Abstract
Background
Access to dermatology and dermatopathology services is scarce in sub-Saharan Africa. Teledermatology provides consultations for healthcare providers in resource-limited settings where specialty medical services are difficult to obtain, and the African Teledermatology Project has helped to bridge the gap in dermatological care in Africa. This program also allows for biopsy specimens to be sent to the USA for processing in cases where the clinical diagnosis is difficult and definitive diagnosis has implications for patient management. This study characterizes conditions diagnosed through clinicopathological correlation in conjunction with photos and tissue submitted to the African Teledermatology Project.
Materials and methods
Retrospective case review of tissue specimens submitted over three years.
Results
Fifty-five biopsy specimens met inclusion criteria and represent cases of malignancy (35%), infection (7%), suspected infection (15%), lichenoid tissue reaction (5%), dermatitis (15%), and other various conditions (18%). Three biopsy specimens were non-diagnostic (5%). Clinicopathological concordance between submitting clinician and biopsy results occurred in 32 out of 55 cases (58%). Clinical and pathological diagnoses differed in 21 out of 55 cases (38%). Kaposi sarcoma (KS) represents the clinical diagnosis most often suspected in the evaluated biopsy specimens (42%) and was correctly recognized clinically in 13 out of 23 cases (57%).
Conclusion
Clinical images may not provide sufficient information to definitively diagnose certain infectious and malignant dermatological conditions submitted through telemedicine consultation. Microscopic examination of skin biopsy specimens is an important adjunct for accurate diagnosis of disease and determination of appropriate treatment strategies.
Introduction
Nations of the developing world face significant physician shortages, particularly specialists such as dermatologists. Sub-Saharan Africa is among the world's most underdeveloped and resource-poor regions, with as few as 10 physicians per 100,000, and no dermatologists in many areas.1–3 The availability of dermatopathology services is even rarer, with only 14% of sub-Saharan African nations reporting the presence of a dermatopathologist in their country.4 In areas where access to dermatology and dermatopathology is scarce, general clinicians rely heavily on clinical judgment and are rarely able to submit skin biopsies for histological interpretation.
The African Teledermatology Project was initiated through a collaboration between the Departments of Dermatology at the University of Pennsylvania, USA, and the Medical University of Graz, Austria, with additional collaboration from Mbarara University of Science and Technology and Makerere University in Uganda, the Botswana-UPenn Partnership, the Baylor International Pediatric AIDS Initiative (BIPAI), the American Academy of Dermatology, and the University of Queensland in Brisbane, Australia, in 2007. Participating African medical centers in Botswana, Eritrea, Kenya, Lesotho, Liberia, Malawi, Mozambique, Nigeria, South Africa, Swaziland, Tanzania, and Uganda have submitted approximately 700 clinical cases for review since the project began in 2007. Clinical photographs along with patient history are posted on the web-based application (http://africa.telederm.org) for secure internet review by teledermatologists who provide feedback and suggestions on management and diagnosis.1,2
The use of teledermatology consultation for second opinion service has been shown to be useful as a means of generating discussion and confirmation of diagnosis, as well as management decisions.5 The African Teledermatology Project has demonstrated the usefulness of a teledermatology network in improving the provision of care for skin disease in resource-limited settings. In most cases, diagnosis and management decisions can be reached with the clinical history and clinical photographs alone. However, in certain situations, such as dermatoses of the immunosuppressed, histology provides information imperative to determining definitive diagnosis and safe treatment strategies. Anecdotal evidence and case reports highlight the protean manifestations of human immunodeficiency virus (HIV)-associated dermatoses and underscore the value of histopathological confirmation of diagnosis prior to the initiation of treatment strategies.6–8 Treatment courses for antimicrobial and malignant conditions are complex in this patient population, and if the incorrect empiric therapy is initiated, the underlying disease process can worsen.6
The African Teledermatology Project has helped to bridge the gap in access to dermatological specialty care in Africa through store and forward teledermatology consultation services. This teledermatology program also allows for biopsy specimens to be performed and sent to the USA for processing in cases where the clinical diagnosis is difficult and definitive diagnosis has implications for management of the patient. This study seeks to characterize the conditions diagnosed through clinicopathological correlation in conjunction with photos and tissue submitted to the African Teledermatology Project. We will categorize the diagnoses rendered; determine the timeliness of specimen acquisition and diagnosis; evaluate the concordance of the clinical diagnosis of the submitting clinician and histological diagnosis; and determine the possible clinical impact of such information on the ultimate course of patient care and treatment recommendations. The types of specimens sent represent clinically challenging situations where tissue examination is imperative to determining the safest and best course of treatment for skin diseases, and the African Teledermatology Project presents a unique solution for the definitive diagnosis of difficult dermatology cases in resource-limited settings.
Materials and methods
This is a retrospective case review of cases submitted to the African Teledermatology Project where corresponding biopsies were also sent to the USA for analysis and definitive diagnosis. All biopsy specimens originating as consultations from Africa in the University of Pennsylvania Department of Dermatology, Division of Dermatopathology, were evaluated. This includes all tissue specimens sent for processing and subsequent pathological consultation in the three-year period from 2007 to 2009.
The age, gender, and country of origin (Fig. 1) of each patient were recorded. The clinical history outlined in the teledermatology consultation and the corresponding pathology report, as well as the histological diagnoses rendered, were evaluated by both authors. In cases where two or more biopsy specimens were taken from a single patient, each biopsy was counted separately if the corresponding diagnoses were different. In settings where the diagnosis of both specimens was identical, the biopsies were aggregated and counted once, as they represented two samples of an identical process. Based upon the biopsy site and microscopic description, biopsies were excluded if they did not include skin as part of the specimen. Biopsies were not excluded because of patient age, race, or economic status.
Figure 1.
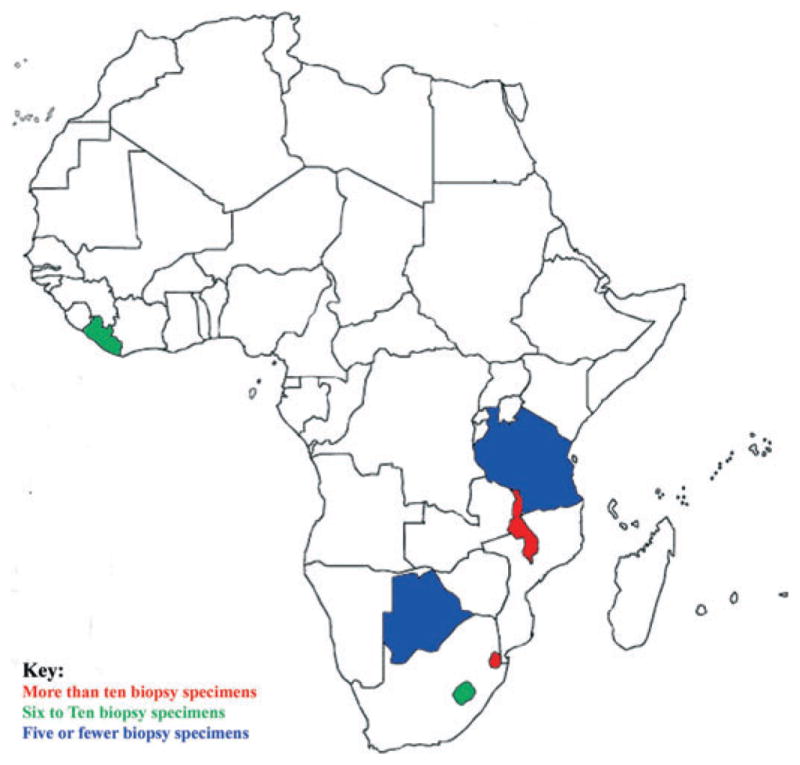
Map of Africa indicating biopsy specimen countries of origin. Fifty-five biopsies from: Botswana (1), Lesotho (7), Liberia (7), Swaziland (11), Tanzania (1), and Malawi (26)
Results
Fifty-nine cases were identified for review and, of these cases, 55 met both the inclusion and exclusion parameters. The biopsy specimens represent 22 females and 31 males (one female and one male each with two biopsies).
The diagnoses rendered in the 55 cases can be divided into seven general categories: malignancy; infection; suspected infection; lichenoid tissue reaction; dermatitis; other; and non-diagnostic biopsies (Table 1). Kaposi sarcoma (KS) represents 79% of malignant biopsies (15/19) and 27% of all biopsy specimens (15/55). Infections represent 22% (12/55) of biopsy specimens, including confirmed infections based upon organism identification in four specimens and suggestive histology despite negative stains in eight specimens. Infections include filariasis, fungal infections, and leprosy. Dermatitis represents 15% of biopsy samples (8/55), and includes eczema, dermatitis not otherwise specified (NOS), folliculitis, psoriasis, and lichen simplex chronicus. Other diagnoses represent 18% of biopsy specimens and include: congenital ichthyosiform erythroderma; a suspected immunobullous disorder; connective tissue disorder; vasculitis; pyogenic granuloma; gout; normal lymph node; scar; and cysts.
Table 1.
Classification of biopsy specimens by type
| Biopsy classification | Total # | ||
|---|---|---|---|
| Malignancy | Kaposi sarcoma | 15 | 19 |
| SCCIS | 2 | ||
| Metastatic SCC | 1 | ||
| CTCL | 1 | ||
| Infection | Blastomycosis | 1 | 4 |
| Majocci granuloma | 1 | ||
| Filarial disease | 1 | ||
| Lepromatous leprosy | 1 | ||
| Suspected infection | Secondary syphilis | 2 | 8 |
| Filarial disease | 1 | ||
| Other NOS | 5 | ||
| Lichenoid tissue reaction | 3 | ||
| Dermatitis | Eczema | 3 | 8 |
| Dermatitis NOS | 1 | ||
| Folliculitis | 1 | ||
| Psoriasis | 1 | ||
| Lichen simplex chronicus | 2 | ||
| Other | Cornification disorder (CIE) | 1 | 10 |
| Immunobullous (BP or EBA) | 1 | ||
| Lupus | 1 | ||
| Vasculitis | 1 | ||
| Pyogenic granuloma | 1 | ||
| Gout | 1 | ||
| Lymph node | 1 | ||
| Scar | 1 | ||
| Cyst | 2 | ||
| Non-diagnostic | 3 | ||
| Total biopsies | 55 |
Each major category is further subdivided by specific diagnosis where available.
BP, bullous pemphigoid; CIE, congenital ichthyosiform erythroderma; CTCL, cutaneous T-cell lymphoma; EBA, epidermolysis bullosa acquisita; NOS, not otherwise specified; SCC, squamous cell carcinoma; SCCIS, squamous cell carcinoma in situ
Only 5% of biopsies were non-diagnostic (3/55). Of these biopsies, one represented a superficial sample of mostly epidermis. The clinically suspected malignant process, KS, could not be excluded in the absence of dermis. For the remaining two biopsies, infectious etiologies were suspected clinically. Stains performed for bacteria, mycobacteria, and fungi did not reveal organisms, and the histological pattern was not suspicious for an infectious process.
In 32 of 55 cases (58%), the clinical diagnoses suspected by the submitting clinician and the pathological diagnoses were consistent. In 21 of 55 cases (38%), the clinical and pathological diagnoses conflicted (Table 2), and in the remaining two of 55 cases (4%), insufficient clinical data were provided through the teledermatology program. Of the 21 cases that lacked clinical and pathological concordance, 11 (52%) represent cases clinically suspicious for malignancy. One case of a clinically suspected vulvar squamous cell carcinoma (SCC) revealed no evidence of malignancy, and histology suggested an infectious etiology. KS was clinically suspected in the remaining 10 cases, and histological examination revealed three cases (30%) of lichenoid tissue reaction. Two cases of clinically suspected KS were histologically identified as infectious processes, namely filarial disease and histoid lepromatous leprosy.
Table 2.
Clinical and histopathological diagnoses were discordant in 21 cases
| Clinical diagnosis (# cases) | Histological diagnosis (# cases) |
|---|---|
| Malignancy (11) | Lichenoid tissue reaction (3) |
| Infection (2) | |
| Suspect infection (1) | |
| Vasculitis (1) | |
| Dermatitis (1) | |
| Normal tissue (1) | |
| Infection (3) | Benign (1) |
| Malignant (1) | |
| Non-diagnostic (1) | |
| Not infection (1) | Infection (1) |
| Dermatitis (2) | Infection (2) |
| Benign/no pathology (3) | Malignant (1) |
| Infection (1) | |
| Metabolic (1) | |
| Immunobullous (1) | Dermatitis (1) |
KS represents the clinical diagnosis most often suspected in the evaluated biopsy specimens, comprising 23 of 55 biopsies (42%). It was correctly recognized clinically in 13 of 23 cases (57%).
Discussion
Enabling healthcare providers in resource-limited settings to submit skin biopsy specimens in difficult clinical cases that have been submitted for teledermatology evaluation has allowed for definitive diagnosis and appropriate management of patients with serious dermatological conditions. In countries where pathology services are limited or unavailable, the transport of fixed tissue to an experienced pathology laboratory may be a feasible option for histological analysis of skin biopsies. In the biopsies submitted for analysis through the African Teledermatology Project, the clinical diagnosis was confirmed by histological examination in 58% of cases and provided useful information in the management of patients with both malignant and benign conditions. Over half of cases confirmed malignancies, of which 81% (13/16) represent HIV-associated KS in patients with very low CD4 counts. Prior to initiating the scarcely available and costly chemotherapy in resource-limited settings, histological diagnosis is imperative. In the one case of tumoral stage cutaneous T-cell lymphoma, the confirmation of a malignant process and exclusion of infectious etiology allowed for targeted therapy and avoidance of unnecessary complications. Histological confirmation of benign diagnoses is equally important: confirmation of multiple steatocystomas on the chest of a young African man permitted reassurance and prevented the proposed surgical excision and subsequent deformation of the entire chest wall. Clinicopathological correlation and histological confirmation allow for the delivery of high-quality health care and avoidance of empiric treatments that may lead to worsening of the condition and unnecessary costs. In order to optimize the use of resources, clinicopathological correlation should be reserved for the most difficult cases with management implications.
KS diagnoses deserve special attention given the prevalence of HIV infection in patients presenting to dermatology clinics in sub-Saharan Africa. The HIV status of 50% of the 345 consultation cases submitted through the African Teledermatology Project is known, and of these 174 cases, 71.8% were HIV-infected and/or had HIV/AIDS-associated skin conditions.2 This prevalence explains clinicians' high suspicion for KS diagnoses in biopsy specimens and the common request for special stains to confirm the suspected diagnosis. Prior studies have demonstrated up to 90–100% sensitivity and 100% specificity for monoclonal antibodies to HHV-8 (Fig. 2).9–11
Figure 2.
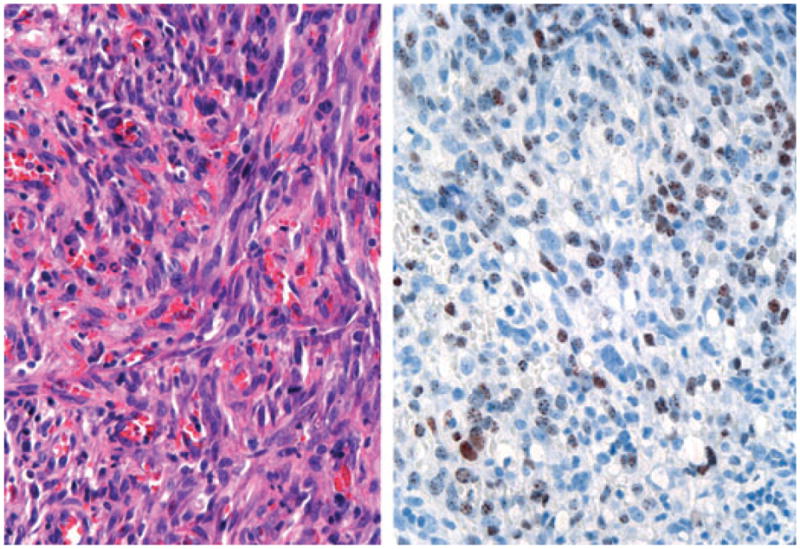
Histology demonstrating KS, with an atypical spindle cell proliferation with extravasated red blood cells (left) and positive nuclear HHV-8 staining (right). (Original power × 400)
Over half of cases lacking clinicopathological correlation (11 out of 21) involved clinically suspected or microscopically confirmed KS. In 10 cases, KS was suspected clinically but revealed different disease processes histologically. Three of these 10 cases represent lichenoid tissue reactions with prominent pigment incontinence on histology. Clinically these cases represent flat hyper-pigmented patches and thin plaques, which mimic early patch stage KS. The case of vasculitis by histology had a clinical history highly suggestive of KS; the clinical lesion presented as a nodule on the lower extremity of an HIV+ patient with a CD4 count of 200. Three cases suspected of KS clinically were confirmed as infections and, in these cases, appropriate antimicrobial therapy resulted in clinical improvement.
In only one histologically confirmed case of KS was the diagnosis not clinically suspected or included in the differential diagnosis and clinical history. This particular single lesion presented on the chin of a child and initially improved on oral antibiotic therapy but recurred as an ulcerated nodule. In another case, the clinician had a low suspicion for KS based upon the patient's CD4 count of 950 (Fig. 3). KS was listed on the differential diagnosis and was confirmed by histology and HHV-8+ staining. These cases underscore the important role of histology in definitively diagnosing a serious dermatological disease in immunocompromised patients in whom the differential diagnosis is broad and the management is complex.
Figure 3.
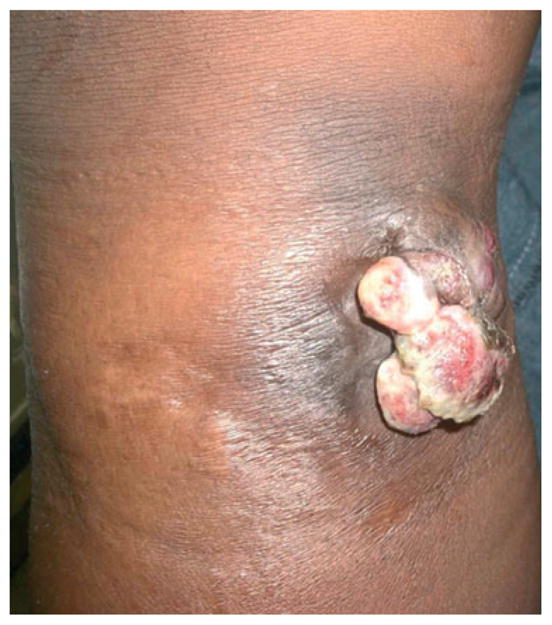
Patient with a CD4 count of 950 with nodule ultimately diagnosed as KS
There are limitations to this study. First, the retrospective nature and inclusion criteria permit us only to look at cases submitted in consultation and therefore may not represent the true population burden of disease in the local areas from which they derive. Second, scarcity of resources in Africa, namely biopsy kits and formalin, create an inherent selection bias for only the most challenging cases that require histological assistance for diagnosis. Selection of only the most difficult cases might explain, in part, the relatively low rate of clinicopathological correlation. Furthermore, clinicopathological correlation requires the presence of accurate clinical information, which often was not included with the case. Third, not all biopsy specimens resulted in a clearly defined histological diagnosis. The transportation time of the biopsies from Africa to the USA was on average less than one week; however, in rare cases the time from obtaining the specimen to arrival in the processing laboratory exceeded one month. In transport, one of the non-diagnostic cases was lost for weeks and arrived dry without formalin in the biopsy cup. Finally, this study does not evaluate the impact of diagnostic data derived from histological examination. Clinicians submitting specimens are responsible for identifying and managing the patients in question, and due to the method by which specimens are sent, there are no structured mechanisms in place to inform dermatopathologists of the ultimate patient outcome. Further investigation and research in these areas is required.
This study highlights the significant contribution dermatopathology services can provide in the diagnosis and treatment of skin disease in the developing world. Efforts to expand dermatological care to rural parts of the developing world will rely heavily on partnerships between local clinics and urban academic medical centers, as well as creative implementation of technology and reappropriation of resources. Store and forward teledermatology can be used to provide dermatological care and education in settings where no specialists exist. Histological examination of skin biopsy specimens in difficult clinical cases can assist in definitive diagnosis. In countries where there are no histological services, one solution is the shipment of wet tissue to experienced laboratories. In order to ship the tissue in a cost-effective manner in resource-limited settings, innovative specimen containers, such as old medicine vials, are often used to hold formalin-fixed tissue (Fig. 4). The obvious logistic limitations to this practice are the time to send specimens and cost of shipment.
Figure 4.
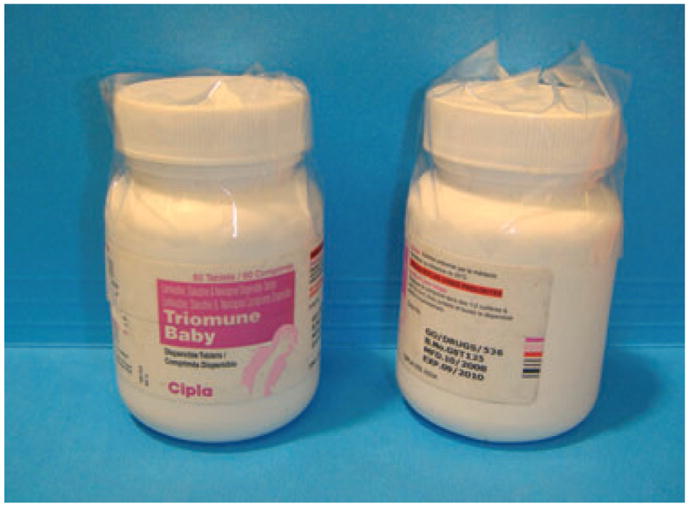
Creative solutions to resource scarcity include the reuse of empty medicine vials as histology specimen containers
We have examined other possibilities for histological evaluation of skin specimens, including wet tissue processing at local sites and the use of teledermatopathology. Eighty-six percent of Sub-Saharan African countries report the presence of histology or lab processing service; however, the extensive time to receive results is often prohibitive.4 Digital images of scanned slides could be reviewed in a store-forward fashion over the existing web-based African Teledermatology Project in a manner similar to that currently used for clinical images; however, this would require local tissue processing, slide scanning technology, and internet with sufficient bandwidth. Alternatively, a robotic microscope can be installed at the local site, and slides may be loaded to be reviewed remotely in real time by a remote expert. A robotic microscope has been established in Botswana in conjunction with the University of California Los Angeles and the University of Pennsylvania and is currently in use by one author (CLK) in the USA. This method, however, also relies on the ability to process tissue locally as well as a local pathologist with whom to partner and collaborate.
These exciting technologies offer several possible solutions to increasing access to dermatopathology services and expert physician consultation as an adjunct to clinical teledermatology. Numerous modalities need to be evaluated and researched to develop the best strategies to increase access to care in the developing world and allow for clinicopathological correlation. Dermatopathology solutions also will need to be tailored to specific sites, as their capabilities and access to resources vary greatly.
Acknowledgments
Funding sources: None.
We extend special thanks to our colleagues at the following institutions for their valuable contributions: the Baylor College of Medicine Bristol-Myers Squibb Children's Clinical Centre of Excellence (COE), Swaziland; the Baylor College of Medicine Abbott Fund Children's Clinical COE, Malawi; the Baylor College of Medicine Bristol-Myers Squibb Children's Clinical COE, Lesotho; the Botswana-Baylor Children's COE, the Princess Marina Hospital, Gaborone, Botswana; the John F. Kennedy Memorial Hospital in Monrovia, Liberia; and the Kiliminjaro Christian Medical Centre.
Footnotes
Disclosures: The authors have no conflict of interest to declare. Dr. Tsang's research time was supported by the American Society of Dermatopathology Mentorship Award.
References
- 1.Kaddu S, Soyer HP, Gabler G, et al. The Africa Teledermatology Project: preliminary experience with a sub-Saharan teledermatology and e-learning program. J Am Acad Dermatol. 2009;61:155–157. doi: 10.1016/j.jaad.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Weinberg J, Kaddu J, Gabler G, et al. The African Teledermatology Project: providing access to dermatologic care and education in sub-Saharan Africa. Pan African Med J. 2009;3:16. [PMC free article] [PubMed] [Google Scholar]
- 3.Schmid-Grendelmeier P, Doe P, Pakenham-Walsh N. Teledermatology in sub-Saharan Africa. Curr Probl Dermatol. 2003;32:233–246. doi: 10.1159/000067349. [DOI] [PubMed] [Google Scholar]
- 4.Tsang MW, Kovarik CL. Global access to dermatopathology services: physician survey of availability and needs in sub-Saharan Africa. J Am Acad Dermatol. 2010 Aug;63(2):346–8. doi: 10.1016/j.jaad.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 5.Lozzi GP, Soyer HP, Massone C, et al. The additive value of second opinion teleconsulting in the management of patients with challenging inflammatory, neoplastic skin diseases: a best practice model in dermatology. J Eur Acad Dermatol Venerol. 2007;21:30–34. doi: 10.1111/j.1468-3083.2006.01846.x. [DOI] [PubMed] [Google Scholar]
- 6.Arkin LM, Cox CM, Kovarik CL. Kaposi's sarcoma in the pediatric population: the critical need for a tissue diagnosis. Pediatr Infect Dis J. 2009;28:426–428. doi: 10.1097/INF.0b013e318193ee21. [DOI] [PubMed] [Google Scholar]
- 7.Trindade MA, Valente NY, Manini MI, et al. Two patients coinfected with Mycobacterium leprae and human immunodeficiency virus type 1 and naive for antiretroviral therapy who exhibited type 1 leprosy reactions mimicking the immune reconstitution inflammatory syndrome. J Clin Microbiol. 2006;44:4616–4618. doi: 10.1128/JCM.01425-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Handisurya A, Rieger A, Bago-Horvath Z, et al. Rapid progression of an anal Buschke-Lowenstein tumour into a metastasising squamous cell carcinoma in an HIV-infected patient. Sex Transm Infect. 2009;85:261–263. doi: 10.1136/sti.2008.034959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robin YM, Guillou L, Michels JJ, et al. Human herpesvirus 8 immunostaining: a sensitive and specific method for diagnosing Kaposi sarcoma in paraffin-embedded sections. Am J Clin Pathol. 2004;121:330–334. doi: 10.1309/96U1-6LRR-AN5H-WWVE. [DOI] [PubMed] [Google Scholar]
- 10.Cheuk W, Wong KO, Wong CS, et al. Immunostaining for human herpesvirus 8 latent nuclear antigen-1 helps distinguish Kaposi sarcoma from its mimickers. Am J Clin Pathol. 2004;121:335–342. doi: 10.1309/B8TC-0LBV-H8XY-5MFV. [DOI] [PubMed] [Google Scholar]
- 11.Wada DA, Perkins SL, Tripp S, et al. Human herpesvirus 8 and iron staining are useful in differentiating Kaposi sarcoma from interstitial granuloma annulare. Am J Clin Pathol. 2007;127:263–270. doi: 10.1309/GMH9CENH4909AWVB. [DOI] [PubMed] [Google Scholar]


