsequently, all of the mice were injected with saline; and their behavior was monitored for an additional 60 min (see Fig. 4). On day 2 their activity in the open-field was again monitored for the initial 90 min, prior to being injected with either saline or cocaine (see Fig. 4). (A) Time course of distance traveled during the first 90 min in the chamber by WT and DAT-LE mice day 1 (left panel) and day 2 (right panel). ***p<0.001, DAT-LE vs WT (B) Total distance traveled by the two genotypes in the first 30 min (novelty) in the chamber on days 1 and 2. ***p<0.001, DAT-LE vs WT.
Greater locomotor activation of DAT-LE mice by cocaine
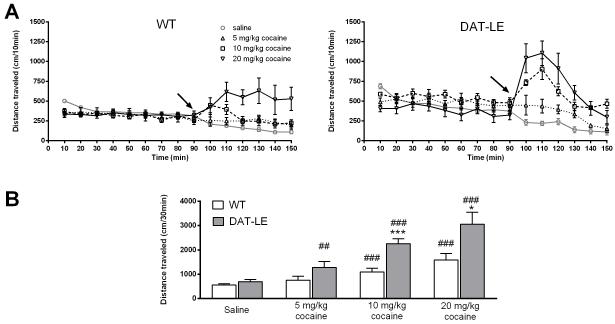
After the 90 min-habituation period in the open field box, DAT-LE and WT mice were injected with saline on day 1 and with one of three doses of cocaine on day 2. Locomotor activity was recorded for 60 min post-injection on both days (Fig. 4). Fig. 4A shows the entire time course of the experiment for locomotor responses on day 1 to saline (1 ml/kg, i.p.) and on day 2 to the three doses of cocaine (5, 10 and 20 mg/kg; i.p.) in the DAT-LE and WT mice. Upon comparing the responses of the mice on days 1 and 2 within each group (N = 6/group), we found that there was a significant effect of drug [F(2, 30) = 5.24, p < 0.05] and a difference in genotype [F(1, 30) = 10.77, p < 0.01], but the genotype × drug interaction did not reach significance [F(2, 30) = 1.38, p = 0.27]. Upon post hoc analysis using Tukey HSD test, we found no differences between genotypes after the injection of saline on day 1 (Fig. 4A). In contrast, on day 2, 10 mg/kg cocaine potentiated locomotor activity of the DAT-LE mice to a greater extent than the WT mice (p < 0.05; Fig. 4A). Interestingly, over the entire 60 min after injection, 5 and 20 mg/kg cocaine produced similar effects in both genotypes (Fig. 4A). However, the majority of cocaine-induced activation occurs within the first 30 min after injection. Thus, we analyzed the drug effect as a function of distance traveled during first 30 min and found that both 10 and 20 mg/kg cocaine stimulated DAT-LE mice to a much greater extent than the WT mice (p < 0.001 and p < 0.05 respectively; Fig. 4B). A similar trend was observed for the 5 mg/kg dose of cocaine. Overall, in DAT-LE mice all doses of cocaine elicited higher locomotor activity, as compared to saline within their respective treatment groups (p < 0.05), while in the WT mice only 10 and 20 mg/kg cocaine increased locomotor activity over saline-treatment (p < 0.05).
Fig. 4. Dose-effect of acute cocaine exposure on locomotor activity in DAT-LE and WT mice.
Following the 90-min habituation of the mice to the open field chamber on day 2 (see Fig. 3), WT (A, left panel) and DAT-LE (A right panel) mice were injected with a single dose of cocaine (arrow; 5, 10 or 20 mg/kg; mean values ± SEM, N = 6 for each dose and each genotype). Following injection, their locomotor activity was monitored for an additional 60 min. See Results for statistical analyses. Locomotor activity on day 1 following saline treatment is shown as a single group only for reference (N = 18). (B) Total distance traveled in the first 30 min after saline or cocaine injection (mean values ± SEM; * p<0.05, ***p<0.001, DAT-LE vs WT; ## p<0.01, ###p<0.001, cocaine vs saline within the respective genotype).
Lower Vmax and higher Vmax/Bmax values for DA uptake into striatal synaptosomes from DAT-LE mice
We next examined the functionality of the mutant DAT in DAT-LE mice by measuring [3H]DA/DA uptake kinetics in striatal synaptosomes (Fig. 5). We measured uptake kinetics in groups of control DAT-LE and WT mice that had been injected with saline on two consecutive days but never tested behaviorally (N = 4/genotype). Following the behavioral experiments, striatal synaptosomes were also prepared from DAT-LE and WT mice at 150 min post cocaine injection, a time when any residual cocaine in the tissue should be negligible. To account for day-to-day variations in absolute uptake values, we compared Vmax and Km values for the DAT-LE mice as a percentage of the WT mouse values obtained on the same assay day. No significant genotype differences were detected in either parameter following saline or any of the doses of cocaine tested, suggesting no persistent regulation of the DAT following cocaine exposure (data not shown). Thus, we pooled the striatal DA uptake results within the two genotypes, resulting in N = 22/genotype (Fig. 5).
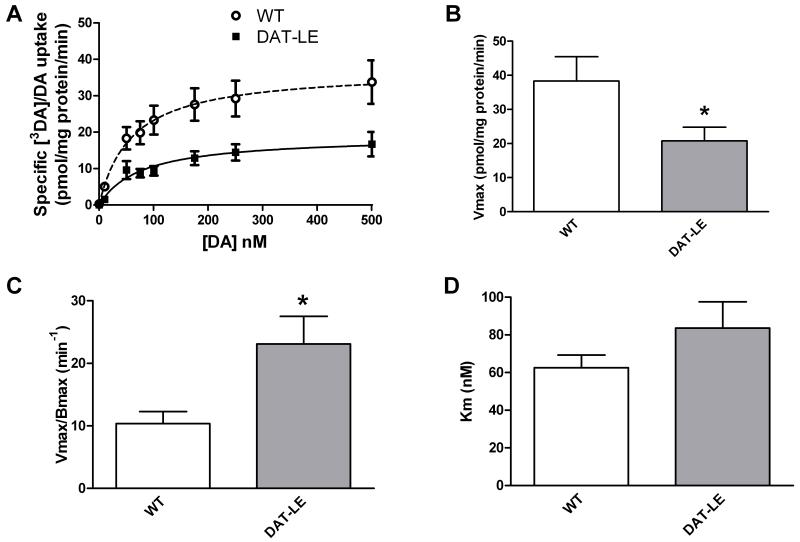
Fig. 5. DA uptake kinetics in striatal synaptosomes of DAT-LE and WT mice.
(A) DA uptake levels were measured in the presence of increasing [3H]DA/DA concentrations in striatal synaptosomes prepared from WT and DAT-LE mice injected with saline (N = 4/genotype) or cocaine (N = 6/genotype) 150 min after injection. Since no treatment group differences in the uptake parameters were found within each genotype, the results were pooled (mean values ± SEM, N = 22/genotype). Values for Vmax (B), uptake turnover rate (C; Vmax/Bmax) and Km (D) were determined from the curves in panel A. The group mean Bmax values (Fig. 2) were used to calculate uptake turnover rate in panel C. * p<0.05, DAT-LE vs WT.
Relative to the Vmax value of 38.3 ± 7.1 pmol [3H]DA/DA taken up/mg protein/min in WT mice, the DAT-LE mice showed a significantly lower Vmax value of 20.8 ± 4.0 pmol/mg protein/min (Fig. 5B). Taking into account the reduced number of DAT binding sites in the DAT-LE mouse striatum (Fig. 2) an overall increase in uptake turnover rate (Vmax/Bmax) was revealed. This rate in DAT-LE mice was 23.1 ± 4.4 DA taken up/DAT binding site/min, as compared to 10.4 ± 1.9 in WT mice (Fig. 5C). On the other hand, Km values for uptake in DAT-LE mice did not differ significantly from those for WT mice (Fig. 5D). Km for WT mice was 62.51 ± 6.77 nM, while DAT-LE mice showed Km of 83.63 ± 13.89 nM (p value = 0.1789).
Discussion
We found that mice expressing ~35% of WT striatal DATs, as measured with both immunoblotting and radioligand binding, were hyperactive in a novel open-field environment and showed higher cocaine-induced locomotor activation at several doses. These results are all consistent with a loss of DAT function, which was confirmed by a 46% reduction in the maximal DA uptake velocity into striatal synaptosomes from DAT-LE mice, as compared to WT mice. Taking into account both Vmax and Bmax values for DATs, however, revealed that the DA uptake turnover rate of the DAT-LE mice was increased by 2-fold; suggesting a compensatory functional up-regulation of maximal DA uptake. Nonetheless, our behavioral findings are consistent with the conclusion that such a marked reduction in DAT levels contributes to higher levels of extracellular DA, thereby resulting in both greater baseline and cocaine-induced activation. We do not know how DA release may be altered in these mice. Extracellular DA reflects the combination of both DA release and uptake, and previous studies have shown that cocaine promotes stimulation-evoked DA release preferentially in the nucleus accumbens (Wu et al., 2001). Thus, in order to understand the relative contributions of DA uptake and release to cocaine responsiveness in this model, it will be informative to investigate cocaine-mediated striatal DA release in DAT-LE mice in the future.
Transgenic mouse models with reduced DAT levels offer several advantages for studying the function of the transporter and its regulation by psychostimulants like cocaine. Several mouse models with low DAT levels have been studied in the past. These models have either used ES cell-targeted genetic manipulations (Giros et al., 1996; Zhuang et al., 2001) or transient knock-down approaches via RNA interference (RNAi; Salahpour et al., 2007; Thakker et al., 2004). In making a HA-DAT mouse model for studies of DAT endocytosis, our first knock-in mouse unexpectedly had reduced DAT protein expression (Rao et al., 2012). Here, we took advantage of this unique DAT-LE mouse model in order to perform behavioral and functional studies, comparing it to age- and sex-matched WT littermates.
Although the DAT-LE mice were able to breed normally, the body weights of the adult (2-4 months) female and male mice were reduced by ~10% (Figs. 1C & D). The body weights of DAT knockout mice were reduced to a greater extent, by 43% (Bliziotes et al., 2000; Bosse et al., 1997). Interestingly, however mice expressing 10% (Beeler et al., 2012; Zhuang et al., 2001) or ~50% (Giros et al., 1996) of WT DAT did not show any abnormalities in body weight.
Western blotting revealed a 58% reduction in striatal DAT protein levels in DAT-LE mice (Figs. 1A & B) whereas [3H]WIN 35,428 binding studies showed a 73% reduction in the striatal DAT number (Bmax; Fig. 2). It is likely that these 15% differences are due to the different methods of tissue preparations (solubilization in detergent vs. homogenization in buffer). Also, the antibody and radioligand likely bind to different sites on DAT. In any case, the DAT-LE mice appear to have ~35% of the normal compliment of striatal DATs. In the mesencephalic region of these mice, lower molecular weight DAT protein was found (Fig. 1A). This DAT species is recognized by N- and C-terminal specific antibodies but not HA11 antibody (Fig. 1A), suggesting this DAT form has an internal deletion encompassing a part of the extracellular loop 2 and HA sequence. Since we did not find this mutant DAT in the striatal region, it is likely that this DAT variant was mis-folded and retained in the ER in the somatodendritic compartment of the DA neurons. Numerous previous mutagenesis studies have shown the high susceptibility of ER retention of DAT to even small changes in extracellular loops of the DAT molecule (Miranda et al., 2004). Alternatively, short DAT form could be delivered to the axons but then turned over very rapidly. This explanation is less likely because axons of DA neurons lack lysosomes, the site of degradation of membrane proteins (Rao et al., 2011, 2012). Regardless of the nature of the trafficking defect of the short DAT form, this defect is rescued upon excision of the floxed neomycin cassette from the intron 3 (Rao et al., 2012), suggesting that the defect is not due to the insertion of the HA sequence.
DAT homozygous and heterozygous knockout mice have been shown to have reduced TH levels but the normal physiological activity of TH is preserved (Jones et al., 1998). In contrast, DAT-LE mice had normal TH protein expression (Fig. 1A). The level of TH activity in these mice remains to be tested. Nonetheless, our observations indicate that distinct neuroadaptive changes have occurred in the DAT-LE mice that differ from the DAT knockout animals. Hence, these mice presented us with a unique model to study the transporter function and sensitivity to cocaine.
Monitoring locomotor activity in the open-field showed that in a novel environment the DAT-LE mice were hyperactive as compared to WT littermates (Fig. 3). These results are similar to a previously studied mouse model with 10% DAT protein levels (Zhuang et al., 2001). Interestingly, DAT heterozygous knockout mice with a Bmax of 55% of WT did not show markedly different locomotor responsiveness to a novel environment (Giros et al., 1996; Jones et al., 1998). This suggests that the threshold for DAT expression level for manifestation of the novelty-induced hyperactive phenotype falls between 35% and 55% of WT levels.
Since DAT is essential for the action of the drug cocaine, we examined its effect in our mouse model. Despite the higher basal locomotor activity of DAT-LE mice, cocaine-induced hyperactivity was preserved and even magnified in the DAT-LE mice (Fig. 4). These mice displayed significantly greater locomotor activity in response to an i.p. injection of 5, 10 or 20 mg/kg cocaine, as compared to saline. Additionally, 10 and 20 mg/kg cocaine had a greater locomotor stimulating effect in these mice than in the WT mice. Hence, our results suggest that a reduction in DAT protein levels is associated with greater acute cocaine-induced hyperactivity, consistent with what we have seen in Sprague-Dawley rats (Nelson et al., 2009). Interestingly, in mice with 10% residual DAT, 5 and 10 mg/kg cocaine produced a greater locomotor activation than in WT; but there was no genotype difference to a dose of 20 mg/kg cocaine (Tilley et al., 2007). On the other hand, in DAT heterozygous mice, 10 and 40 mg/kg cocaine elicited locomotor responses that were similar to the WT mice (Giros et al., 1996; Jones et al., 1998). Again, our DAT-LE mice with 35% striatal DATs exhibit an intermediate phenotype compared to these two previously studied mouse models. It will also be interesting to study the response of our intermediate DAT expression mouse to different doses of amphetamine, which have been studied in other DAT mutant models.
Functional studies of [3H]DA uptake into striatal synaptosomes revealed a 46% decrease in maximal DAT-mediated uptake velocity in the DAT-LE mice, as compared to WT. On the other hand, Km values reflecting affinity for substrate in the DAT-LE mice were similar to WT, as were Kd values for the cocaine congener [3H]WIN 35,428. Considering the reduced number of DATs along with the reduced DAT function in the DAT-LE mice, an overall 2-fold increase in DA uptake turnover (Vmax/Bmax) was found. Since membrane binding measures the majority of cellular DATs, this number likely under-estimates the function per transporter. We also do not know if the cellular distribution of DATs differs between DAT-LE and WT mice. Nevertheless, our results strongly suggest that neuroadaptive changes have occurred in the DAT-LE mice that have either up-regulated DAT activity or that involve other biogenic amine transporters to compensate for the low DAT numbers. Further, cocaine also increases evoked striatal DA release (Oleson et al., 2009; Wu et al., 2001), and we do not know what neuroadaptive changes in DA release may have occurred in the DAT-LE mice during development. In any case, our results suggest that the increased DAT activity in the DAT-LE mice is insufficient to reduce extracellular DA levels to those in WT mice, resulting in novelty-induced hyperactivity and greater behavioral activation in the DAT-LE mice in response to moderate doses of cocaine that would be expected to only partially inhibit normal striatal DAT activity.
Acknowledgements
This work was supported by NIH grants R01 DA004216 (NRZ), R01 DA014204 (AS, NRZ), K05 DA015050 (NRZ), and F32 DA029357 (AR). We would also like to thank Mr. Christopher Ng, Mr. Gaynor Larson, and Ms. Umarani Pugazhenthi (Endocrinology PCR Core, University of Colorado Denver) for technical assistance, and Dr. Diana Simmons for help with the statistical analysis. The authors have no conflicts of interest to declare.
Abbreviations
- ANOVA
analysis of variance
- AU
arbitrary units
- Bmax
maximal number of binding sites
- CPP
conditioned place preference
- DA
dopamine
- DAT
dopamine transporter
- ER
endoplasmic reticulum
- HA11
haemagglutinin
- HET
heterozygous
- Kd
binding affinity
- Km
uptake affinity
- LE
low expresser
- NET
norepinephrine transporter
- RNAi
RNA interference
- SERT
serotonin transporter
- TH
tyrosine hydroxylase
- Vmax
maximal uptake velocity
- WT
wildtype
References
- Allen RM, Everett CV, Nelson AM, Gulley JM, Zahniser NR. Low and high locomotor responsiveness to cocaine predicts intravenous cocaine conditioned place preference in male Sprague-Dawley rats. Pharmacol Biochem Behav. 2007;86:37–44. doi: 10.1016/j.pbb.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler JA, Frazier CRM, Zhuang X. Dopaminergic enhancement of local food-seeking is under global homeostatic control. Eur J Neurosci. 2012;35:146–159. doi: 10.1111/j.1460-9568.2011.07916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliziotes M, McLoughlin S, Gunness M, Fumagalli F, Jones SR, Caron MG. Bone histomorphometric and biochemical abnormalities in mice homozygous for deletion of the dopamine transporter gene. Bone. 2000;26:15–19. doi: 10.1016/s8756-3282(99)00232-x. [DOI] [PubMed] [Google Scholar]
- Bosse R, Fumagalli F, Jaber M, Giros B, Gainetdinov RR, Wetsel WC, Missale C, Caron MG. Anterior pituitary hypoplasia and dwarfism in mice lacking the dopamine transporter. Neuron. 1997;19:127–138. doi: 10.1016/s0896-6273(00)80353-0. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen R, Han DD, Gu HH. A triple mutation in the second transmembrane domain of mouse dopamine transporter markedly decreases sensitivity to cocaine and methylphenidate. J Neurochem. 2005;94:352–359. doi: 10.1111/j.1471-4159.2005.03199.x. [DOI] [PubMed] [Google Scholar]
- Chen N, Reith ME. Substrates and inhibitors display different sensitivity to expression level of the dopamine transporter in heterologously expressing cells. J Neurochem. 2007;101:377–388. doi: 10.1111/j.1471-4159.2006.04384.x. [DOI] [PubMed] [Google Scholar]
- Chen R, Tilley MR, Wei H, Zhou F, Zhou F-M, Ching S, Quan N, Stephens RL, Hill ER, Nottoli T, Han DD, Gu HH. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc Natl Acad Sci USA. 2006;103:9333–9338. doi: 10.1073/pnas.0600905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBlasi A, O’Reilly K, Motulsky HJ. Calculating receptor number from binding experiments using same compound as radioligand and competitor. Trends Pharmacol Sci. 1989;10:227–229. doi: 10.1016/0165-6147(89)90266-6. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Miner LL, Perry MP, Revay RS, Sharpe LG, Przedborski S, Kostic V, Philpot RM, Kirstein CL, Rothman RB, Schindler CW, Uhl GR. Cocaine reward and MPTP toxicity: alteration by regional variant dopamine transporter overexpression. Brain Res Mol Brain Res. 1999;73:37–49. doi: 10.1016/s0169-328x(99)00235-1. [DOI] [PubMed] [Google Scholar]
- Drgon T, Lin Z, Wang GJ, Fowler J, Pablo J, Mash DC, Volkow N, Uhl GR. Common human 5′ dopamine transporter (SLC6A3) haplotypes yield varying expression levels in vivo. Cell Mol Neurobiol. 2006;26:875–889. doi: 10.1007/s10571-006-9014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Hall FS, Li XF, Sora I, Xu F, Caron M, Lesch KP, Murphy DL, Uhl GR. Cocaine mechanisms: enhanced cocaine, fluoxetine and nisoxetine place preferences following monoamine transporter deletions. Neuroscience. 2002;115:153–161. doi: 10.1016/s0306-4522(02)00379-2. [DOI] [PubMed] [Google Scholar]
- Hanania T, Gulley JM, Salaz DO, Larson GA, Zahniser NR. Role of the dopamine transporter in the differential cocaine-induced locomotor activation of inbred long-sleep and short-sleep mice. Neuropsychopharmacology. 2004;29:1814–1822. doi: 10.1038/sj.npp.1300501. [DOI] [PubMed] [Google Scholar]
- Janowsky A, Mah C, Johnson RA, Cunningham CL, Phillips TJ, Crabbe JC, Eshleman AJ, Belknap JK. Mapping genes that regulate density of dopamine transporters and correlated behaviors in recombinant inbred mice. J Pharmacol Exp Ther. 2001;298:634–643. [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci U S A. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian MA, Li Y, Zhen J, Meyer E, Hai N, Christen HJ, Hoffmann GF, Jardine P, von Moers A, Mordekar SR, O’Callaghan F, Wassmer E, Wraige E, Dietrich C, Lewis T, Hyland K, Heales S, Jr., Sanger T, Gissen P, Assmann BE, Reith ME, Maher ER. Clinical and molecular characterisation of hereditary dopamine transporter deficiency syndrome: an observational cohort and experimental study. Lancet Neurol. 2011;10:54–62. doi: 10.1016/S1474-4422(10)70269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little KY, Kirkman JA, Carroll FI, Clark TB, Duncan GE. Cocaine use increases [3H]WIN 35428 binding sites in human striatum. Brain Res. 1993;628:17–25. doi: 10.1016/0006-8993(93)90932-d. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Kreek MJ. Acute tolerance to the dopamine response induced by a binge pattern of cocaine administration in male rats: an in vivo microdialysis study. J Pharmacol Exp Ther. 1994;268:916–921. [PubMed] [Google Scholar]
- Mandt BH, Schenk S, Zahniser NR, Allen RM. Individual differences in cocaine-induced locomotor activity in male Sprague-Dawley rats and their acquisition of and motivation to self-administer cocaine. Psychopharmacology (Berl) 2008;201:195–202. doi: 10.1007/s00213-008-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandt BH, Zahniser NR. Low and high cocaine locomotor responding male Sprague-Dawley rats differ in rapid cocaine-induced regulation of striatal dopamine transporter function. Neuropharmacology. 2010;58:605–612. doi: 10.1016/j.neuropharm.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BJ, Naughton BJ, Thirtamara-Rajamani K, Yoon DJ, Han DD, Devries AC, Gu HH. Dopamine transporter inhibition is necessary for cocaine-induced increases in dendritic spine density in the nucleus accumbens. Synapse. 2011;65:490–496. doi: 10.1002/syn.20865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash DC, Pablo J, Ouyang Q, Hearn WL, Izenwasser S. Dopamine transport function is elevated in cocaine users. J Neurochem. 2002;81:292–300. doi: 10.1046/j.1471-4159.2002.00820.x. [DOI] [PubMed] [Google Scholar]
- Miranda M, Sorkina T, Grammatopoulos TN, Zawada WM, Sorkin A. Multiple molecular determinants in the carboxyl terminus regulate dopamine transporter export from endoplasmic reticulum. J Biol Chem. 2004;279(29):30760–70. doi: 10.1074/jbc.M312774200. [DOI] [PubMed] [Google Scholar]
- Nelson AM, Larson GA, Zahniser NR. Low or high cocaine responding rats differ in striatal extracellular dopamine levels and dopamine transporter number. J Pharmacol Exp Ther. 2009;331:985–997. doi: 10.1124/jpet.109.159897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Salek J, Bonin KD, Jones SR, Budygin EA. Real-time voltammetric detection of cocaine-induced dopamine changes in the striatum of freely-moving mice. J Neurosci Meth. 2009;467:144–146. doi: 10.1016/j.neulet.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Richards TL, Simmons D, Zahniser NR, Sorkin A. Epitope-tagged dopamine transporter knock-in mice reveal rapid endocytic trafficking and filopodia targeting of the transporter in dopaminergic axons. FASEB J. 2012;26:1921–1933. doi: 10.1096/fj.11-196113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Simmons D, Sorkin A. Differential subcellular distribution of endosomal compartments and the dopamine transporter in dopaminergic neurons. Mol Cell Neurosci. 2011;46(1):148–58. doi: 10.1016/j.mcn.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Acute cocaine differentially alters accumbens and striatal dopamine clearance in low and high cocaine locomotor responders: behavioral and electrochemical recordings in freely moving rats. J Pharmacol Exp Ther. 2002;302:1201–1211. doi: 10.1124/jpet.102.035816. [DOI] [PubMed] [Google Scholar]
- Salahpour A, Medvedev IO, Beaulieu J-M, Gainetdinov RR, Caron MG. Local knockdown of genes in the brain using small interfering RNA: a phenotypic comparison with knockout animals. Biol Psychiatry. 2007;61:65–69. doi: 10.1016/j.biopsych.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Thakker DR, Natt F, Hüsken D, Maier R, Müller M, van der Putten H, Hoyer D, Cryan JF. Neurochemical and behavioral consequences of widespread gene knockdown in the adult mouse brain by using nonviral RNA interference. Proc Natl Acad Sci USA. 2004;101:17270–17275. doi: 10.1073/pnas.0406214101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Han DD, Gu HH, Caine SB. Lack of cocaine self-administration in mice expressing a cocaine-insensitive dopamine transporter. J Pharmacol Exp Ther. 2009;331:204–211. doi: 10.1124/jpet.109.156265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley MR, Cagniard B, Zhuang X, Han DD, Tiao N, Gu HH. Cocaine reward and locomotion stimulation in mice with reduced dopamine transporter expression. BMC Neurosci. 2007;8:42. doi: 10.1186/1471-2202-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Hall FS, Sora I. Cocaine, reward, movement and monoamine transporters. Mol Psychiatry. 2002;7:21–26. doi: 10.1038/sj.mp.4000964. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, Hitzemann R, Shea CE. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997;386:827–830. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Kuhar MJ, Carroll FI, Garris PA. Preferential increases in nucleus accumbens dopamine after systemic cocaine administration are caused by unique characteristics of dopamine neurotransmission. J Neurosci. 2001;21(16):6338–47. doi: 10.1523/JNEUROSCI.21-16-06338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A. 2001;98:1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]