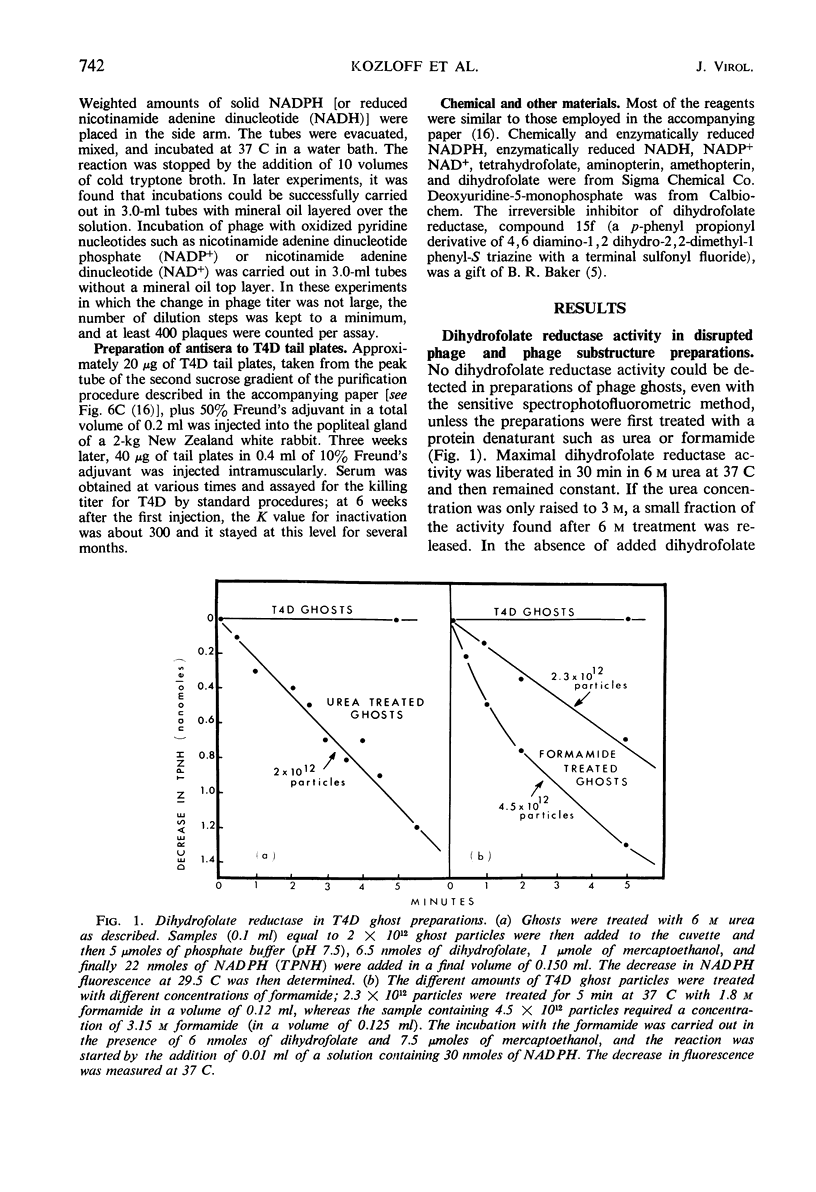
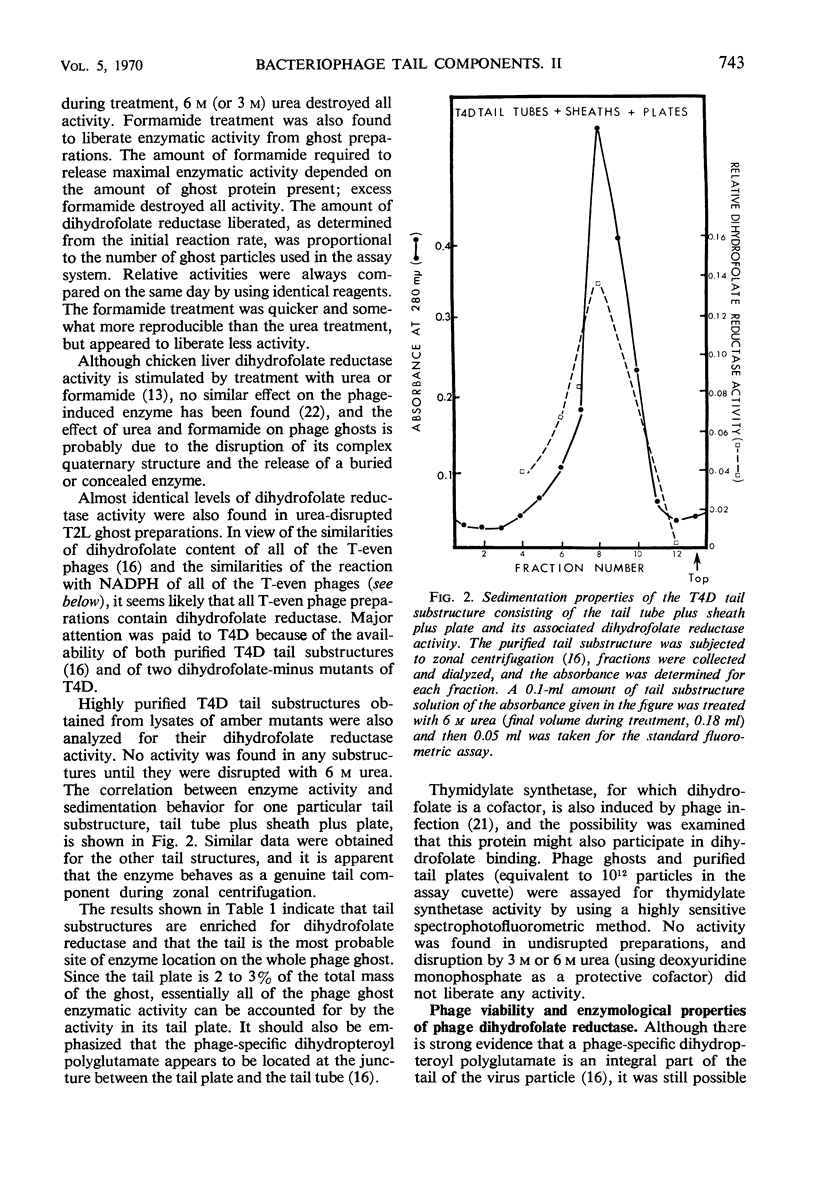
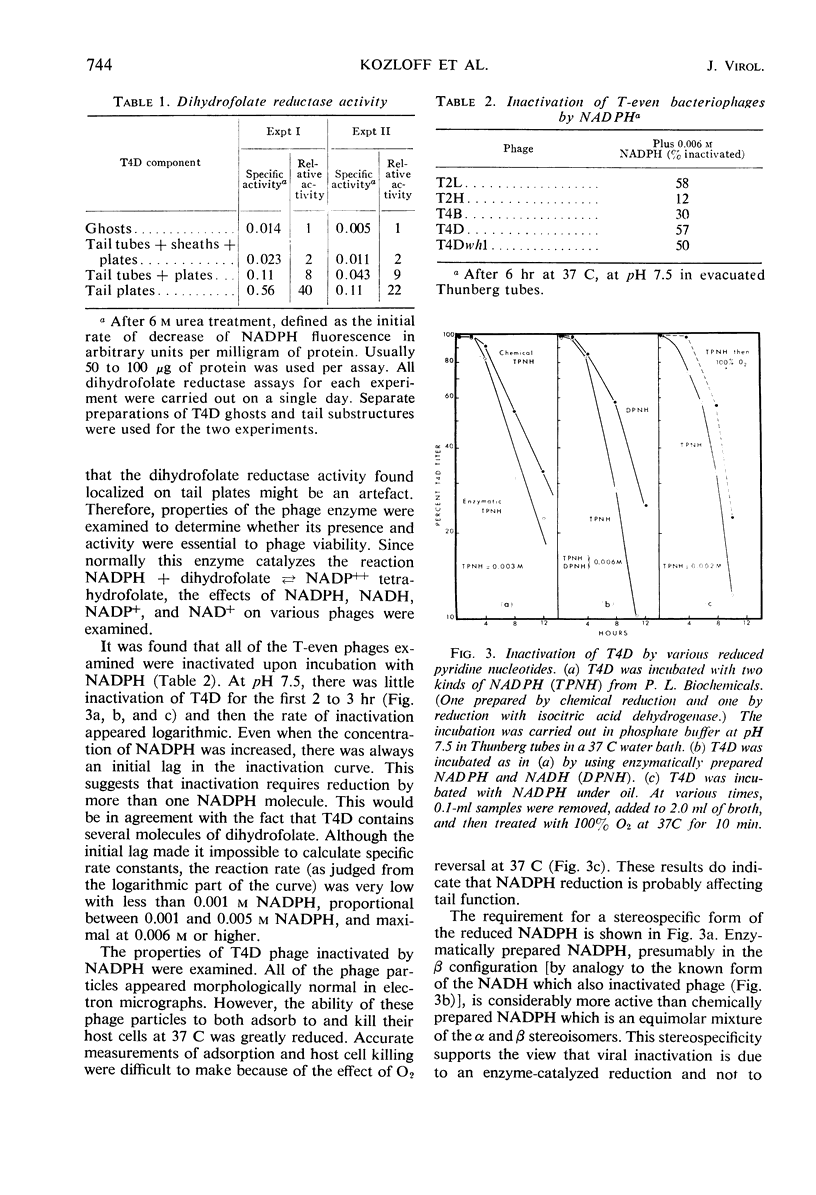
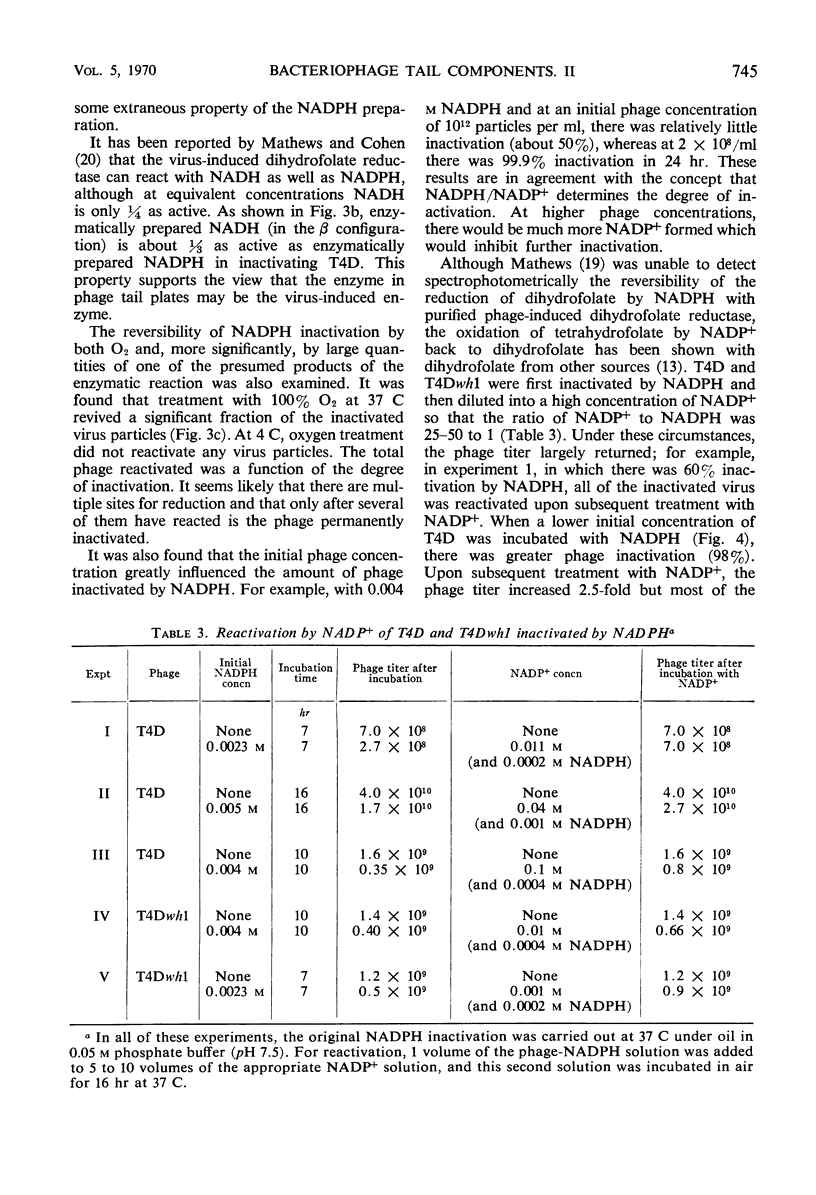
Abstract
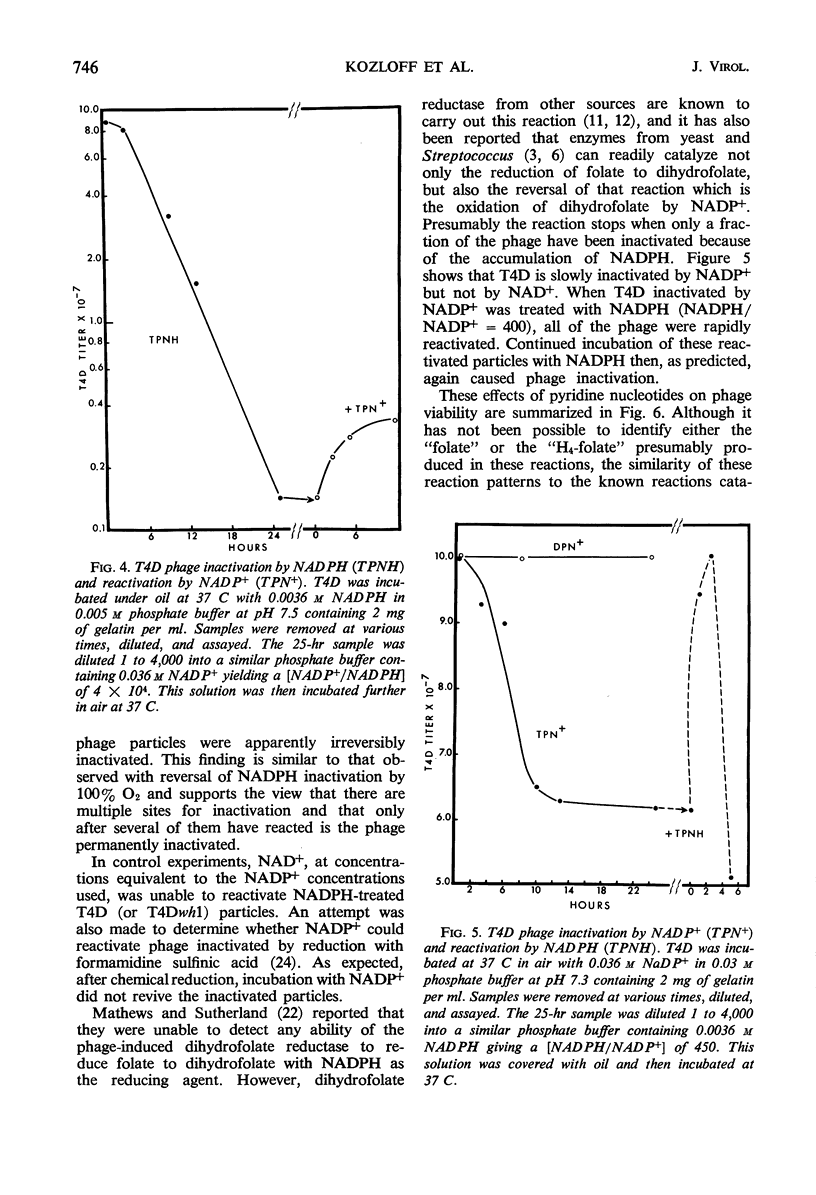
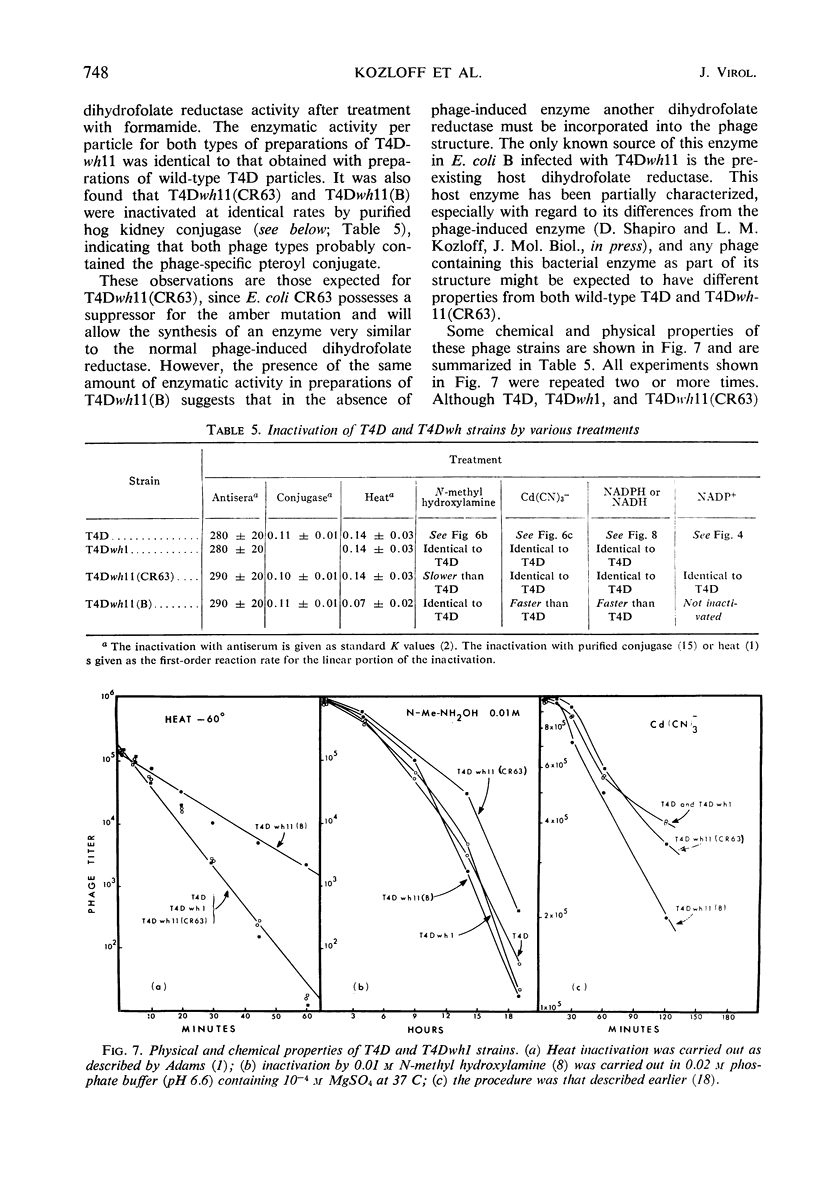
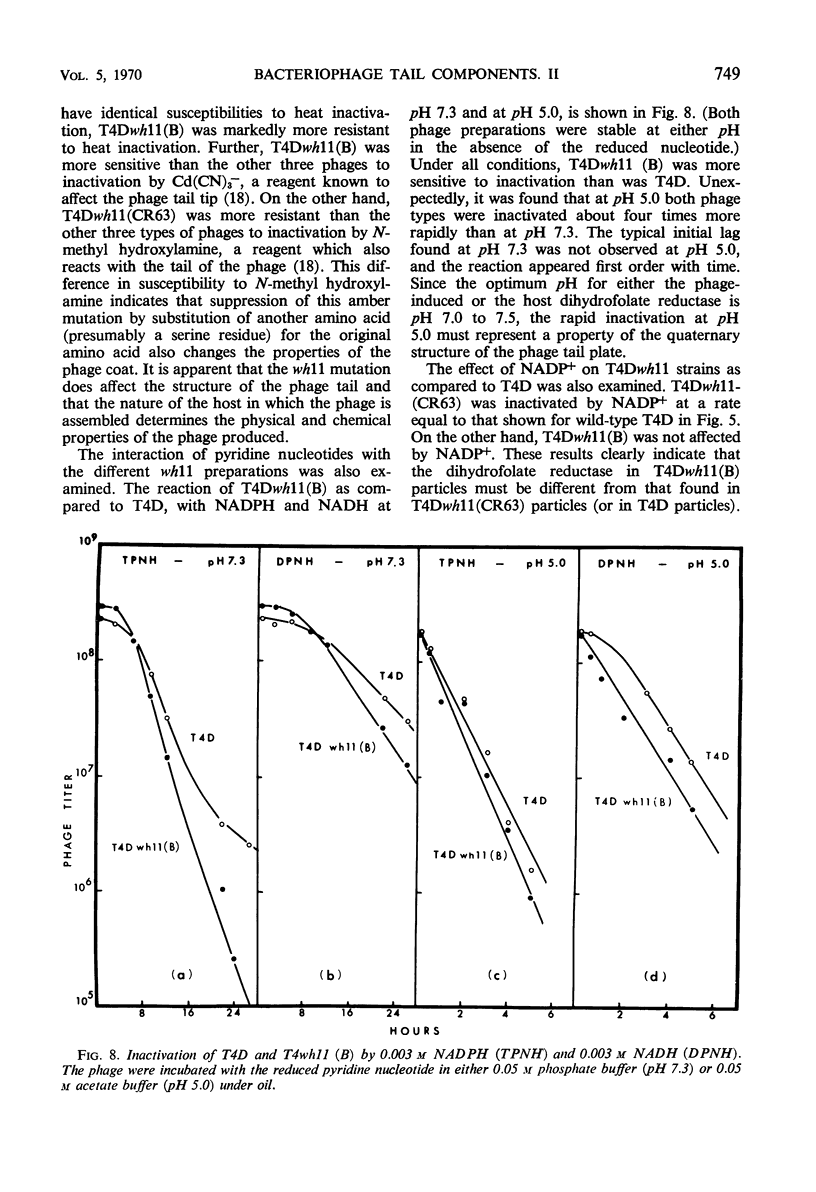
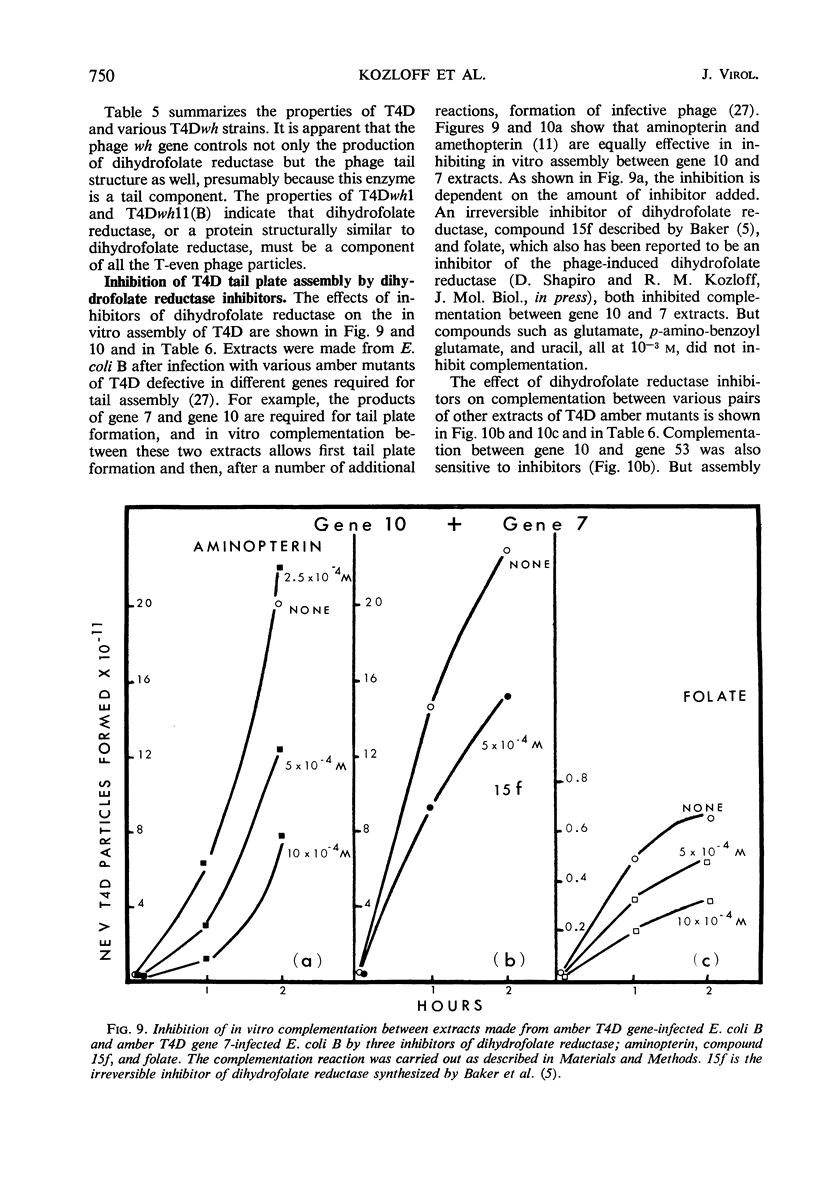
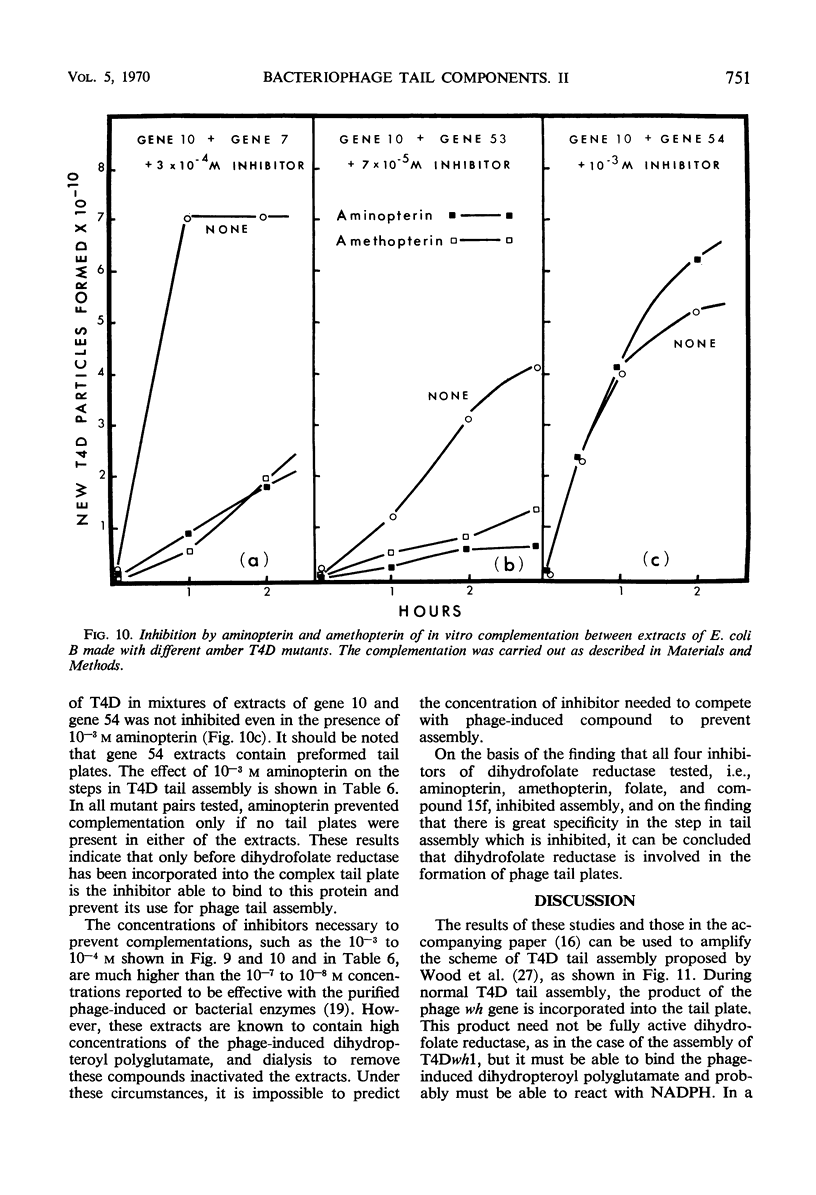
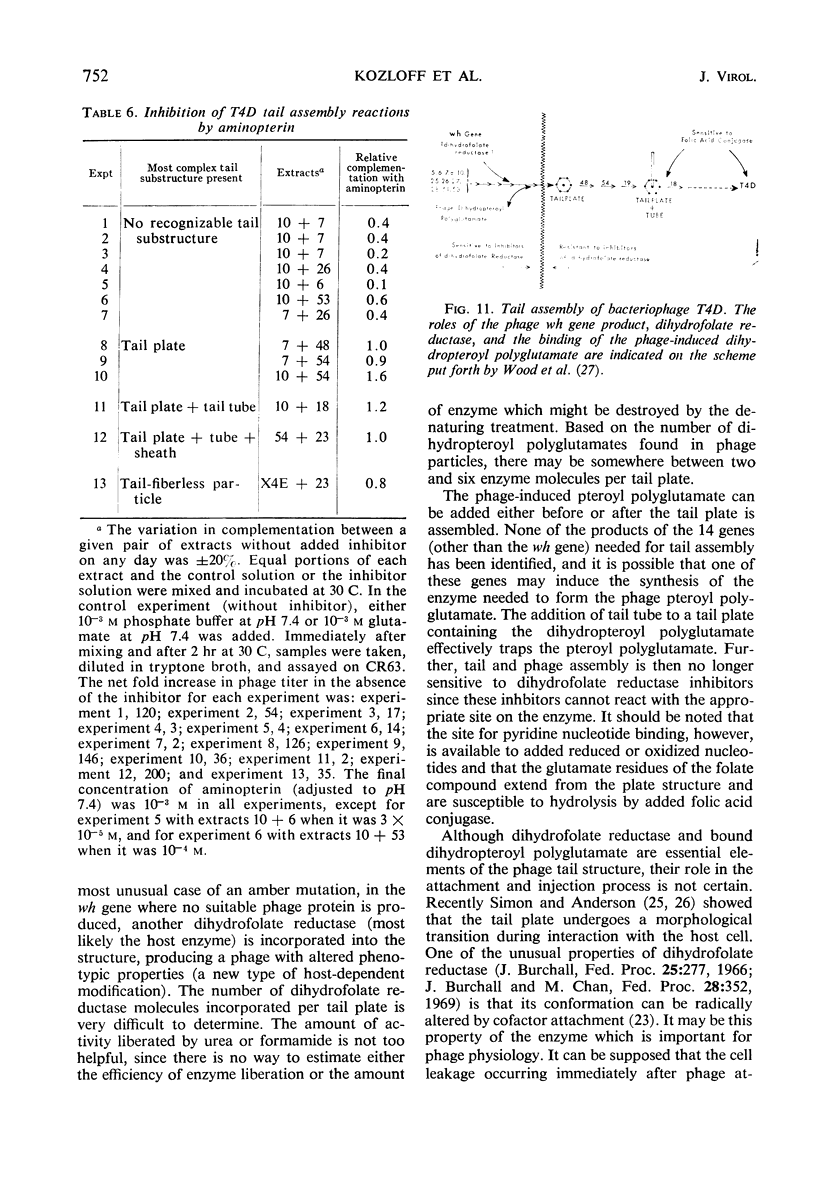
The protein component of the T-even bacteriophage coat which binds the phage-specific dihydropteroyl polyglutamate has been identified as the phage-induced dihydrofolate reductase. Dihydrofolate reductase activity has been found in highly purified preparations of T-even phage ghosts and phage substructures after partial denaturation. The highest specific enzymatic activity was found in purified tail plate preparations, and it was concluded that this enzyme was a structural component of the phage tail plate. Phage viability was directly correlated with the enzymological properties of the phage tail plate dihydrofolate reductase. All reactions catalyzed by this enzyme which changed the oxidation state of the phage dihydrofolate also inactivated the phage. Properties of two T4D dihydrofolate reductase-negative mutants, wh1 and wh11, have been examined. Various lines of evidence support the view that the product of the wh locus of the phage genome is normally incorporated into the phage tail structure. The effects of various dihydrofolate reductase inhibitors on phage assembly in in vitro complementation experiments with various extracts of conditional lethal T4D mutants have been examined. These inhibitors were found to specifically block complementation when added to extracts which did not contain preformed tail plates. If tail plates were present, inhibitors such as aminopterin, did not affect further phage assembly. This specific inhibition of tail plate formation in vitro confirms the analytical and genetic evidence that this phage-induced “early” enzyme is a component of the phage coat.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht A. M., Pearce F. K., Suling W. J., Hutchison D. J. Folate reductase and specific dihydrofolate reductase of the amethopterin-sensitive Streptococcus faecium var. durans. Biochemistry. 1969 Mar;8(3):960–967. doi: 10.1021/bi00831a029. [DOI] [PubMed] [Google Scholar]
- Baker B. R., Lourens G. J. Irreversible enzyme inhibitors. CV. Differential irreversible inhibition of vertebrate dihydrofolic reductases by derivatives of 4,6-diamino-1,2-dihydro-2,2-dimethyl-1-phenyl-s-triazines substituted with a terminal sulfonyl fluoride. J Med Chem. 1967 Nov;10(6):1113–1122. doi: 10.1021/jm00318a029. [DOI] [PubMed] [Google Scholar]
- Bush M. E., Donaldson K. O. Tetrahydrofolate dehydrogenase from bakers' yeast. Biochem Biophys Res Commun. 1965 Sep 8;20(5):635–640. doi: 10.1016/0006-291x(65)90447-x. [DOI] [PubMed] [Google Scholar]
- Edgar R. S., Wood W. B. Morphogenesis of bacteriophage T4 in extracts of mutant-infected cells. Proc Natl Acad Sci U S A. 1966 Mar;55(3):498–505. doi: 10.1073/pnas.55.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREESE E., BAUTZ-FREESE E., BAUTZ E. Hydroxylamine as a mutagenic and inactivating agent. J Mol Biol. 1961 Apr;3:133–143. doi: 10.1016/s0022-2836(61)80040-5. [DOI] [PubMed] [Google Scholar]
- HUENNEKENS F. M. The role of dihydrofolic reductase in the metabolism of one-carbon units. Biochemistry. 1963 Jan-Feb;2:151–159. doi: 10.1021/bi00901a027. [DOI] [PubMed] [Google Scholar]
- Hall D. H. Mutants of bacteriophage T4 unable to induce dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1967 Aug;58(2):584–591. doi: 10.1073/pnas.58.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. H., Tessman I., Karlström O. Linkage of T4 genes controlling a series of steps in pyrimidine biosynthesis. Virology. 1967 Mar;31(3):442–448. doi: 10.1016/0042-6822(67)90224-3. [DOI] [PubMed] [Google Scholar]
- KAUFMAN B. T. Activation of dihydrofolic reductase by urea and formamide. Biochem Biophys Res Commun. 1963 Mar 25;10:449–453. doi: 10.1016/0006-291x(63)90378-4. [DOI] [PubMed] [Google Scholar]
- KOZLOFF L. M., LUTE M., HENDERSON K. Viral invasion. I. Rupture of thiol ester bonds in the bacteriophage tail. J Biol Chem. 1957 Sep;228(1):511–528. [PubMed] [Google Scholar]
- King J. Assembly of the tail of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):231–262. doi: 10.1016/0022-2836(68)90007-7. [DOI] [PubMed] [Google Scholar]
- Kozloff L. M., Lute M., Crosby L. K., Rao N., Chapman V. A., DeLong S. S. Bacteriophage tail components. I. Pteroyl polyglutamates in T-even bacteriophages. J Virol. 1970 Jun;5(6):726–739. doi: 10.1128/jvi.5.6.726-739.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozloff L. M., Lute M., Crosby L. K., Wong R., Stern B. Critical arginine residue for maintaining the bacteriophage tail structure. J Virol. 1969 Feb;3(2):217–227. doi: 10.1128/jvi.3.2.217-227.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozloff L. M., Lute M. Folic acid, a structural component of T4 bacteriophage. J Mol Biol. 1965 Jul;12(3):780–792. doi: 10.1016/s0022-2836(65)80327-8. [DOI] [PubMed] [Google Scholar]
- MATHEWS C. K., COHEN S. S. Virus-induced acquisition of metabolic function. VI. Dihydrofolate reductase, a new phage-induced enzyme. J Biol Chem. 1963 Feb;238:853–855. [PubMed] [Google Scholar]
- MATHEWS C. K., SUTHERLAND K. E. COMPARATIVE BIOCHEMISTRY OF BACTERIAL AND PHAGE-INDUCED DIHYDROFOLATE REDUCTASES. J Biol Chem. 1965 May;240:2142–2147. [PubMed] [Google Scholar]
- Mathews C. K. Evidence that bacteriophage-induced dihydrofolate reductase in a viral gene product. J Biol Chem. 1967 Sep 25;242(18):4083–4086. [PubMed] [Google Scholar]
- Mell G. P., Martelli M., Kirchner J., Huennekens F. M. Multiple forms of dihydrofolate reductase. Biochem Biophys Res Commun. 1968 Oct 10;33(1):74–79. doi: 10.1016/0006-291x(68)90257-x. [DOI] [PubMed] [Google Scholar]
- SHASHOUA V. E. FORMAMIDINE SULFINIC ACID AS A BIOCHEMICAL REDUCING AGENT. Biochemistry. 1964 Nov;3:1719–1720. doi: 10.1021/bi00899a021. [DOI] [PubMed] [Google Scholar]
- Simon L. D., Anderson T. F. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. I. Attachment and penetration. Virology. 1967 Jun;32(2):279–297. doi: 10.1016/0042-6822(67)90277-2. [DOI] [PubMed] [Google Scholar]
- Simon L. D., Anderson T. F. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. II. Structure and function of the baseplate. Virology. 1967 Jun;32(2):298–305. doi: 10.1016/0042-6822(67)90278-4. [DOI] [PubMed] [Google Scholar]
- Wood W. B., Edgar R. S., King J., Lielausis I., Henninger M. Bacteriophage assembly. Fed Proc. 1968 Sep-Oct;27(5):1160–1166. [PubMed] [Google Scholar]



