Abstract
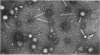
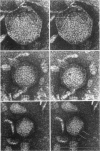
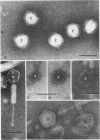
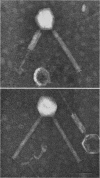
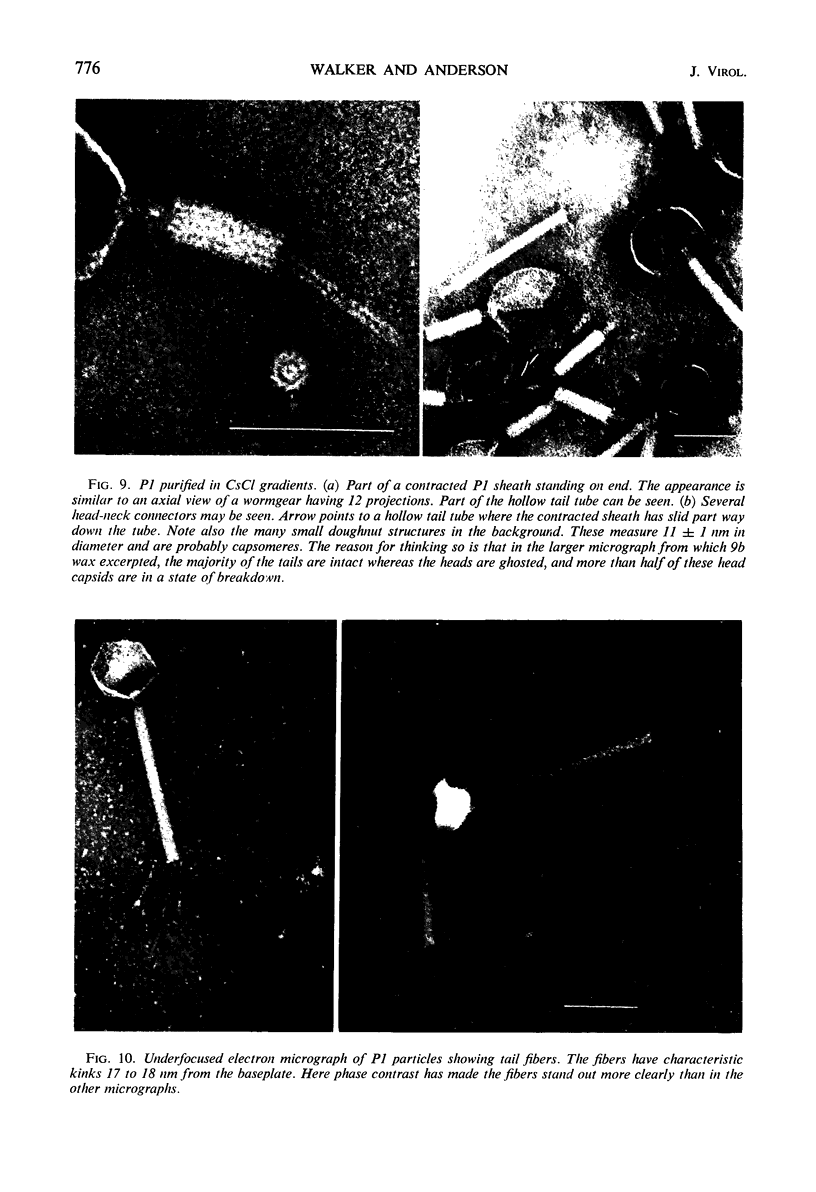
Lysates of P1 from all hosts tested contained at least three morphological variants with respect to head size. These were termed “big” (P1B), “small” (P1S), and “minute” (P1M). Since successive clonings of plaques isolated on many different hosts failed to change the proportions of the variants, we concluded that the production of variants was a function of the P1 genome rather than that of the host. In the electron microscope, the heads appeared to be icosadeltahedra, having face-to-face head diameters of 86 ± 2 nm, 65 ± 2 nm, and 47 ± 2 nm. Assuming the head capsids to be composed of the same protein subunits, these diameters were compatible with T = 16, 9, and 4 with a lattice constant (intercapsomere distance) of 12 to 13 nm. The tails of all variants were morphologically indistinguishable. Each consisted of a hollow tail tube surrounded by a contractile sheath attached to the head by means of a “head-neck connector” which could be a specialized vertex capsomere. In CsCl gradients, a number of bands were observed. One band contained the majority of P1B particles and 99% of the plaque-forming units. Two other bands contained P1S particles whose densities suggested a content of about 40 and 60% of the complete P1B genome. The less dense of these two bands also contained defective P1B particles with a calculated content of only 60% of the complete genome. The P1S particles tested injected their deoxyribonucleic acid (DNA) into host cells and killed them. Genetic markers contained in this band could be rescued by infectious P1B particles, confirming the evidence of Ikeda and Tomizawa that this fraction contains P1 DNA.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON T. F. The morphology and osmotic properties of bacteriophage systems. Cold Spring Harb Symp Quant Biol. 1953;18:197–203. doi: 10.1101/sqb.1953.018.01.030. [DOI] [PubMed] [Google Scholar]
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRENNER S., HORNE R. W. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959 Jul;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Remsen C. C. Bacteriophage T2 as seen with the freeze-etching technique. Virology. 1970 Mar;40(3):703–718. doi: 10.1016/0042-6822(70)90215-1. [DOI] [PubMed] [Google Scholar]
- Boy de la Tour E., Kellenberger E. Aberrant forms of the T-even phage head. Virology. 1965 Oct;27(2):222–225. doi: 10.1016/0042-6822(65)90163-7. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- COHEN D. A variant of phage P2 originating in Escherichia coli, strain B. Virology. 1959 Jan;7(1):112–126. doi: 10.1016/0042-6822(59)90180-1. [DOI] [PubMed] [Google Scholar]
- CUMMINGS D. J., KOZLOFF L. M. Various properties of the head protein of T2 bacteriophage. J Mol Biol. 1962 Jul;5:50–62. doi: 10.1016/s0022-2836(62)80060-6. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Chapman V. A., DeLong S. S. Disruption of T-even bacteriophages by dimethyl sulfoxide. J Virol. 1968 Jun;2(6):610–620. doi: 10.1128/jvi.2.6.610-620.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISERLING F. A., BOYDELATOUR E. CAPSOMERES AND OTHER STRUCTURES OBSERVED ON SOME BACTERIOPHAGES. Pathol Microbiol (Basel) 1965;28:175–180. [PubMed] [Google Scholar]
- HERSHEY A. D., CHASE M. Independent functions of viral protein and nucleic acid in growth of bacteriophage. J Gen Physiol. 1952 May;36(1):39–56. doi: 10.1085/jgp.36.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Tomizawa J. I. Transducing fragments in generalized transduction by phage P1. 3. Studies with small phage particles. J Mol Biol. 1965 Nov;14(1):120–129. doi: 10.1016/s0022-2836(65)80234-0. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Tomizawa J. I. Transducing fragments in generalized transduction by phage P1. I. Molecular origin of the fragments. J Mol Biol. 1965 Nov;14(1):85–109. doi: 10.1016/s0022-2836(65)80232-7. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Tomizawa J. Prophage P1, and extrachromosomal replication unit. Cold Spring Harb Symp Quant Biol. 1968;33:791–798. doi: 10.1101/sqb.1968.033.01.091. [DOI] [PubMed] [Google Scholar]
- Israel J. V., Anderson T. F., Levine M. in vitro MORPHOGENESIS OF PHAGE P22 FROM HEADS AND BASE-PLATE PARTS. Proc Natl Acad Sci U S A. 1967 Feb;57(2):284–291. doi: 10.1073/pnas.57.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., BOLLE A., BOYDELATOUR E., EPSTEIN R. H., FRANKLIN N. C., JERNE N. K., REALE SCAFATI A., SECHAUD J. FUNCTIONS AND PROPERTIES RELATED TO THE TAIL FIBERS OF BACTERIOPHAGE T4. Virology. 1965 Jul;26:419–440. doi: 10.1016/0042-6822(65)90006-1. [DOI] [PubMed] [Google Scholar]
- KLUG A., BERGER J. E. AN OPTICAL METHOD FOR THE ANALYSIS OF PERIODICITIES IN ELECTRON MICROGRAPHS, AND SOME OBSERVATIONS ON THE MECHANISM OF NEGATIVE STAINING. J Mol Biol. 1964 Dec;10:565–569. doi: 10.1016/s0022-2836(64)80081-4. [DOI] [PubMed] [Google Scholar]
- King J. Assembly of the tail of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):231–262. doi: 10.1016/0022-2836(68)90007-7. [DOI] [PubMed] [Google Scholar]
- Krimm S., Anderson T. F. Structure of normal and contracted tail sheaths of T4 bacteriophage. J Mol Biol. 1967 Jul 28;27(2):197–202. doi: 10.1016/0022-2836(67)90015-0. [DOI] [PubMed] [Google Scholar]
- LABAW L. W., MOSLEY V. M. Demonstration of striated fibers in the capsule of the Lisbonne strain of lysogenic Escherichia coli. J Bacteriol. 1954 May;67(5):576–584. doi: 10.1128/jb.67.5.576-584.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Mölbert E., Showe M., Kellenberger E. Form-determining function of the genes required for the assembly of the head of bacteriophage T4. J Mol Biol. 1970 Apr 14;49(1):99–113. doi: 10.1016/0022-2836(70)90379-7. [DOI] [PubMed] [Google Scholar]
- Moody M. F. Structure of the sheath of bacteriophage T4. I. Structure of the contracted sheath and polysheath. J Mol Biol. 1967 Apr 28;25(2):167–200. doi: 10.1016/0022-2836(67)90136-2. [DOI] [PubMed] [Google Scholar]
- Moody M. F. Structure of the sheath of bacteriophage T4. II. Rearrangement of the sheath subunits during contraction. J Mol Biol. 1967 Apr 28;25(2):201–208. doi: 10.1016/0022-2836(67)90137-4. [DOI] [PubMed] [Google Scholar]
- Moody M. F. The shape of the T-even bacteriophage head. Virology. 1965 Aug;26(4):567–576. doi: 10.1016/0042-6822(65)90319-3. [DOI] [PubMed] [Google Scholar]
- Mosig G. A map of distances along the DNA molecule of phage T4. Genetics. 1968 Jun;59(2):137–151. doi: 10.1093/genetics/59.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academy of Sciences. Science. 1960 Nov 18;132(3438):1488–1501. doi: 10.1126/science.132.3438.1488. [DOI] [PubMed] [Google Scholar]
- SWANSTROM M., ADAMS M. H. Agar layer method for production of high titer phage stocks. Proc Soc Exp Biol Med. 1951 Nov;78(2):372–375. doi: 10.3181/00379727-78-19076. [DOI] [PubMed] [Google Scholar]
- Smith K. O., Trousdale M. Multiple-Tailed T4 Bacteriophage. J Bacteriol. 1965 Sep;90(3):796–802. doi: 10.1128/jb.90.3.796-802.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisinger G., Emrich J., Stahl M. M. Chromosome structure in phage t4, iii. Terminal redundancy and length determination. Proc Natl Acad Sci U S A. 1967 Feb;57(2):292–295. doi: 10.1073/pnas.57.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TING R. C. The specific gravity of transducing particles of bacteriophage P1. Virology. 1962 Feb;16:115–121. doi: 10.1016/0042-6822(62)90286-6. [DOI] [PubMed] [Google Scholar]
- Thomas C. A., Jr The rule of the ring. J Cell Physiol. 1967 Oct;70(2 Suppl):13–33. doi: 10.1002/jcp.1040700404. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., HEARST J. E. Equilibrium sedimentation of macromolecules and viruses in a density gradient. Fortschr Chem Org Naturst. 1962;20:373–422. [PubMed] [Google Scholar]