Abstract
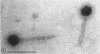
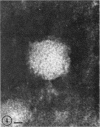
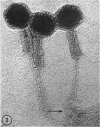
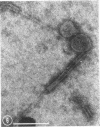
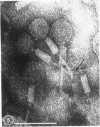
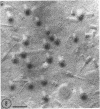
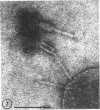
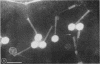
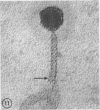
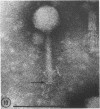
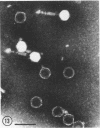
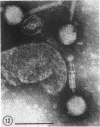
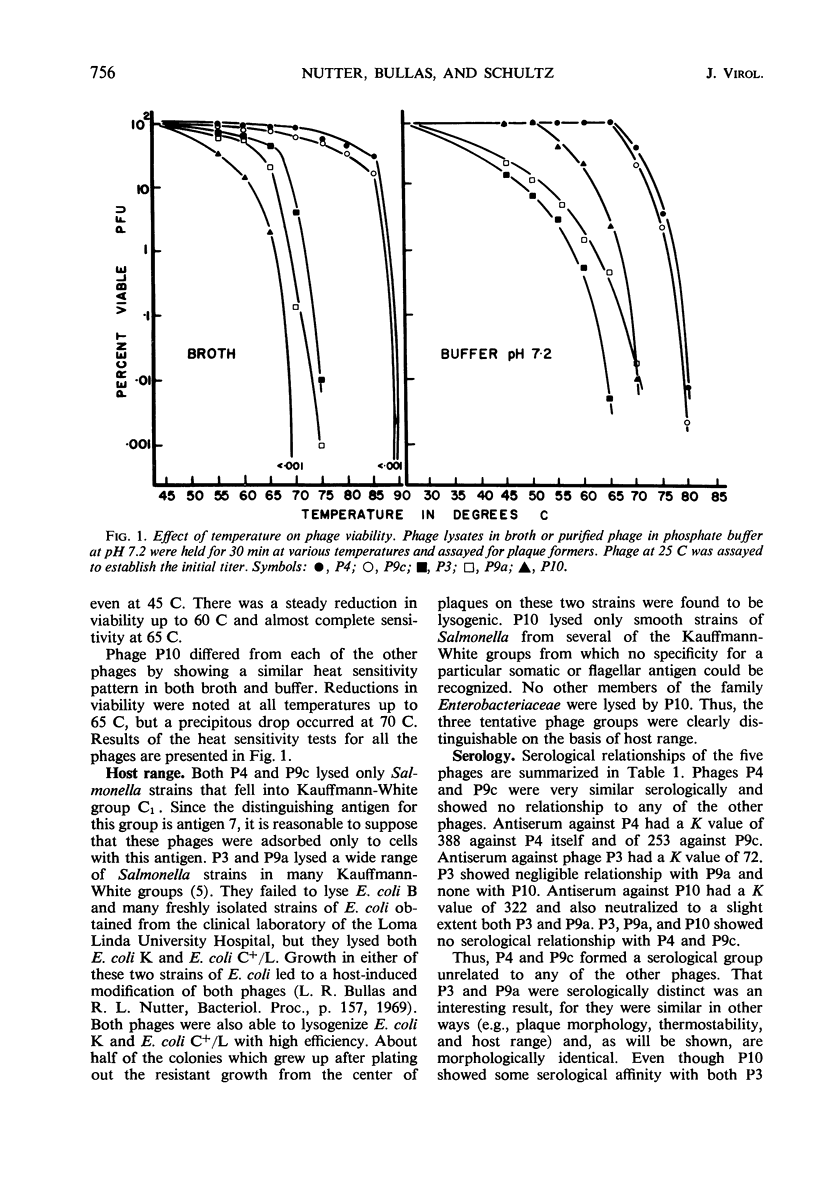
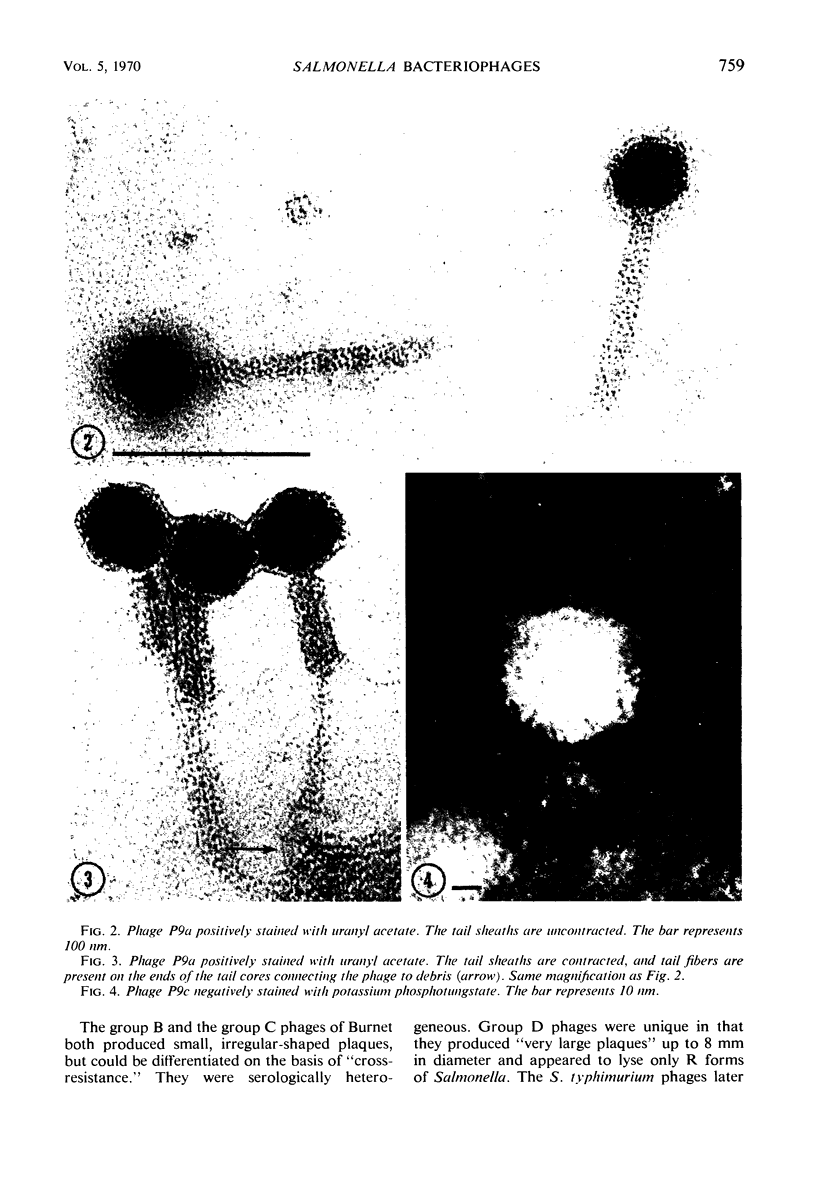
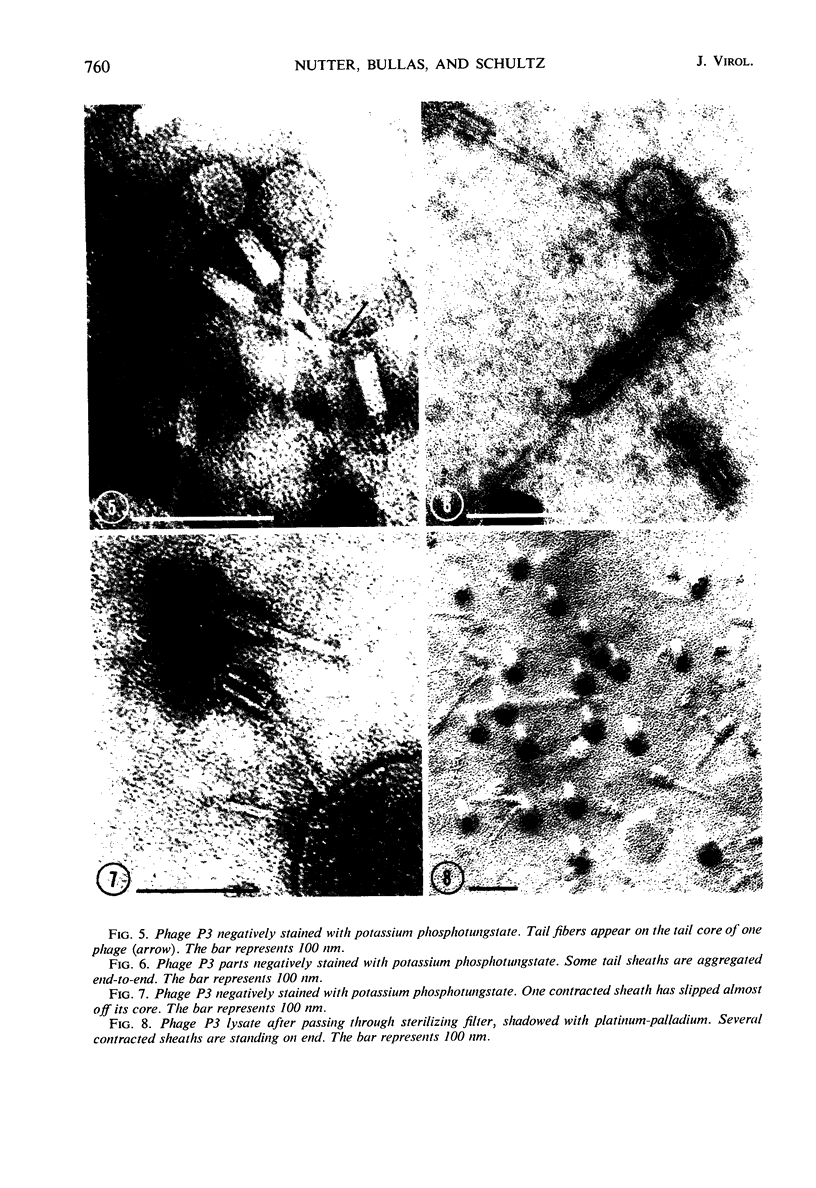
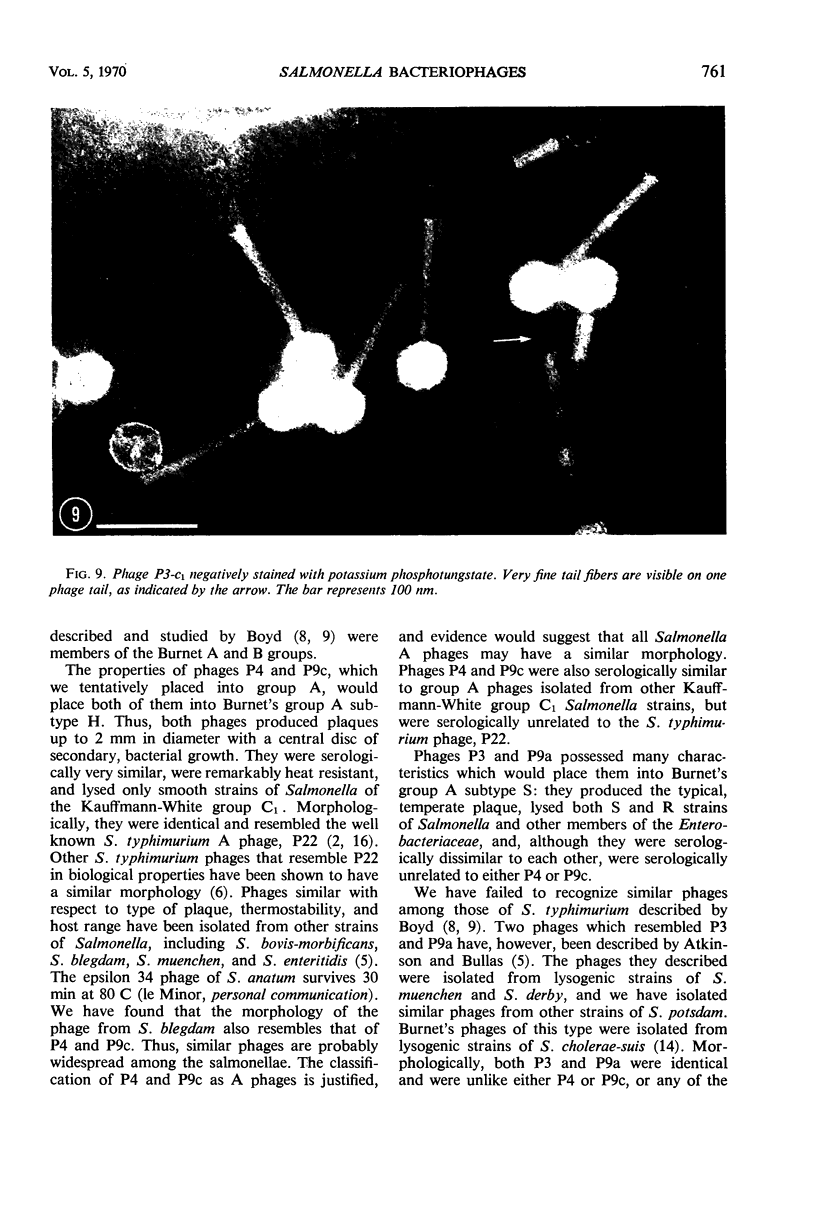
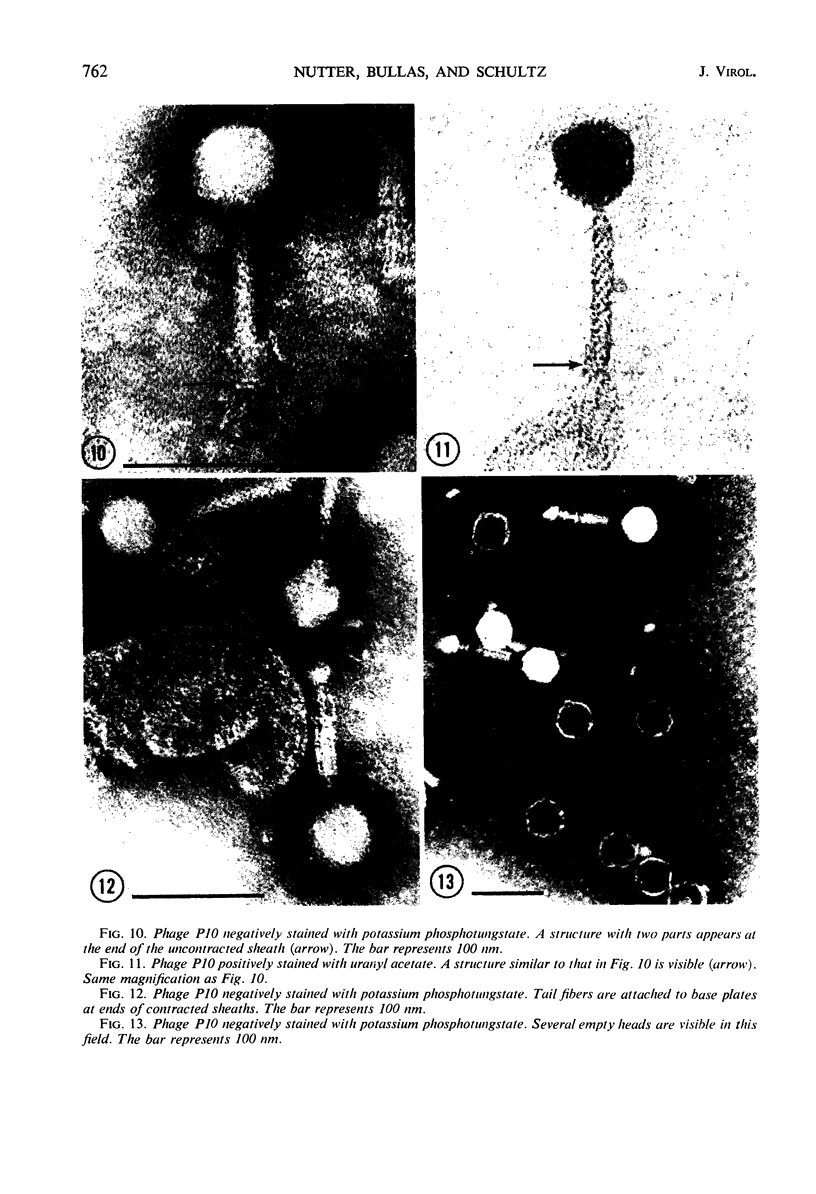
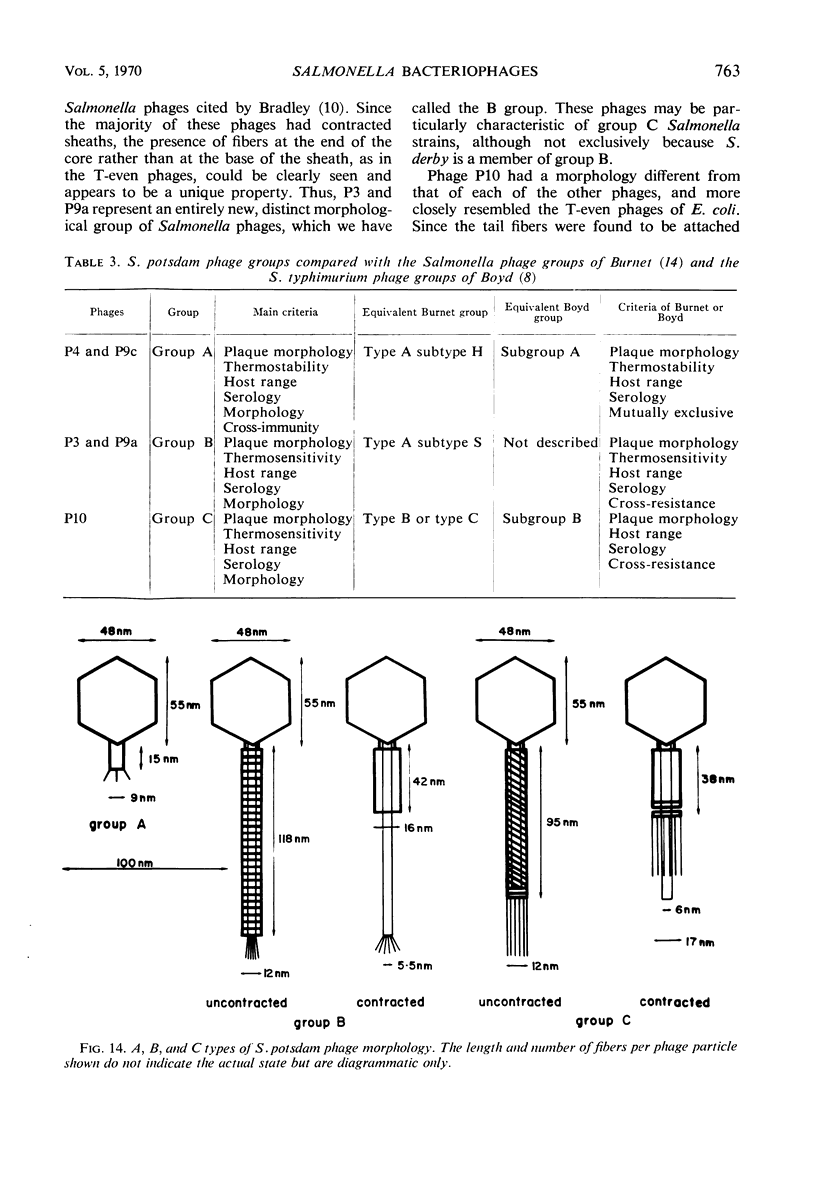
Five bacteriophages were isolated from lysogenic strains of Salmonella potdam. On the basis of plaque morphology, thermostability, serology, host range, one-step growth parameters, and phage morphology, they were divided into three groups: group A, phages P4 and P9c; group B, phages P3 and P9a; and group C, phage P10. Group A phages had a hexagonal head 55 nm in diameter with a short tail 15 nm long. These phages were particularly characterized by high thermostability, lack of serological relationship with any of the other phages, and restriction of lysis to other Salmonella strains of Kauffmann-White group C1. Group B phages had a head identical in size and shape to that of the A phages, but they possessed a tail 118 nm long with a contractile sheath. A unique feature was the occurrence of tail fibers at the end of the core rather than at the base of the sheath. These phages were considerably less thermostable, had extended host ranges, and were serologically distinct from each other but unrelated to the A phages. The group C phage, P10, had a head identical to that of the A and B phages. It had a tail 95 nm in length, with tail fibers attached to a base plate at the end of a contractile sheath. P10 was highly sensitive to heat, lysed only smooth strains of Salmonella, and showed a degree of serological relationship to both B phages. The relationship of these phage groups to previous Salmonella phage grouping schemes is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON N., BULLAS L. R. Lysogenicity and lysis patterns in the Salmonellas. X. S. potsdam. Aust J Exp Biol Med Sci. 1956 Dec;34(6):461–469. doi: 10.1038/icb.1956.56. [DOI] [PubMed] [Google Scholar]
- ATKINSON N., BULLAS L. R. Salmonella bacteriophages. 6. Some heat-resistant phages. Aust J Exp Biol Med Sci. 1957 Jun;35(3):193–208. [PubMed] [Google Scholar]
- ATKINSON N., BULLAS L. R. Salmonella bacteriophages. V. Some bacteriophages of S. potsdam. Aust J Exp Biol Med Sci. 1956 Dec;34(6):455–460. doi: 10.1038/icb.1956.55. [DOI] [PubMed] [Google Scholar]
- BOWEN G. Kinetic studies on the mechanism of photoreactivation of bacteriophage T2 inactivated by ultraviolet light. Ann Inst Pasteur (Paris) 1953 Jan;84(1):218–221. [PubMed] [Google Scholar]
- BOYD J. S. K. The symbiotic bacteriophages of Salmonella typhi-murium. J Pathol Bacteriol. 1950 Oct;62(4):501–517. doi: 10.1002/path.1700620402. [DOI] [PubMed] [Google Scholar]
- BOYD J. S. The type-A phages of Salmonella typhi-murium: two new types. J Pathol Bacteriol. 1954 Oct;68(2):311–314. doi: 10.1002/path.1700680202. [DOI] [PubMed] [Google Scholar]
- Bezdek M., Amati P. Properties of P22 and A related Salmonella typhimurium phage. I. General features and host specificity. Virology. 1967 Feb;31(2):272–278. doi: 10.1016/0042-6822(67)90171-7. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- Israel J. V., Anderson T. F., Levine M. in vitro MORPHOGENESIS OF PHAGE P22 FROM HEADS AND BASE-PLATE PARTS. Proc Natl Acad Sci U S A. 1967 Feb;57(2):284–291. doi: 10.1073/pnas.57.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lwoff A., Tournier P. The classification of viruses. Annu Rev Microbiol. 1966;20:45–74. doi: 10.1146/annurev.mi.20.100166.000401. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Melnick J. L., Rongey R., Mayor H. D. Physical assay and growth cycle studies of a defective adeno-satellite virus. J Virol. 1967 Feb;1(1):171–180. doi: 10.1128/jvi.1.1.171-180.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rullas L. R., Zuccarelli A. J., Nutter R. L. Aging effect on phage particles leading to an increase in burst size. Nature. 1967 Dec 30;216(5122):1308–1308. doi: 10.1038/2161308a0. [DOI] [PubMed] [Google Scholar]
- Simon L. D., Anderson T. F. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. I. Attachment and penetration. Virology. 1967 Jun;32(2):279–297. doi: 10.1016/0042-6822(67)90277-2. [DOI] [PubMed] [Google Scholar]