Abstract
Mouse embryonic stem (mES) cells are pluripotent cells that can be propagated in vitro with leukemia inhibitory factor (LIF) and serum. Intracellular signaling by LIF is principally mediated by activation of STAT-3, although additional pathways for self-renewal have been described. Here, we identified a novel role for Insulin receptor substrate-1 (IRS-1) as a critical factor in mES cells self-renewal and differentiation. IRS-1 is expressed and tyrosyl phosphorylated during mES cells self-renewal. Differentiation of mES cells, by LIF withdrawal, is associated with a marked reduction in IRS-1 expression. Targeting of IRS-1 by si-IRS-1 results in a severe reduction of Oct-4 protein expression and alkaline phosphatase activity, markers of undifferentiated mES cells. IRS-1 targeting does not interfere with LIF-induced STAT-3 phosphorylation, but negatively affects protein kinase B (PKB/AKT) and glycogen synthase kinase-3 (GSK-3β) phosphorylation, which are downstream effectors of the LIF-mediated PI3K signaling cascade. Targeting of IRS-1 also results in a marked down regulation of Id-1 and Id-2 proteins expression, which are important components for self-renewal of ES cells. Conversely, over expression of IRS-1 inhibits mES cell differentiation. Taken together, these results suggest that expression and activity of IRS-1 are critical to the maintenance of the self-renewal program in mES cells.
Embryonic stem cells can grow indefinitely in vitro without immortalizing or transforming agents (Evans, 1981; Martin, 1981). They are tumorigenic when injected in syngenic mice, developing teratocarcinomas (Evans, 1981; Martin, 1981; Toomey et al., 1997; Nozaki et al., 1999), and they are pluripotent maintaining the capacity for differentiation into a wide range of cells and tissues in vitro and in vivo (Bradley et al., 1984). Upon leukemia inhibitory factor (LIF) withdrawal, mouse embryonic stem (mES) cells differentiate forming three-dimensional structures called embryoid-bodies (EBs; Keller, 1995). In many ways, EBs mimic normal mouse embryonic development, differentiating over the course of a few days into derivatives of all three germ layers both in vitro and in chimaeric mice (Mummery et al., 1990; Keller, 1995).
LIF acts by binding to a LIFR-gp130 signaling complex that activates at least two downstream pathways: the Jak–STAT pathway (Boeuf et al., 1997; Niwa et al., 1998; Matsuda et al., 1999; Raz et al., 1999) and the Ras–Raf–MEK–ERK pathway (Ernst et al., 1996; Burdon et al., 1999). Activation of Jak-STAT pathway has been shown to be critical for ES cell self-renewal (Saxton et al., 1997; Starr et al., 1997; Niwa et al., 1998; Qu and Feng, 1998; Matsuda et al., 1999), while activation of ERK pathway not only is dispensable for self-renewal (Ernst et al., 1996; Burdon et al., 1999), but appears to promote differentiation (Burdon et al., 1999). Therefore it has been proposed that the balance between LIF-mediated STAT3 activation and ERK signals is important in the determination of ES cells fate (Burdon et al., 2002). However, LIF does not act alone but requires the presence of serum to maintain self-renewal (Nichols et al., 1990).
Other intracellular signaling proteins that are important for the maintenance of self-renewal are the inhibitor of differentiation (Id) proteins, which are rapidly down-regulated upon differentiation of ES cells (Ying et al., 2003). Bone morphogenic protein (BMP) has been found to stimulate the transcription of Id proteins (Ruzinova and Benezra, 2003), and can replace serum requirement for mES cell self-renewal in vitro (Ying et al., 2003). Moreover, mES cells overexpressing Id-1 are able to self-renew in serum free condition solely with LIF supplementation (Ying et al., 2003).
Recently, the PI3-K pathway has been identified as playing a critical role in the maintenance of self-renewal in ES cells (Paling et al., 2004). LIF-mediated activation of PI3K results in Akt dependent phosphorylation of GSK-3β at serine 21 and 9, which inhibits GSK-3β activity (Paling et al., 2004). Inhibition of GSK-3β activity has been found to repress ES cells differentiation even in the absence of LIF, in both mouse and human ES cells (Sato et al., 2004). Expression levels of the transcriptional factors Oct-4, Nanog, and alkaline phosphatase (AP) activity are markers of self-renewing mES cells and are down-regulated during differentiation (Ying et al., 2003; Saito et al., 2004).
Insulin receptor substrate-1 (IRS-1) is the major substrate for insulin and insulin-like growth factor-I (IGF-I) receptors, and mediates much of the metabolic, mitogenic, and anti-apoptotic actions of their respective ligands (White, 1998). IRS-1 has a mass of approximately 165 kDa. It contains amino-terminal pleckstrin homology (PH) and phosphotyrosine (PTB) domains, the latter binding the NPXY motif of the insulin and IGF-I receptors. The mitogenic signal of an activated IRS-1 is largely mediated by PI3K activation, which in turn activates the AKT/p70/S6k/mTor pathway and/or leads to the expression of Id proteins (Belletti et al., 2001, 2002; Prisco et al., 2001). Although IRS-1 has been shown to be a downstream target of LIF (Argetsinger et al., 1995), its role in self-renewal and differentiation in ES cells is unknown. Activation of IRS-1 occurs by tyrosine phosphorylation at multiple sites within the carboxy-terminal domain which possesses multiple Src homology-2 (SH2) domains. IRS-1 is tyrosine phosphorylated in response to several ligands in addition to insulin and IGF-I, including prolactin, growth hormone, IL-2, 6, 9, 13 and 15, interferon α, β and γ, angiotensin (White, 1998), and LIF (Argetsinger et al., 1995). Intracellular activation of IRS-1 can occur via JAK (as in the case of interferon-α and LIF; Cengel and Freund, 1999; Argetsinger et al., 1995). Phosphorylated SH2 domains bind several proteins with signaling functions including Grb-2, Nck, Crk, PI 3-kinase, SH-PTP, and Fyn. We found that IRS-1 is expressed and activated during mES cell self-renewal, and directly interferes with differentiation. We show that IRS-1 expression and activation are critical for the self-renewing program of mES cells, and we demonstrate it by two different approaches: IRS-1 targeting and over expression of IRS-1 in mES cells.
Materials and Methods
Cell culture
E14Tg2A mouse embryonal stem (ES) cells, derived from 2.5-day blastocyst, were obtained from BayGenomics, University of California, Davis, CA. Cells were cultured on tissue culture plates (BD Falcon, Franklin Lakes, NJ) coated with 0.1% (v/v) porcine gelatin (Sigma-Aldrich Corp, St. Louis, MO) in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Grand Island, NY) in presence of 15% fetal bovine serum (ES-tested Hyclone, Perbio, Logan, UT), 0.1 mM 2-β-mercaptoethanol, 0.1 mM non-essential amino acids, 2 mM glutamine, 0.1 mM sodium pyruvate, and 1,000 units/ml murine LIF (Chemicon International Inc., Temecula, CA). Cells (passages 16–40) were trypsinized and re-plated every 2nd day and re-fed daily. For differentiation of ES cells as EB, cells were trypsinized and plated on bacteriological dishes at 1.6×105 cells/cm2 upon removal of LIF in medium without or with 50 ng/ml of IGF-1 (Invitrogen) and harvested at the indicated time points. For short-term stimulation with LIF or IGF-1 (30 min) cells were washed three times in Hank’s buffered saline solution and incubated in SFM (DMEM supplemented with 0.1% BSA and 50 μg/ml transferrin) for 3 h before stimulation.
Retroviral infection
Retroviral infection of ES cells has been carried out as previously described (Prisco et al., 2004). The construct used to generate retroviral supernatant was MSCVpuro IRS-1 carrying the mouse IRS-1 cDNA, kind gift of Dr. R. Baserga (Thomas Jefferson University, Philadelphia, PA). Selection was carried out as mixed population by the presence of (2 μg/ml) of puromycin in the growing medium.
Alkaline phosphatase assays
AP assays were performed as previously described (Paling et al., 2004). Briefly, mES cells were plated 3 × 103 cells per gelatin coated 35 mm dishes in medium without or with LIF 1,000 units/ml (Chemicon) or IGF-I 50 ng/ml (Invitrogen) and harvested at the indicated times. AP staining was performed using Fast Red TR salt™ (Sigma) reagent, according to the manufacturer’s protocol. AP positive colonies were scored in triplicate for each experiment. Experiments were performed three times.
Small interfering RNAs
Small interfering RNAs (siGENOME SMARTpool IRS1 and siCONTROL non-targeting (NT) siRNA #2 as a negative control) were synthetized by Dharmacon RNA Technologies, Lafayette, CO. After seeding for 24 h, cells were transfected with 100 nM/L of siRNAs, using LipofectAMINE 2000 (Invitrogen), as recommended by the manufacturer’s instructions, and cultured in LIF containing medium. Cells were subjected to a second round of transfection after 2 days, prior performing experiments. Down regulation of IRS-1 was determined by Western blot.
Cell growth
Parental cells, control cells transfected with non-targeting siRNA (NA), and cells transfected with si-IRS-1, were plated 3 × 103 cells/ gelatin coated 35 mm and grown up to day 4. Total cell number was determined by trypan-blue exclusion. Cells were scored in triplicate for each experiment. Experiments were performed two times.
Western blot and immunoprecipitation
Cell extracts, Western blot and immunoprecipitation were performed by standard procedures previously described (Navarro et al., 2001; Prisco et al., 2004). Antibodies used were: anti-IRS1, Id1, Id2, phospho-Akt1/2/3 (Ser473), Oct4, IGF-IR(β), InsulinRβ, and PY99 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); phospho-(Tyr608) IRS1 (Calbiochem, EMD Biosciences, Inc. San Diego, CA); phospho-(Ser307) IRS1 (Upstate Biotecnology, Inc. Charlottesville, VA); Akt, GSK-3β, phospho-(Ser6) GSK-3β, STAT3, phospho-(Tyr705) STAT3, and Grb-2 (Cell Signaling Techology, Inc., Danvers, MA).
RT-PCR
RNA was extracted using the RNeasy Kit (Qiagen, Valencia, CA), and RT-PCR was performed using 100 ng of total RNA and the one-step RT-PCR kit (Roche Diagnostics GmbH, Mannheim, Germany), following the manufacturers protocol. The following primers were used: mouse IGF-II: forward 5′-cgttaggcctggatcaagatgc and reverse 5′-cgataccactgttagcgcag; mouse IGF-IR forward 5′-gagtacttgctgctcttccgag and reverse 5′-tccaagatgagagaccagtc; mouse insulin receptor forward: 5′-gacatccggaacaacctgac and reverse: 5′-tcagctgtgcagccatgtgac; mouse IRS-1 forward: 5′-gactacatgaccatggacatag and reverse 5′-cgagtaggtgctgagaaggtc; mGAPDH forward: 5′-ggagccaaacccct-catcatctc and reverse: 5′-gaggggccatccacagtcttct.
Results
Self-renewal and differentiation of mES cells
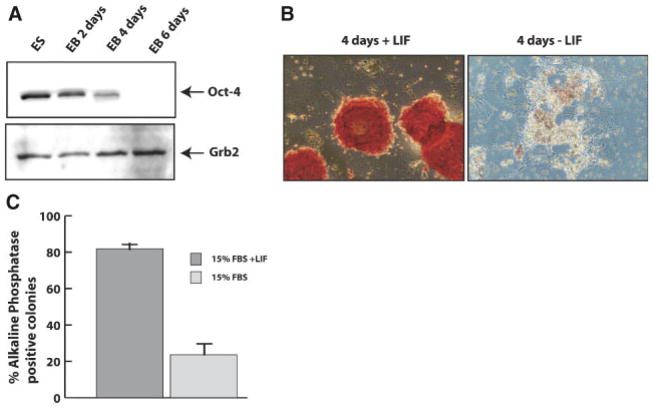
Expression levels of the transcriptional factor Oct-4 and AP activity are well-known markers of self-renewing mES cells and are down-regulated during differentiation (Ying et al., 2003; Saito et al., 2004). In Figure 1A, we confirm that in our cell system Oct-4 protein is highly expressed in self-renewing mES cells and is down-regulated upon LIF withdrawal and differentiation as EBs. After 4 days of the LIF withdrawal, levels of Oct-4 are substantially decreased, becoming barely detectable at day 6. Similarly, colonies generated from mES cells cultured in gelatin coated plates, in the presence of LIF, show high levels of AP (Fig. 1B, left part), which rapidly declines by day 4 following LIF withdrawal (Fig. 1B, right part). LIF withdrawal results in the generation of colonies whose cells are enlarged, spread and flattened on the plate, with few red stained cells. Quantitatively, AP colony assay (Fig. 1C) shows that in the presence of LIF, ~80% of cells form positive colonies, while 4 days after LIF withdrawal the number of positive colonies is decreased to ~25%.
Fig. 1.
mES cells differentiation. A: mES cells were differentiated as EBs at the indicated times following LIF withdrawal. Oct-4 protein expression was detected by Western blot. Grb-2 was used to verify equal loading. Results are representative of three independent experiments. B: Alkaline phosphatase colony assay was performed as described in Materials and Methods. Representative fields of alkaline phosphatase colony assay in mES cells cultured in the presence (left part) or absence (right part) of LIF for 4 days. C: Quantitative analysis of AP colony assay. The number of positive colonies is expressed as percent on total colonies scored and represents the mean ±SD of three separate experiments performed in triplicate. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
IRS-1, IGF-I receptor and insulin receptor expression in mES cells
We investigated protein and mRNA expression levels of IRS-1 in mES cells during their growth and differentiation as EBs up to day 6 after LIF withdrawal. IRS-1 protein is present in self-renewing cells and markedly down-regulated upon LIF withdrawal and induction of differentiation (Fig. 2A, upper part). Since IRS-1 is known to be one of the major substrates for insulin and IGF-I receptors, the expression levels of these two receptors were also investigated. Both IGF-I and insulin receptors proteins are expressed in self-renewing mES cells and up regulated upon LIF withdrawal and induction of differentiation (Fig. 2A second and third parts, respectively). In a similar experiment, we assayed levels of IRS-1 mRNA in self-renewing and differentiating conditions. Figure 2B (upper part) shows that IRS-1 mRNA is present in self-renewing cultures, and still detected during differentiation (unlike protein levels), suggesting that IRS-1 expression is controlled post-transcriptionally. IGF-I and Insulin receptors mRNA increased during differentiation of mES cells (Fig. 2B, second and third parts), in accordance with their protein expression profiles.
Fig. 2.
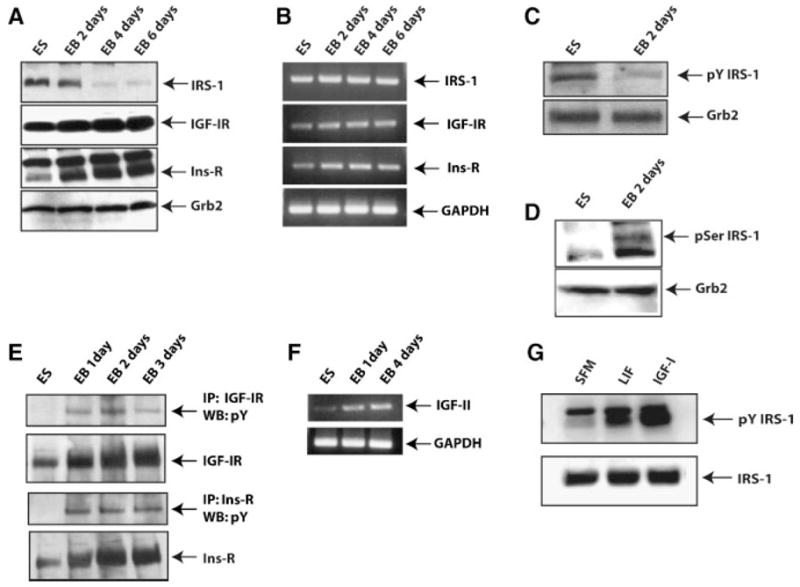
IRS-1, IGF-IR, and Ins-R expression and phosphorylation in mES cells. A,B: Protein and mRNA expression profiles of IRS-1, IGF-IR, and Ins-R in self-renewing and differentiating ES cells at the indicated time points. Grb-2 and GAPDH were assayed in Western blot and RT-PCR analysis, respectively, to verify equal loading. C,D: ES cells were induced to differentiate. Lysates from cells, at indicated time points from LIF withdrawal, were probed for pY-IRS-1 and pSer-IRS-1 by Western blotting. Grb-2 protein level was used to verify equal loading. E: IGF-IR or Ins-R were immunoprecipitated from ES cells or EBs, and the Western blots probed with a phosphotyrosine antibody. F: mRNA expression of IGF-II and GAPDH by RT-PCR in ES cells and EBs (1 and 4 days). G: mES cells were starved of FBS and LIF for 3 h prior to the addition of LIF (1,000 ng/ml) or IGF-I (50 ng/ml). Lysates were obtained after 30 min, and pY-IRS-1 and IRS-1 visualized by Western blotting.
We next asked whether IRS-1 was tyrosine phosphorylated under self-renewing conditions. We assayed the basal phosphorylation status of IRS-1 on tyrosine residues using an antibody against phosphotyrosine 608 of IRS-1. This tyrosine residue has been found to be important for the binding of IRS-1 to the p85 regulatory subunit of PI3K (Yamamoto-Honda et al., 1996; Esposito et al., 2003; Valverde et al., 2003). We found that in the presence of LIF, IRS-1 is tyrosine phosphorylated, and the signal decreases after LIF withdrawal and induction of differentiation (Fig. 2C). However, after LIF withdrawal IRS-1 became phosphorylated at residue serine 307 (Fig. 2D), a site important for the proteasome-mediated degradation of IRS-1 (Pederson et al., 2001).
We asked whether the activation of IRS-1 in self-renewing condition was due to an activated IGF-I and/or insulin receptors. Interestingly, we failed to detect any tyrosine phosphorylation in self-renewing conditions for both IGF-IR (Fig. 2E, upper two parts) and insulin receptor (Fig. 2E, lower two parts). However, both receptors showed tyrosine phosphorylation during ES cells differentiation. Increased phosphorylation of these two receptors was associated with an increase of IGF-II transcripts detected during ES cells differentiation by RT-PCR (Fig. 2F).
To further investigate the ability of LIF to activate IRS-1, serum starved mES cells were stimulated with LIF, and the results of such experiment are illustrated in Figure 2G. Treatment with IGF-I was used as positive control. After serum starvation of mES cells, LIF treatment transiently increased IRS-1 tyrosine phosphorylation, although less efficiently than the treatment with IGF-I.
IRS-1 targeting induces differentiation of mES cells in self-renewing conditions
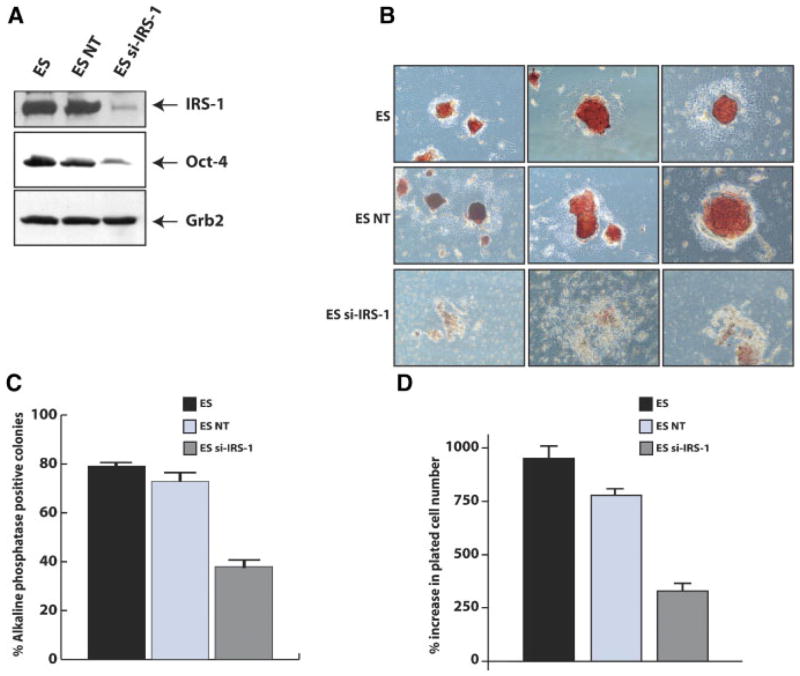
Role of IRS-1 in self-renewal of mES cell was investigated using siRNA approaches. First, we asked whether targeting of IRS-1 could induce differentiation in medium that sustains self-renewal of mES cells, and second, whether IRS-1 targeting could inhibit PI3K-dependent signals, especially phosphorylation of AKT and GSK-3β that have been reported to control mES cell self-renewal (Paling et al., 2004). Cells, ES parental, and transfected with non-targeting siRNA, or with si-IRS-1, were plated in gelatin-coated dishes in medium supplemented with LIF and serum, and assayed after 5 days for Oct-4 protein expression and AP activity. Targeting of IRS-1 (Fig. 3A) decreased the expression of Oct-4 in self-renewing conditions. Degree of differentiation and morphology of colonies generated in medium supplemented with LIF and serum (self-renewing conditions) by AP colony assay (Fig. 3B) showed that after 5 days in culture, parental, and control (NT) cells (upper and middle row) grow in large and spherical colonies, typical of undifferentiated phenotype, retaining AP activity. Conversely, at the same time point, cells transfected with si-IRS-1 appeared differentiated (Fig. 3B, lower row). Quantification of the AP positive colonies shows that, approximately 80% of parental and control (NT) were undifferentiated, while targeting of IRS-1 caused a significant reduction (approximately 40%) in the proportion of undifferentiated colonies (Fig. 3C). As a consequence of the impairment in self-renewing properties, we asked whether targeting of IRS-1 could also affect proliferation of these cells in medium supplemented with LIF and FBS. Cells (parental, control (NT), and si-IRS-1 transfected), were seeded on gelatin coated plates and grown for 4 days. As shown in Figure 3D, parental and control (NT) cells grew at a comparable rate, whereas IRS-1 targeted cells grew at much slower rate, consistent with the fact that they spontaneously differentiate.
Fig. 3.
IRS-1 targeting inhibits mES cells self-renewal. A: Western blot analysis showing IRS-1 (upper part) and Oct-4 (lower part) proteins expression in parental, control(NT), and IRS-1 targeted ES cells. Grb-2 protein expression was used as loading control. Results are representative of three independent experiments. B: Alkaline phosphatase colony assay showing that IRS-1 targeting affects the morphology of colonies generated after 4 days in medium supplemented with LIF. Representative fields of parental (upper part), control (NT; middle parts) and IRS-1 targeted ES cells (lower parts). C: Same experiment as in (B); AP positive colony numbers are expressed as percent on total number of colonies scored and represents the Mean ±SD of three separate experiments performed in triplicate. D: IRS-1 targeting affects the proliferation of mES cells in LIF containing medium. Total cell number was determined by trypan-blue exclusion after 4 days from cell plating. Results are representative of two independent experiments performed in triplicate. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
IRS-1 targeting inhibits LIF mediated PI3K depending signals
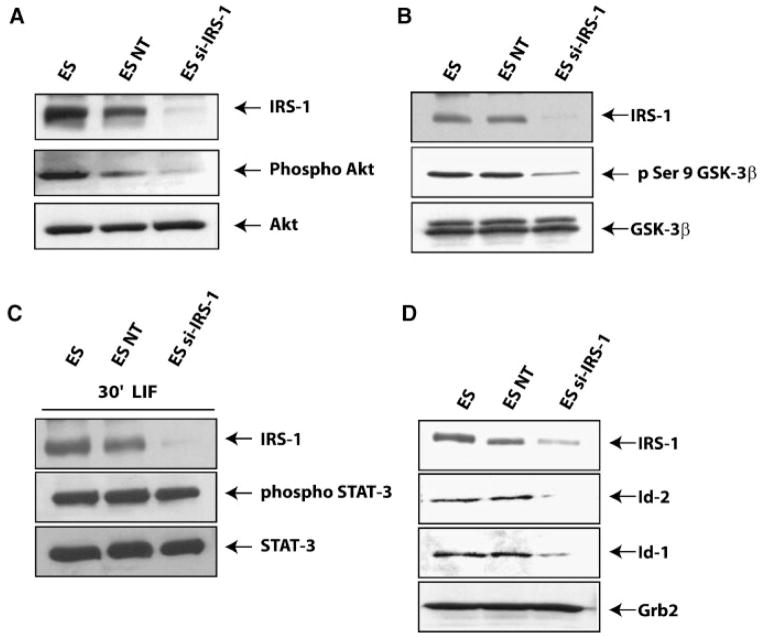
We determined the effect of IRS-1 targeting on the PI3K pathway by analyzing the phosphorylation levels of Akt and GSK-3β in cells grown in the presence of LIF and serum. Under these conditions, as expected, both parental and control (NT) cells showed phosphorylation of AKT on serine 473 (Fig. 4A). Instead, IRS-1 targeting drastically reduced AKT phosphorylation, while total amount of AKT protein was unchanged. Similarly, phosphorylation of GSK-3β on serine 9 was impaired in si-IRS-1 transfected cells, when compared to control cells (Fig. 4B). The total amount of GSK-3β protein was not affected by siRNA targeting. These results are consistent with our hypothesis that IRS-1 mediates the LIF-induced activation of PI3K-dependent signals in self-renewing ES cells. We also found that IRS-1 targeting does not affect STAT3 phosphorylation on tyrosine 705 (Fig. 4C), indicating that IRS-1 and STAT3 act on different pathways.
Fig. 4.
Effect of IRS-1 targeting on LIF-mediated PI3K depending signals. A: Western blot showing IRS-1 protein expression (upper part), Akt phosphorylation on serine 473 (middle part), and total Akt protein (lower part), in parental, control (NT), and IRS-1 targeted ES cells. B: Same experiment as in (A) to detect phosphorylation of GSK-3β on serine 9, as well as total amount of GSK-3β protein. C: IRS-1 targeting has no effect on LIF-induced STAT-3 phosphorylation. Total amount of STAT3 protein was used as loading control. D: Western blots showing the levels of both Id-1 and Id-2 protein expression. Grb-2 protein levels were used as loading control. Figures are representative of three independent experiments.
In a similar experimental setting we evaluated the effect of IRS-1 targeting on the expression levels of both Id-1 and Id-2 proteins, which are known to be expressed in self-renewing ES cells and down-regulated when these cells are differentiating (Ying et al., 2003). As shown in Figure 4D, expression levels of both Id-1 and Id-2 proteins were down-regulated by IRS-1 targeting in medium supplemented with LIF and serum.
Forced expression and activation of IRS-1 maintains self-renewal of mES cells
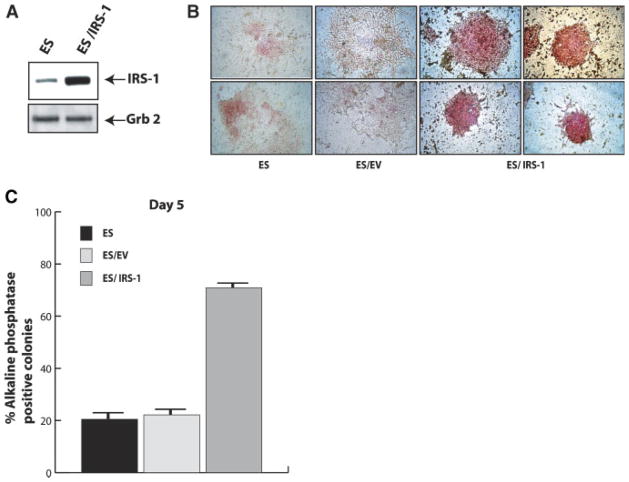
We examined whether forced expression and activation of IRS-1 was able to prevent or delay mES cell differentiation induced by LIF withdrawal. We introduced mouse IRS-1 cDNA in ES cells by retroviral vector as described in material and methods. Figure 5A shows levels of expression of IRS-1 in the selected mixed population. In the next series of experiments activation of IRS-1 was achieved by addition of IGF-I, since there is no LIF in differentiating conditions. AP colony assay was performed to determine cell morphology and extent of differentiation. Figure 5B shows morphology of the colonies generated after 4 days from LIF withdrawal. Parental ES cells and control ES/EV cells (transfected with empty vector, EV) appear clearly differentiated, flattened and spread on the plate, with few red stained cells. Conversely, ES/IRS-1 cells are strongly reactive to AP and grew in compact colonies. Already at day 4, ES and ES/EV cells showed a reduced number of AP positive colonies (about 20%). Instead, ~75% of the colonies generated from ES/IRS-1 cells maintained AP reactivity (Fig. 5C). Furthermore, we assayed if ES/IRS-1 cells were able to maintain Oct-4 expression level in differentiating conditions. ES, ES/EV, and ES/IRS-1 cells were seeded in Petri dishes in medium without LIF to generate EBs. IGF-I was added to the differentiating medium to insure IRS-1 activation. Cells were grown as EBs up to day 6, and protein lysates were probed for the presence of Oct-4. As shown in Figure 6A and B, when compared to parental and control EV cells, forced expression and activation of IRS-1 is able to maintain the levels of Oct-4 at least up to day 6 from LIF withdrawal. IRS-1 protein levels are rapidly down-regulated in differentiating cells, but still present in undifferentiated cells.
Fig. 5.
Effect of forced expression and activation of IRS-1 on ES cells differentiation. A: IRS-1 expression in mES cells transduced with a retrovirus carrying mouse IRS-1 cDNA. B: Representative field showing the morphology of colonies generated from ES, ES empty vector, and ES/IRS-1 cells after 4 days from LIF withdrawal and IGF-I supplementation. C: Quantification of AP colony assay at day 5 after LIF withdrawal. The number of positive colonies is expressed as percent on total colonies scored and represents the mean ±SD of three separate experiments performed in triplicate. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Fig. 6.

Overexpression of IRS-1 maintains Oct-4 protein expression. A: Parental and mES cells with a forced expression of IRS-1 were induced to differentiate as EBs after LIF withdrawal, in the presence of IGF-I. Lysates were separated by SDS–PAGE and probed for the presence of Oct-4 and IRS-1 at the indicated time points. B: Same experiment as previous showing Oct-4 expression level in mES cells infected with the EV. These results are representative of three independent experiments. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Finally, we assayed for the presence of both Id-1 and Id-2 proteins at early times from LIF withdrawal and IGF-I supplementation. As shown in Figure 7A, in ES cells both Id-1 and Id-2 proteins are rapidly down-regulated as early as 3 h from the induction of differentiation, and this event is concomitant with down regulation of IRS-1. On the contrary, ES/IRS-1 cells were able to maintain high levels of Id1 and Id2 proteins (Fig. 7B).
Fig. 7.
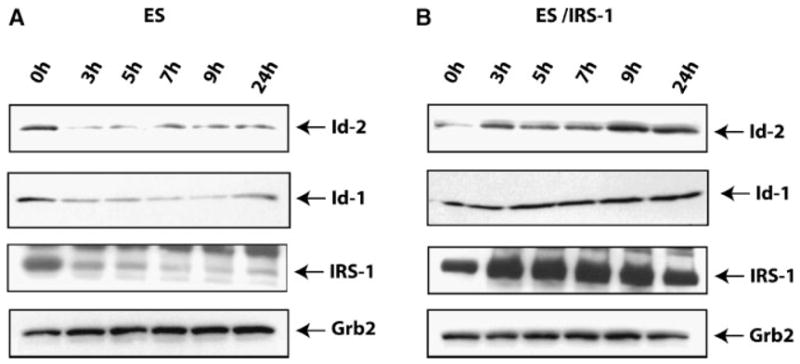
Overexpression of IRS-1 maintains ID-1 and ID-2 proteins expression. A,B: Parental and ES/IRS-1 cells were induced to differentiate as EBs after LIF withdrawal, in the presence of IGF-I. Western blot shows the expression of Id-1 and Id-2 proteins at the indicated time points. Row 3 in both pictures indicates the level of IRS-1. Grb-2 protein expression was used as loading control (row 4). Results are representative of three independent experiments.
Discussion
We have identified a role for IRS-1 in the regulation of self-renewal and differentiation of mES cells. Our novel findings are summarized as follows: (1) IRS-1 is expressed and activated in self-renewing ES cells; (2) IRS-1 protein expression levels are down-regulated upon LIF withdrawal and induction of ES cell differentiation; (3) LIF induces tyrosine phosphorylation of IRS-1 in self-renewing ES cells; (4) Reduction of IRS-1 expression by siRNA causes differentiation of ES cells even in the presence of LIF, down-regulating Oct4 protein expression and AP activity; (5) Targeting of IRS-1 impairs the capacity of LIF to induce PI3K dependent signals, resulting in a decrease of AKT and GSK-3β phosphorylation; (6) IRS-1 targeting results also in the down regulation of both Id1 and Id2 proteins expression; (7) Overexpression and activation of IRS-1 sustain self-renewal of mES cells and maintain Id-1 and Id-2 protein expression.
Although there is a substantial literature on the role of IRS-1 in cell proliferation and differentiation (Wang et al., 1993; Kim et al., 1998; Shima et al., 1998; Bohni et al., 1999; Valentinis et al., 2000; Brogiolo et al., 2001; Sadowski et al., 2001), information on its functions in ES cells is lacking. In the current study we show that the presence and activation of IRS-1 is critical for LIF mediated self-renewal of ES cells. IRS-1 is expressed and activated in ES cells cultured in medium supplemented with LIF, and its protein level is rapidly down-regulated after LIF withdrawal and induction of differentiation. Since IRS-1 mRNA is still detectable after LIF withdrawn and induction of differentiation, we reason that the control of IRS-1 expression is at posttranscriptional level. Removal of LIF induces changes on IRS-1 phosphorylation status from tyrosine phosphorylation to serine phosphorylation, which has been reported to be one of the first steps for IRS-1 degradation via proteasome pathway (Pederson et al., 2001).
IRS-1 knockout mice are over 50% smaller at birth than their wild-type littermates, and exhibit diabetes (Araki et al., 1994; Tamemoto et al., 1994). There are several related IRS family members, including IRS-2, IRS-3, IRS-4, Gab-10, and p62dok. IRS-2 has overlapping signaling potential, but does not restore insulin sensitivity in IRS-1 knockout mice (Suzuki et al., 2004). Recent studies have implicated a role for IRS-1 as a negative regulator of differentiation (Wang et al., 1993; Kim et al., 1998; Sadowski et al., 2001). Activated IRS-1 is a frequent event associated with different kind of human tumors (breast cancer, leiomyomas, Wilms’ tumors, rhabdomyosarcomas, liposarcomas, and adrenal cortical carcinomas; Chang et al., 2002).
LIF activates IRS-1 during self-renewal probably through Jak2, as it has been reported in fibroblast (Argetsinger et al., 1995). Insulin and the IGF-I receptors are unlikely to be responsible for IRS-1 activation during self-renewal, since these two receptors are not phosphorylated under this condition. Instead, activation of IGF-I and insulin receptors, detected after 24 h from LIF withdrawal, suggests a potential role for these receptors in the differentiation program of ES cells. This is in accord with previous reports that the IGF-IR, without IRS-1, sends a differentiation signal (Valentinis et al., 1999; Baserga, 2000). IGF-I and insulin receptors phosphorylation was also concomitant with increased levels of IGF-II transcripts detected upon LIF withdrawal and induction of ES cell differentiation. Interestingly, while insulin has not been detectable in any pre-implantation stage mouse embryos (Shi et al., 1994), increased levels of IGF-II has been proposed as differentiation marker for ES cells (Mummery et al., 1990). Thus our data are consistent with IGF-II activation of insulin and IGF-I receptors during mES cell differentiation. It is also interesting that in the presence of LIF, IGF-1R, InsR, and IGF-II expression seem to be down-regulated.
Our aim was to understand the role of IRS-1 in LIF mediated self-renewal of ES cells, and we employed siRNA technique to reduce IRS-1 levels in ES cells cultured in the presence of LIF. Targeting of IRS-1 induces differentiation of ES cells in the presence of LIF, whereas cells should self-renew. This has been determined by down-regulation of Oct4 transcription factor and AP activity of the generated colonies.
LIF has been reported to activate the PI3K pathway (Paling et al., 2004) resulting in Akt-mediated phosphorylation of GSK-3β on serine 9 and 21 residues, which inhibits the activity of this molecule. Several reports indicate that the PI3K pathway has a prominent role in the regulation of self-renewal of ES cells (Paling et al., 2004; Watanabe et al., 2006). IRS-1 is a strong activator of PI3K (Myers et al., 1994; Baserga, 2005), and it has binding sites for the p85 regulatory subunit of PI3K (Sun et al., 1993; Yenush et al., 1998). Our findings clearly indicate that IRS-1 mediates LIF activation of PI3K, since targeting of this molecule in ES cells results in the inhibition of both Akt and GSK-3β phosphorylation. Inhibition of GSK-3β activity, by 6-bromoindirubin-3′-oxime (BIO) has been reported to maintain self-renewal of murine and human ES cells in the absence of LIF (Sato et al., 2004). Recently, c-Myc has been identified as the downstream effector of the LIF/STAT3 pathway (Cartwright et al., 2005) in promoting ES self-renewal. After LIF withdrawal, c-Myc becomes the target of GSK-3β leading to its degradation (Cartwright et al., 2005).
Targeting of IRS-1 also impaired the expression of Id proteins, which are dominant negative regulators of transcription factors involved with differentiation. Id proteins have been reported to inhibit differentiation in a variety of cell types (Benezra et al., 1990; Kreider et al., 1992; Lister et al., 1995; Prisco et al., 2001), including ES cells (Ying et al., 2003). Id proteins are rapidly down-regulated upon induction of differentiation, and activation of IRS-1 has been shown to increases their expression levels in other cell systems (Valentinis et al., 2000; Belletti et al., 2001, 2002; Prisco et al., 2001).
We have demonstrated that overexpression and activation of IRS-1 inhibits differentiation of ES cells. ES overexpressing IRS-1 cells were able to maintain an undifferentiated phenotype as shown by sustained levels of Oct-4 and retention of AP activity. We also assayed for Id proteins expression at early times after the switch from LIF to IGF-I and their expression was up-regulated in ES cells overexpressing IRS-1.
In conclusion, we propose that expression and activation of IRS-1 is required for efficient self-renewal of murine ES cells, and that IRS-1 down regulation could represent an important step for the implementation of their differentiation program.
Acknowledgments
This work was supported by NIH grant R01-AA009976 and by FIRB funding.
Literature cited
- Araki E, Lipes MA, Patti ME, Bruning JC, Haag B, III, Johnson RS, Kahn CR. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- Argetsinger LS, Hsu GW, Myers MG, Jr, Billestrup N, White MF, Carter-Su C. Growth hormone, interferon-gamma, and leukemia inhibitory factor promoted tyrosyl phosphorylation of insulin receptor substrate-1. J Biol Chem. 1995;270:14685–14692. doi: 10.1074/jbc.270.24.14685. [DOI] [PubMed] [Google Scholar]
- Baserga R. The contradictions of the insulin-like growth factor 1 receptor. Oncogene. 2000;19:5574–5581. doi: 10.1038/sj.onc.1203854. [DOI] [PubMed] [Google Scholar]
- Baserga R. The insulin-like growth factor-I receptor as a target for cancer therapy. Expert Opin Ther Targets. 2005;9:753–768. doi: 10.1517/14728222.9.4.753. [DOI] [PubMed] [Google Scholar]
- Belletti B, Prisco M, Morrione A, Valentinis B, Navarro M, Baserga R. Regulation of Id2 gene expression by the insulin-like growth factor I receptor requires signaling by phosphatidylinositol 3-kinase. J Biol Chem. 2001;276:13867–13874. doi: 10.1074/jbc.M010509200. [DOI] [PubMed] [Google Scholar]
- Belletti B, Drakas R, Morrione A, Tu X, Prisco M, Yuan T, Casaburi I, Baserga R. Regulation of Id1 protein expression in mouse embryo fibroblasts by the type 1 insulin-like growth factor receptor. Exp Cell Res. 2002;277:107–118. doi: 10.1006/excr.2002.5542. [DOI] [PubMed] [Google Scholar]
- Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: A negative regulator of helix–loop–helix DNA binding proteins. Cell. 1990;61:49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Boeuf H, Hauss C, Graeve FD, Baran N, Kedinger C. Leukemia inhibitory factor-dependent transcriptional activation in embryonic stem cells. J Cell Biol. 1997;138:1207–1217. doi: 10.1083/jcb.138.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohni R, Riesgo-Escovar J, Oldham S, Brogiolo W, Stocker H, Andruss BF, Beckingham K, Hafen E. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS 1-4. Cell. 1999;97:865–875. doi: 10.1016/s0092-8674(00)80799-0. [DOI] [PubMed] [Google Scholar]
- Bradley A, Evans M, Kaufman MH, Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol. 2001;11:213–221. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Burdon T, Stracey C, Chambers I, Nichols J, Smith A. Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells. Dev Biol. 1999;210:30–43. doi: 10.1006/dbio.1999.9265. [DOI] [PubMed] [Google Scholar]
- Burdon T, Smith A, Savatier P. Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol. 2002;12:432–438. doi: 10.1016/s0962-8924(02)02352-8. [DOI] [PubMed] [Google Scholar]
- Cartwright P, McLean C, Sheppard A, Rivett D, Jones K, Dalton S. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development. 2005;132:885–896. doi: 10.1242/dev.01670. [DOI] [PubMed] [Google Scholar]
- Cengel KA, Freund GG. JAK1-dependent phosphorylation of insulin receptor substrate-1 (IRS-1) is inhibited by IRS-1 serine phosphorylation. J Biol Chem. 1999;274:27969–27974. doi: 10.1074/jbc.274.39.27969. [DOI] [PubMed] [Google Scholar]
- Chang Q, Li Y, White MF, Fletcher JA, Xiao S. Constitutive activation of insulin receptor substrate 1 is a frequent event in human tumors: Therapeutic implications. Cancer Res. 2002;62:6035–6038. [PubMed] [Google Scholar]
- Ernst M, Oates A, Dunn AR. Gp130-mediated signal transduction in embryonic stem cells involves activation of Jak and Ras/mitogen-activated protein kinase pathways. J Biol Chem. 1996;271:30136–30143. doi: 10.1074/jbc.271.47.30136. [DOI] [PubMed] [Google Scholar]
- Esposito DL, Li Y, Vanni C, Mammarella S, Veschi S, Della Loggia F, Mariani-Costantini R, Battista P, Quon MJ, Cama A. A novel T608R missense mutation in insulin receptor substrate-1 identified in a subject with type 2 diabetes impairs metabolic insulin signaling. J Clin Endocrinol Metab. 2003;88:1468–1475. doi: 10.1210/jc.2002-020933. [DOI] [PubMed] [Google Scholar]
- Evans MJ. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Keller GM. In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol. 1995;7:862–869. doi: 10.1016/0955-0674(95)80071-9. [DOI] [PubMed] [Google Scholar]
- Kim B, Leventhal PS, White MF, Feldman EL. Differential regulation of insulin receptor substrate-2 and mitogen-activated protein kinase tyrosine phosphorylation by phosphatidylinositol 3-kinase inhibitors in SH-SY5Y human neuroblastoma cells. Endocrinology. 1998;139:4881–4889. doi: 10.1210/endo.139.12.6348. [DOI] [PubMed] [Google Scholar]
- Kreider BL, Benezra R, Rovera G, Kadesch T. Inhibition of myeloid differentiation by the helix–loop–helix protein Id. Science. 1992;255:1700–1702. doi: 10.1126/science.1372755. [DOI] [PubMed] [Google Scholar]
- Lister J, Forrester WC, Baron MH. Inhibition of an erythroid differentiation switch by the helix–loop–helix protein Id1. J Biol Chem. 1995;270:17939–17946. doi: 10.1074/jbc.270.30.17939. [DOI] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in mediumconditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, Yokota T. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J. 1999;18:4261–4269. doi: 10.1093/emboj/18.15.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery CL, van den Eijnden-van Raaij AJ, Feijen A, Freund E, Hulskotte E, Schoorlemmer J, Kruijer W. Expression of growth factors during the differentiation of embryonic stem cells in monolayer. Dev Biol. 1990;142:406–413. doi: 10.1016/0012-1606(90)90362-m. [DOI] [PubMed] [Google Scholar]
- Myers MG, Jr, Grammer TC, Wang LM, Sun XJ, Pierce JH, Blenis J, White MF. Insulin receptor substrate-1 mediates phosphatidylinositol 3′-kinase and p70S6k signaling during insulin, insulin-like growth factor-1, and interleukin-4 stimulation. J Biol Chem. 1994;269:28783–28789. [PubMed] [Google Scholar]
- Navarro M, Valentinis B, Belletti B, Romano G, Reiss K, Baserga R. Regulation of Id2 gene expression by the type 1 IGF receptor and the insulin receptor substrate-1. Endocrinology. 2001;142:5149–5157. doi: 10.1210/endo.142.12.8548. [DOI] [PubMed] [Google Scholar]
- Nichols J, Evans EP, Smith AG. Establishment of germ-line-competent embryonic stem (ES) cells using differentiation inhibiting activity. Development. 1990;110:1341–1348. doi: 10.1242/dev.110.4.1341. [DOI] [PubMed] [Google Scholar]
- Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki T, Masutani M, Watanabe M, Ochiya T, Hasegawa F, Nakagama H, Suzuki H, Sugimura T. Syncytiotrophoblastic giant cells in teratocarcinoma-like tumors derived from Parp-disrupted mouse embryonic stem cells. Proc Natl Acad Sci USA. 1999;96:13345–13350. doi: 10.1073/pnas.96.23.13345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paling NR, Wheadon H, Bone HK, Welham MJ. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J Biol Chem. 2004;279:48063–48070. doi: 10.1074/jbc.M406467200. [DOI] [PubMed] [Google Scholar]
- Pederson TM, Kramer DL, Rondinone CM. Serine/threonine phosphorylation of IRS-1 triggers its degradation: Possible regulation by tyrosine phosphorylation. Diabetes. 2001;50:24–31. doi: 10.2337/diabetes.50.1.24. [DOI] [PubMed] [Google Scholar]
- Prisco M, Peruzzi F, Belletti B, Baserga R. Regulation of Id gene expression by type I insulin-like growth factor: Roles of Stat3 and the tyrosine 950 residue of the receptor. Mol Cell Biol. 2001;21:5447–5458. doi: 10.1128/MCB.21.16.5447-5458.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisco M, Maiorana A, Guerzoni C, Calin G, Calabretta B, Voit R, Grummt I, Baserga R. Role of pescadillo and upstream binding factor in the proliferation and differentiation of murine myeloid cells. Mol Cell Biol. 2004;24:5421–5433. doi: 10.1128/MCB.24.12.5421-5433.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu CK, Feng GS. Shp-2 has a positive regulatory role in ES cell differentiation and proliferation. Oncogene. 1998;17:433–439. doi: 10.1038/sj.onc.1201920. [DOI] [PubMed] [Google Scholar]
- Raz R, Lee CK, Cannizzaro LA, d’Eustachio P, Levy DE. Essential role of STAT3 for embryonic stem cell pluripotency. Proc Natl Acad Sci USA. 1999;96:2846–2851. doi: 10.1073/pnas.96.6.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzinova MB, Benezra R. Id proteins in development, cell cycle and cancer. Trends Cell Biol. 2003;13:410–418. doi: 10.1016/s0962-8924(03)00147-8. [DOI] [PubMed] [Google Scholar]
- Sadowski CL, Choi TS, Le M, Wheeler TT, Wang LH, Sadowski HB. Insulin Induction of SOCS-2 and SOCS-3 mRNA expression in C2C12 skeletal muscle cells is mediated by Stat5*. J Biol Chem. 2001;276:20703–20710. doi: 10.1074/jbc.M101014200. [DOI] [PubMed] [Google Scholar]
- Saito S, Liu B, Yokoyama K. Animal embryonic stem (ES) cells: Self-renewal, pluripotency, transgenesis and nuclear transfer. Hum Cell. 2004;17:107–115. doi: 10.1111/j.1749-0774.2004.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Saxton TM, Henkemeyer M, Gasca S, Shen R, Rossi DJ, Shalaby F, Feng GS, Pawson T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 1997;16:2352–2364. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi CZ, Collins HW, Buettger CW, Garside WT, Matschinsky FM, Heyner S. Insulin family growth factors have specific effects on protein synthesis in preimplantation mouse embryos. Mol Reprod Dev. 1994;37:398–406. doi: 10.1002/mrd.1080370406. [DOI] [PubMed] [Google Scholar]
- Shima H, Pende M, Chen Y, Fumagalli S, Thomas G, Kozma SC. Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J. 1998;17:6649–6659. doi: 10.1093/emboj/17.22.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr R, Novak U, Willson TA, Inglese M, Murphy V, Alexander WS, Metcalf D, Nicola NA, Hilton DJ, Ernst M. Distinct roles for leukemia inhibitory factor receptor alpha-chain and gp130 in cell type-specific signal transduction. J Biol Chem. 1997;272:19982–19986. doi: 10.1074/jbc.272.32.19982. [DOI] [PubMed] [Google Scholar]
- Sun XJ, Crimmins DL, Myers MG, Jr, Miralpeix M, White MF. Pleiotropic insulin signals are engaged by multisite phosphorylation of IRS-1. Mol Cell Biol. 1993;13:7418–7428. doi: 10.1128/mcb.13.12.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Tobe K, Aoyama M, Inoue A, Sakamoto K, Yamauchi T, Kamon J, Kubota N, Terauchi Y, Yoshimatsu H, Matsuhisa M, Nagasaka S, Ogata H, Tokuyama K, Nagai R, Kadowaki T. Both insulin signaling defects in the liver and obesity contribute to insulin resistance and cause diabetes in Irs2(−/−) mice. J Biol Chem. 2004;279:25039–25049. doi: 10.1074/jbc.M311956200. [DOI] [PubMed] [Google Scholar]
- Tamemoto H, Kadowaki T, Tobe K, Yagi T, Sakura H, Hayakawa T, Terauchi Y, Ueki K, Kaburagi Y, Satoh S, et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature. 1994;372:182–186. doi: 10.1038/372182a0. [DOI] [PubMed] [Google Scholar]
- Toomey JR, Kratzer KE, Lasky NM, Broze GJ., Jr Effect of tissue factor deficiency on mouse and tumor development. Proc Natl Acad Sci USA. 1997;94:6922–6926. doi: 10.1073/pnas.94.13.6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentinis B, Romano G, Peruzzi F, Morrione A, Prisco M, Soddu S, Cristofanelli B, Sacchi A, Baserga R. Growth and differentiation signals by the insulin-like growth factor 1 receptor in hemopoietic cells are mediated through different pathways. J Biol Chem. 1999;274:12423–12430. doi: 10.1074/jbc.274.18.12423. [DOI] [PubMed] [Google Scholar]
- Valentinis B, Navarro M, Zanocco-Marani T, Edmonds P, McCormick J, Morrione A, Sacchi A, Romano G, Reiss K, Baserga R. Insulin receptor substrate-1, p70S6K, and cell size in transformation and differentiation of hemopoietic cells. J Biol Chem. 2000;275:25451–25459. doi: 10.1074/jbc.M002271200. [DOI] [PubMed] [Google Scholar]
- Valverde AM, Arribas M, Mur C, Navarro P, Pons S, Cassard-Doulcier AM, Kahn CR, Benito M. Insulin-induced up-regulated uncoupling protein-1 expression is mediated by insulin receptor substrate 1 through the phosphatidylinositol 3-kinase/Akt signaling pathway in fetal brown adipocytes. J Biol Chem. 2003;278:10221–10231. doi: 10.1074/jbc.M209363200. [DOI] [PubMed] [Google Scholar]
- Wang LM, Keegan AD, Li W, Lienhard GE, Pacini S, Gutkind JS, Myers MG, Jr, Sun XJ, White MF, Aaronson SA, et al. Common elements in interleukin 4 and insulin signaling pathways in factor-dependent hematopoietic cells. Proc Natl Acad Sci USA. 1993;90:4032–4036. doi: 10.1073/pnas.90.9.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Umehara H, Murayama K, Okabe M, Kimura T, Nakano T. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene. 2006;25:2697–2707. doi: 10.1038/sj.onc.1209307. [DOI] [PubMed] [Google Scholar]
- White MF. The IRS-signalling system: A network of docking proteins that mediate insulin action. Mol Cell Biochem. 1998;182:3–11. [PubMed] [Google Scholar]
- Yamamoto-Honda R, Honda Z, Ueki K, Tobe K, Kaburagi Y, Takahashi Y, Tamemoto H, Suzuki T, Itoh K, Akanuma Y, Yazaki Y, Kadowaki T. Mutant of insulin receptor substrate-1 incapable of activating phosphatidylinositol 3-kinase did not mediate insulin-stimulated maturation of Xenopus laevis oocytes. J Biol Chem. 1996;271:28677–28681. doi: 10.1074/jbc.271.45.28677. [DOI] [PubMed] [Google Scholar]
- Yenush L, Zanella C, Uchida T, Bernal D, White MF. The pleckstrin homology and phosphotyrosine binding domains of insulin receptor substrate 1 mediate inhibition of apoptosis by insulin. Mol Cell Biol. 1998;18:6784–6794. doi: 10.1128/mcb.18.11.6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]