Abstract
Little is known about the transition in behaviors from short-term weight loss to maintenance of weight loss. We wanted to determine how short-term and long-term weight loss and patterns of weight change were associated with intervention behavioral targets.
This analysis includes overweight/obese participants in active treatment (n= 507) from the previously published PREMIER trial, an 18-month, multicomponent lifestyle intervention for blood pressure reduction, including 33 intervention sessions and recommendations to self-monitor food intake and physical activity daily. Associations between behaviors (attendance, recorded days/week of physical activity, food records/week) and weight loss of ≥ 5% at 6 and 18 months were examined using logistic regression. We characterized the sample using 5 weight change categories (weight weight stable, weight loss then relapse, late weight loss, and weight loss then maintenance) and analyzed adherence to the behaviors for each category, comparing means with ANOVA.
Participants lost an average of 5.3 ± 5.6 kg at 6 months and 4.0 ± 6.7 kg (4.96% of body weight) by 18 months. Higher levels of attendance, food record completion, and recorded days/week of physical activity were associated with increasing odds of achieving 5% weight loss. All weight change groups had declines in the behaviors over time; however, compared to the other four groups, the weight loss/maintenance group (n= 154) had statistically significant less decline in number of food records/week (48%), recorded days/week of physical activity (41.7%), and intervention sessions attended (12.8%) through 18 months.
Behaviors associated with short-term weight loss continue to be associated with long-term weight loss, albeit at lower frequencies. Minimizing the decline in these behaviors may be important in achieving long-term weight loss.
Introduction
It is generally well accepted that the achievement of successful long term weight loss poses a great challenge to many patients. Prior studies have typically regarded short term weight loss as net weight loss within six to twelve months, and long term weight loss as net weight loss at or beyond twelve months1–3. Although the barriers for poor rates of long term success remain poorly defined, possible hypotheses include poor self-efficacy, decreased resting energy expenditure, hormonal changes associated with weight loss, negative environmental influences, and declining adherence to key lifestyle behaviors4–6. Few studies have investigated whether the same behaviors that produce short term weight loss also translate into long term weight loss or weight maintenance, or how changes in behavior frequency (i.e., increasing or decreasing) affect change in weight.
Numerous studies have established that behavioral modification of key lifestyle behaviors results in short term weight loss. For example, high levels of physical activity, defined as 2500 kcal/week, and modification of dietary intake (i.e., calorie restriction) using a variety of approaches can lead to clinically significant weight loss within 20 weeks7–12. Other key behaviors associated with greater amounts of short term weight loss include attendance at behavioral group intervention sessions and self-monitoring of food intake8.
Fewer studies provide information on behaviors associated with long term maintenance of weight loss. For example, data from the National Weight Control Registry provide insight into behaviors associated with long term weight loss maintenance, such as high physical activity levels, persistent self-monitoring practices, and decreased television viewing13–15. Similarly, in the Diabetes Prevention Program, participants assigned to intensive lifestyle modification achieved and maintained clinically significant weight loss for over three years, utilizing strategies including reduced dietary fat and total energy intake, recording daily food intake and physical activity, and consistent attendance at educational sessions and individual counseling16. Wing et al analyzed the predictors of maintaining large volume weight loss among participants from the STOP Regain trial and found that decreased physical activity was associated with regain while daily weighing was important for maintenance. Finally, a recent randomized controlled trial by Sacks et al showed that for 645 participants who completed a behavioral weight loss intervention, a change in body weight from baseline to 2 years was associated with attendance at group sessions. Participants lost an average of 0.2 kg for every session attended17.
Despite the aforementioned associations, we still know very little about the behavioral transition to long term maintenance of weight loss. Better long term weight loss strategies could be defined if it was understood how certain behaviors predictive of short term weight loss change over time and the resulting associations with long term weight loss. In this context, we investigated the PREMIER study population to determine whether adherence to components of a lifestyle intervention predicted short term (defined as 6 months) and long term (defined as 18 months) weight loss. The PREMIER study examined the effects of two multicomponent lifestyle interventions on lowering blood pressure, when compared to an advice only control group, over an eighteen month period21. In this study, we assessed the relationship between weight loss at 6 and 18 months to intervention attendance rates, food record completion, and recorded days/week of physical activity. In addition, we examined how patterns of weight change were associated with changes in behavior frequency.
Methods and Procedures
The study design of the PREMIER trial has been described previously21, 22. Briefly, this was a multicenter trial conducted at four different clinical sites that included over 800 adults with either pre-hypertension or Stage I hypertension, not currently on antihypertensive medication. The purpose of the study was to assess the effects of lifestyle modification interventions on various outcomes including blood pressure, weight, dietary fat and sodium intake. Subjects were randomly assigned to one of three groups: an advice only group consisting of a single 30 minute informational session, an intervention group based on established, traditional lifestyle recommendations, or an intervention group based on established lifestyle recommendations plus adherence to the DASH diet. The “established lifestyle recommendations” included weight loss of at least 6.8 kg, 180 minutes per week of moderate physical activity, a daily maximum of 100 mmol of dietary sodium, and a daily maximum alcohol consumption of two drinks for men and one drink for women. The DASH diet required a daily intake of 9–12 servings of fruits and vegetables with 2–3 servings of low-fat dairy products, and daily fat intake limited to less than 25% of total calories.
Participants had the opportunity to attend a total of eighteen sessions during the initial six months including fourteen group meetings and four individual intervention sessions. For the next twelve months, monthly group sessions along with three individual sessions were offered, for a total of fifteen sessions. Attendance rates were calculated as a percent of the number of sessions attended out of the maximum number of sessions provided. Participants were instructed to record food intake and physical activity minutes daily using a Food and Fitness Diary. At each behavioral session, intervention facilitators collected Food and Fitness Diaries from study participants. Facilitators logged the number of days that food intake and physical activity was recorded by the study participant. Participants were given credit for self-monitoring regardless of the quality (i.e., completeness, details, accuracy) of information recorded. Finally, blinded study staff weighed participants, who wore light clothing and no shoes, on a calibrated scale at baseline, six, and eighteen months. Staff used a calibrated wall-mounted stadiometer to measure participants’ height; body mass index (BMI) was calculated as kilograms per meter squared (kg/m2).
For the purpose of this study, we included only those participants in the PREMIER trial who were at least overweight (BMI ≥ 25 m/kg2) and assigned to an active intervention group (n=507). Outcomes of interest included weight change from baseline to six months, from six to eighteen months, and from baseline to eighteen months. Predictive variables at six and 18 months included number of food records per week, reported days/week of physical activity, and attendance rates for behavioral sessions.
Statistical analysis
We examined 5 distinct weight change patterns among the participants: weight gained, weight stable, weight loss then relapse, late weight loss, and weight loss and maintenance. We used 5% weight change as the cut point to categorize weight change at both 6 and 18 month follow-up: gained (≥5% of randomization weight), stable (±4.9% of randomization body weight) and lost (≤5% of randomization weight). Then, we defined the 5 overall weight change patterns, which were discrete and mutually exclusive, based on weight change at months 6 and 18. Those who were classified in the weight gained category were weight stable or had a weight gain of ≥5% at six months and ended the study with weight gain. Weight stable participants gained or lost less than 5% of their randomization weight at both 6 and 18 months. Individuals in the weight loss and relapse category initially lost 5% or more of their randomization weight in 6 months; however, by 18 months they had not maintained a weight loss of at least 5%. Those in the weight loss and maintenance category lost 5% or more of their randomization weight by 6 months and maintained this weight loss through 18 months. Finally, late weight loss participants were at or above their randomization weight at 6 months but subsequently lost 5% or more of their weight by 18 months.
We used SPSS software to conduct basic descriptive statistics for the three behaviors of interest: food records, physical activity records and attendance. We used simple t-test to detect differences within groups, and ANOVA to detect differences among groups. We also created a logistic regression model, using 5% of baseline weight loss as a cut point to create a dichotomous variable (5% weight loss achieved [yes or no]), to determine an odds ratio for each behavior of interest at both six months and eighteen months. We did not control for treatment group, as there was no significant weight change difference between the two groups in the PREMIER study21. Analyses were adjusted for baseline BMI, gender, and race.
Results
The baseline characteristics are shown in Table 1. The mean age was 50 years old, with the majority of the participants being female. Over one-third of participants self identified as African American. On average, participants were considered stage I obese, with nearly even distribution of participants across weight categories of overweight, obese stage I, and obese stage II.
Table 1.
Baseline characteristics for those with body mass index ≥ 25 kg/m2
| N=507 | |
|---|---|
| Age, mean (SD) | 49.8 (8.7) |
| Men (N, %) | 198, 39.1% |
| African American (N, %) | 174, 34.3% |
| Baseline weight in kilograms, mean (SD) | 97.3 (18.5) |
| Baseline BMI, mean (SD) | 33.7 (5.6) |
| Weight Category (N, %) | |
| Overweight (25 ≤ BMI < 30) | 162, 32.0% |
| Obese I (30 ≤ BMI < 35) | 151, 29.8% |
| Obese II (BMI ≥ 35) | 194, 38.3% |
BMI- body mass index
SD-standard deviation
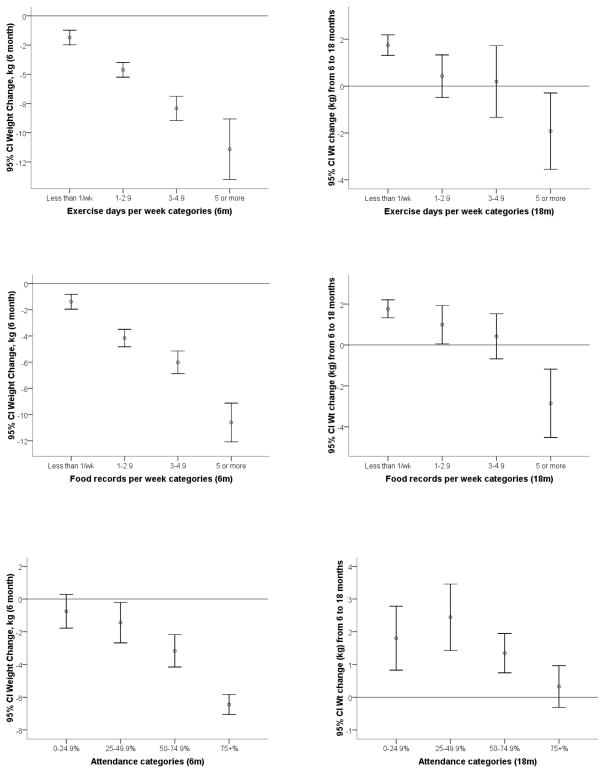
Figures 1a–c depict the mean change in weight of the study sample by number of food records, days of reported physical activity, and attendance rates at 6 and 18 months. In general, at six months, higher levels of adherence to the key behaviors were associated with greater weight loss. The intervention groups were similar in terms of number of days of exercise recorded and group session attendance; however the established recommendations plus DASH diet group had 0.5 day more of food records per week (p=0.006). Beyond six months, there was a decline in the average number of food records per week, days of physical activity, and intervention session attendance rates. At six months, food records were kept an average of 2.8 ± 1.9 days per week, declining to 1.1 ± 1.7 days per week by 18 months. Similarly, exercise decreased from 2.2 ± 1.7 to 0.98 ± 1.5 days per week. Attendance also dropped from 75.1 ± 25.2% to 53.8 ± 33.8%. There were also differences in the pattern of weight change after six months. The mean weight change at six months was −5.3 ± 5.6 kg; however, there was a mean weight gain of 1.2 ± 4.1 kg during the last 12 months of the study. The mean weight loss by 18 months was 4.0 ± 6.7 kg. This represented a net − 4.96 ± 8.0% change in baseline body weight.
Figure 1.
Figure 1a–c. Change in weight at 6 and 18 months by
A: Food records per week
B: Self-reported physical activity patterns
C: Group session attendance
The results of our analysis of the impact of the three key behaviors on achieving at least 5% weight loss at 6 and 18 months are shown in Table 2. Individually, intervention attendance, self-reported physical activity, and food journaling had a strong, positive impact on the odds of achieving at least 5% weight loss at 6 months and at 18 months. Food records had the highest odds at six months; even averaging only 1–2.9 food records per week increased the odds of achieving ≥ 5% weight loss by five-fold. The effect of recording physical activity on the odds of achieving ≥ 5% weight loss was significant at 6 months and appeared to increase at 18 months even within the same categories of recorded days/week.
Table 2.
Relationship between weight loss of at least 5.0% at each follow-up period and behavioral characteristics
| Behavior | N (%) | OR (95% CI) 6 month follow up | Probability of 5.0 % weight loss at 6 months | N (%) | OR (95% CI) 18 month follow up | Probability of 5.0 % weight loss at 18 months |
|---|---|---|---|---|---|---|
| Average food records per week | ||||||
| <1 per week | 105 (20.7) | 1.0 | 0.11 | 351 (69.2) | 1.0 | 0.22 |
| 1–2.9 per week | 193 (38.1) | 6.3 (2.7, 14.5) | 0.43 | 82 (16.2) | 5.5 (3.3, 9.4) | 0.61 |
| 3.0–4.9 per week | 126 (24.9) | 14.2 (6.0, 33.6) | 0.63 | 44 (8.7) | 14.5 (6.4, 32.9) | 0.81 |
| ≥ 5.0 per week | 82 (16.2) | 53.1 (19.6, 144.1) | 0.86 | 30 (5.9) | 16.5 (6.0, 45.4) | 0.83 |
| Recorded days of physical activity | ||||||
| <1 day/week | 148 (29.2) | 1.0 | 0.15 | 364 (71.8) | 1.0 | 0.22 |
| 1.0 –2.9 days/week | 193 (38.1) | 4.2 (2.4, 7.5) | 0.45 | 80 (15.8) | 7.5 (4.3, 13.0) | 0.68 |
| 3.0–4.9 days/week | 131 (25.8) | 10.7 (5.7, 20.1) | 0.69 | 40 (7.9) | 23.1 (8.7, 61.6) | 0.88 |
| ≥ 5.0 days/week | 33 (6.5) | 23.9 (8.1, 71.3) | 0.84 | 23 (4.5) | 11.7 (4.1, 33.0) | 0.78 |
| Meeting attendance goal of 15/18 meetings | ||||||
| No | 218 (42.9) | 1.0 | 0.23 | 388 (76.5) | 1.0 | 0.28 |
| Yes (83.3%) | 289 (57.1) | 4.8 (3.1, 7.3) | 0.61 | 119 (24.5) | 4.5 (2.8, 7.1) | 0.66 |
Adjusted for gender, race and baseline BMI.
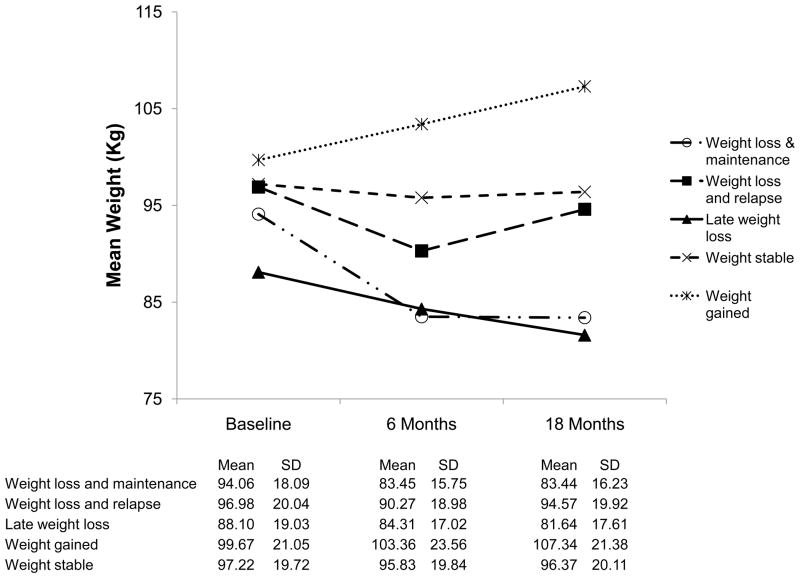
The baseline characteristics of the five weight change groups are described in Table 4, while the mean weight at each follow up time point is illustrated in Figure 2. We observed significant variation between the weight change groups in average number of food records, average number of days of physical activity, and average attendance rate at group sessions (Table 4). Despite differing levels of weight loss at 18 months, all groups had significant declines in weekly food records, recorded days of physical activity, and attendance rates at 18 months compared to 6 months. However, those who lost 5% and maintained their weight loss kept a higher number of food records, reported more exercise days, and attended more intervention sessions than any other group at all time points in the study. Those who lost weight and subsequently relapsed initially completed a greater number of weekly food and physical activity records and had higher attendance rates than those who gained, were weight stable, or had late weight loss. However, by 18 months they had fewer weekly food records and recorded days of physical activity than those who had late weight loss, although the attendance rate between the two groups remained similar (51% vs. 55%). Weight stable participants had similar levels of adherence in all three categories compared to gainers at 6 months, but the decline in food records and attendance by 18 months was statistically smaller and resulted in slightly higher levels of adherence compared to those who gained. While all groups initially maintained an average meeting attendance rate of greater than 60% at six months, only those who maintained weight loss continued to attend greater than 60% of meetings at 18 months.
Table 4.
Behaviors for weight patterns at 6 months and 18 months
| Weight gaineda (n= 18) | Weight loss & relapseb (n= 74) | Stablec (n= 228) | Late weight lossd (n= 33) | Weight loss & maintenancee (n= 154) | |
|---|---|---|---|---|---|
| Mean number of food records/week (SD) | |||||
| 0–6 months | 1.7 (1.2) | 3.2 (1.5) | 1.8 (1.6) | 2.7 (1.6) | 4.2 (1.9) |
| 6–18 months | 0.03 (0.07) | 0.5 (1.0) | 0.5 (1.1) | 1 (1.3) | 2.5 (2.2) |
| Percent change from 6 months (%)(SD) | −99.1(2.4)cde | −86.2 (22.7)de | −82.4 (29.1)ae | −76.1 (40.3)abe | −48 (40.6)abcd |
| Mean number of reported days of physical activity/week (SD) | |||||
| 0–6 months | 1.4 (1.2) | 2.5 (1.3) | 1.5 (1.4) | 2.1 (1.4) | 3.4 (1.6) |
| 6–18 months | 0.1 (0.2) | 0.6 (1.2) | 0.4 (0.9) | 0.7 (0.9) | 2.2 (1.9) |
| Percent change from 6 months (%)(SD) | −96.3 (7.8)de | −83.7 (25.9) de | −79.3 (30)e | −68.8 (40.5) abe | −41.7 (43.3)abcd |
| Attendance Rate (%) (SD) | |||||
| 0–6 months | 63 (27) | 83 (15) | 64 (28) | 75 (26) | 88 (13) |
| 6–18 months | 26 (32) | 51 (28) | 42 (34) | 55 (35) | 76 (23) |
| Percent change from 6 months (%)(SD) | −37.4 (29.2)cde | −32 (25.3)cde | −22.5 (21.8)abe | −19.7 (23)ab | −12.8 (17.8)abc |
SD- standard deviation
Groups labeled with superscripts (a, b, c, d, e); columns with different superscripts indicate significant difference at p< 0.05 by analysis of variance, least significant difference
Figure 2.
Weight change over time according to weight patterns.
Discussion
To our knowledge, this is the first study to examine weight change in association with changes in three vital behaviors for weight loss—food journaling, recording of days/week of physical activity, and session attendance—at six and eighteen months. Our findings offer a unique perspective on how adherence to these practices correlates to five specific weight change patterns. Even though there were differing patterns of weight change, the frequency of all three behaviors between six and eighteen months decreased for all participants. However, those participants who maintained weight loss averaged smaller percentage changes in the number of food journals, recorded days/week of physical activity, and attendance rates than those who gained weight or lost weight but subsequently relapsed. Those who were weight stable or had late weight loss had significantly less decline in their attendance rate compared to those who gained or lost weight but subsequently relapsed. This suggests that the degree of change in key behaviors may have significant influence on weight change patterns in the long term.
Overall our results are encouraging with regard to the amount of effort patients might need to expend to achieve and maintain successful weight loss. A significant number of patients achieved a 5% weight loss with a relatively low number of days per week of recorded physical activity and dietary intake, and incrementally small increases in frequency were associated with even greater odds of losing 5%. In some instances, smaller goals may be viewed as more achievable by patients, thus increasing both willingness and self-efficacy, increasing the chances for successful behavior change25–26. Clinicians may also find themselves more comfortable and confident in their ability to counsel and motivate patients towards behavior change using these modest targets, particularly given that time constraints and futility are commonly cited as barriers to weight loss counseling in daily practice27–28.
Of all of the declines in behaviors, it was of significant interest to see that weight loss was achieved and maintained with lower levels of self-reported physical activity than previously observed in other studies7,18–20. For those attempting to maintain weight loss, the most recent 2008 Physical Activity Guidelines for Americans recommends 60 minutes of moderate or 30 minutes of vigorous physical activity daily 29. The average of approximately two days per week of reported activity for weight loss maintainers in this study is well below the recommendation, yet they still achieved long term weight loss. It may be important to consider the context for this intervention when interpreting these results. Despite the decline in physical activity over time, it appears that maintenance of weight loss may be achieved with lower levels of physical activity when patients consistently practice self-monitoring of dietary intake and attend a structured group behavioral program.
In addition, our findings showed that the frequency of food journaling, recording physical activity, and attendance to group sessions at 18 months more strongly impacts long term weight change than the same frequency at six months. For example, a person recording physical activity 1–2.9 days per week has a 4 times higher odds of losing at least 5% of his or her baseline weight at six months, but a 7-fold increase in odds of losing 5% of baseline weight at 18 months. If adherence to key behaviors will most likely wane over time as seen in this study, we speculate that it may be more effective to offer aggressive counseling and interventions to reinforce adherence after the first six months of a weight loss endeavor. This is especially important because participants were on average gaining weight in the last 12 months of this intervention. This is consistent with other studies that have shown an average regain of 30–35% of weight loss during the year following treatment cessation30–31. In this study, only participants who were in the highest categories for food records, recorded days/week of physical activity, and attendance at behavioral sessions experienced weight loss in the second phase of the study. Given previous findings regarding rates of weight regain over the long term32–34, this does not come as a surprise, but rather supports the idea that over the long term, the focus should switch to prevention of weight regain and helping individuals maintain a significant level of adherence to key behaviors.
This study is not without limitations. First, physical activity was measured by participants’ self-report, which is often overestimated. However, to a large extent, self-report remains the most common way to gauge patients’ physical activity level in current clinical practice, which enhances the generalizability and applicability of our findings. Second, this study assessed the relationship between the observed behaviors and the weight change patterns; as a result, it does not imply cause and effect. While we described associations of clinically meaningful weight loss with fewer than recommended days of journaling (i.e., daily), further research is needed to determine if people randomized to differing prescriptions for journaling of physical activity and dietary intake lead to different weight change outcomes. Additionally, it will be important for future research to determine if increases or even prevention of decreases in the behaviors of self-monitoring dietary intake and physical activity along with group attendance can lead to additional weight loss at later stages (i.e., beyond the acute weight loss stage) in behavioral intervention programs. The early adoption of these behaviors may not translate into late maintenance of these behaviors for many people, potentially creating opportunities to intervene when the potential for recidivism is high. The third limitation relates to the smaller sizes of subgroups that were created based on the weight change cut points. The findings related to the groups that gained or had late weight loss may not be as generalizable as those related to the other groups.
In conclusion, our study illustrates how the frequency of three key behaviors associated with weight loss including food journaling, physical activity, and attendance at behavioral counseling sessions, are associated with weight change over both the short and long term among overweight and obese individuals. We determined that despite an overall decline in frequency of each behavior over time and a relatively infrequent average occurrence of each behavior at 18 months, the majority of participants maintained weight loss and/or prevented weight gain. In addition, our study implies that the change in behavior frequency over a particular time period may be more predictive of weight change patterns than the average behavior frequency at any single time point. Our study also suggests that a relatively small increase in any one behavior can generate a substantial increase in the odds of achieving a 5% decrease in weight. Combined, these findings offer practical and applicable considerations for weight loss counseling, particularly with regard to goal setting and long-term weight loss strategies. Small but consistent steps may be the best route to successful long term weight maintenance.
Table 3.
Baseline characteristics by weight loss categories
| Weight Gained | Weight Stable | Weight Loss & Relapse | Late Weight Loss | Weight Loss & Maintenance | |
|---|---|---|---|---|---|
| N= 18 | N= 228 | N= 74 | N= 33 | N= 154 | |
| Male (N,%) | 5, 28% | 90, 40% | 25, 34% | 11, 33% | 67, 44% |
| African American (N,%) | 10, 56% | 93, 41% | 23, 31% | 14, 32% | 34, 22% |
| Group B1 (N,%) | 9, 50% | 116, 51% | 40, 54% | 16, 49% | 74, 48% |
| Group C2 (N,%) | 9, 50% | 112, 49% | 34, 46% | 17, 51% | 80, 52% |
| Obesity (N, %) | |||||
| Overweight | 3, 17% | 66, 29% | 21, 28% | 10, 30% | 62, 40% |
| Obese I | 8, 44% | 67, 29% | 22, 30% | 15, 46% | 39, 25% |
| Obese II | 7, 39% | 95, 42% | 31, 42% | 8, 24% | 53, 35% |
| Age (mean, SD) | 44, 9.8 | 49, 9.0 | 49, 8.2 | 53, 10 | 51, 7.7 |
Treatment Group B consisted of established lifestyle recommendations
Treatment Group C consisted of established lifestyle recommendations +DASH
Footnotes
No reprints available
Disclosure
PREMIER was supported by National Institutes of Health grants UO1 HL60570, UO1 HL60571, UO1 HL60573, UO1 HL60574, and UO1 HL62828. Dr. Bartfield is supported by grant R25CA047888.
Contributor Information
Jessica K Bartfield, Department of Nutrition Sciences, University of Alabama Birmingham.
Victor Stevens, Center for Health Research, Kaiser Permanente Northwest, Portland, Oregon.
Gerald J Jerome, Department of Kinesiology, Towson University, and Johns Hopkins School of Medicine, Baltimore, MD.
Bryan C. Batch, Sarah W. Stedman Nutrition and Metabolism Center, Duke University Medical Center, Durham, North Carolina
Betty Kennedy, Pennington Biomedical Research Center, Baton Rouge, Louisiana.
William Vollmer, Center for Health Research, Kaiser Permanente Northwest, Portland, Oregon.
David Harsha, Pennington Biomedical Research Center, Baton Rouge, Louisiana.
Lawrence Appel, Johns Hopkins Medical Institutions, Baltimore, Maryland.
Renee Desmond, Department of Preventive Medicine, University of Alabama Birmingham.
Jamy D Ard, Department of Nutrition Sciences, University of Alabama Birmingham.
References
- 1.Bray, George Lifestyle and pharmacological approaches to weight loss: efficacy and safety. J Clin Endocrinol Metab. 2008;93:S81–S88. doi: 10.1210/jc.2008-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norris SL, Zhang X, Avenell A, et al. Long-term effectiveness of lifestyle and behavioral weight loss interventions in adults with type 2 diabetes: A meta-analysis. Am J of Med. 2004;117:762–774. doi: 10.1016/j.amjmed.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Stahre L, Tarnell B, Hakanson C, Hallstrom T. A randomized controlled trial of two weight-reducing short-term group treatment programs for obesity with an 18-month follow-up. Int J of Behav Med. 2007;14:48–55. doi: 10.1007/BF02999227. [DOI] [PubMed] [Google Scholar]
- 4.Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obes Res. 2004;12:151S–162S. doi: 10.1038/oby.2004.282. [DOI] [PubMed] [Google Scholar]
- 5.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1885;332:621–8. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 6.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299:853–5. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- 7.Jeffrey RW, Wing RR, Sherwood NE, Tate DF. Physical activity and weight loss: does prescribing higher physical activity goals improve outcome? Am J Clin Nutr. 2003;78:684–9. doi: 10.1093/ajcn/78.4.684. [DOI] [PubMed] [Google Scholar]
- 8.Hollis JF, Gullion CM, Stevens VJ, et al. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. Am J Prev Med. 2008;35:118–126. doi: 10.1016/j.amepre.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–90. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 10.Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A to Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969–77. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 11.Wadden TA, Foster GD. Behavioral treatment of obesity. Med Clin North Am. 2000;84:441–461. doi: 10.1016/s0025-7125(05)70230-3. [DOI] [PubMed] [Google Scholar]
- 12.National Heart Lung and Blood Institute, National Institute of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Bethesda, Maryland: National Institutes of Health; 1998. [Google Scholar]
- 13.Phelan S, Wyatt HR, Hill JO, Wing RR. Are the eating and exercise habits of successful weight losers changing? Obesity. 2006;14:710–716. doi: 10.1038/oby.2006.81. [DOI] [PubMed] [Google Scholar]
- 14.Butryn M, Phelan S, Hill JO, Wing RR. Consistent self-monitoring of weight: a key component of successful weight loss maintenance. Obesity. 2007;15:3091–3096. doi: 10.1038/oby.2007.368. [DOI] [PubMed] [Google Scholar]
- 15.Raynor DA, Phelan S, Hill JO, Wing RR. Television viewing and long-term weight maintenance: Results from the national weight control registry. Obesity. 2006;14:1816–24. doi: 10.1038/oby.2006.209. [DOI] [PubMed] [Google Scholar]
- 16.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program: Description of lifestyle modification. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sacks FM, Bray GA, Carey VJ, et al. Comparison of Weight-loss Diets with Different Compositions of Fat, Protein, and Carbohydrates. N Engl J Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakicic JM, Marcus BH, Lang W, Janney C. Effect of Exercise on 24-Month Weight Loss Maintenance in Overweight Women. Arch Intern Med. 2008;168:1550–1559. doi: 10.1001/archinte.168.14.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter GR, Brock DW, Byrne NM, Chandler-Laney PC, Del Corral P, Gower BA. Exercise Training Prevents Regain of Visceral Fat for 1 Year Following Weight Loss. Obesity. 2009 doi: 10.1038/oby.2009.316. (advance online publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tate DF, Jeffery RW, Sherwood NE, Wing RR. Long-term weight losses associated with prescription of higher physical activity goals. Are higher levels of physical activity protective against weight regain? Am J Clin Nutr. 2007;85:954–9. doi: 10.1093/ajcn/85.4.954. [DOI] [PubMed] [Google Scholar]
- 21.Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanck E. Effects of comprehensive modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289:2083–93. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 22.Elmer PJ, Obarzanek E, Vollmer WM, et al. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-month results of a randomized trial. Annals of Internal Medicine. 2006;144:485–494. doi: 10.7326/0003-4819-144-7-200604040-00007. [DOI] [PubMed] [Google Scholar]
- 23.Truesdale KP, Stevens J, Jianwen C. Nine-year changes in cardiovascular disease risk factors with weight maintenance in the atherosclerosis risk in communities cohort. Am J Epidemiol. 2007;165:890–900. doi: 10.1093/aje/kwk072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens J, Truesdale KP, McClain JE, Cai J. The definition of weight maintenance. Int J of Obes. 2006;30:391–399. doi: 10.1038/sj.ijo.0803175. [DOI] [PubMed] [Google Scholar]
- 25.Sallit J, Ciccazzo M, Dixon Z. A cognitive-behavioral weight control program improves eating and smoking behaviors in weight-concerned female smokers. J Am Diet Assoc. 2009;109(8):1398–405. doi: 10.1016/j.jada.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Teixeira PJ, Going SB, Houtkooper LB, et al. Pretreatment predictors of attrition and successful weight management in women. Int J Obes Relat Metab Disord. 2004;28(9):1124–33. doi: 10.1038/sj.ijo.0802727. [DOI] [PubMed] [Google Scholar]
- 27.Tsui JI, Dodson K, Jacobson TA. Cardiovascular disease prevention counseling in residency: resident and attending physician attitudes and practices. J Natl Med Assoc. 2004;96:1080–1083. 1088–1091. [PMC free article] [PubMed] [Google Scholar]
- 28.Kushner RF. Barriers to providing nutrition counseling by physicians: a survey of primary care practitioners. Prev Med. 1995;24:546–552. doi: 10.1006/pmed.1995.1087. [DOI] [PubMed] [Google Scholar]
- 29.U. S. Department of Health and Human Services. Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report. Washington, DC: 2008. [Google Scholar]
- 30.Wadden TA, Foster GD. Behavioral treatment of obesity. Med Clin North Am. 2000;84:441–62. doi: 10.1016/s0025-7125(05)70230-3. [DOI] [PubMed] [Google Scholar]
- 31.Kramer FM, Jeffery RW, Forster JL, Snell MK. Long-term follow-up of behavioral treatment for obesity: Patterns of weight regain in men and women. Int J Obes Relat Metab Disord. 1989;13:123–36. [PubMed] [Google Scholar]
- 32.Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21:323–41. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- 33.Wadden TA, Sternberg JA, Letizia KA, Stundard AJ, Foster GD. Treatment of obesity by very-low-calorie diet, behavior therapy, and their combination: a five-year perspective. Int J Obes. 1989;51:167–72. [PubMed] [Google Scholar]
- 34.Brownell KD, Jeffrey RW. Improving long-term weight loss: pushing the limits of treatment. Behav Ther. 1987;18:353–74. [Google Scholar]
- 35.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]