Abstract
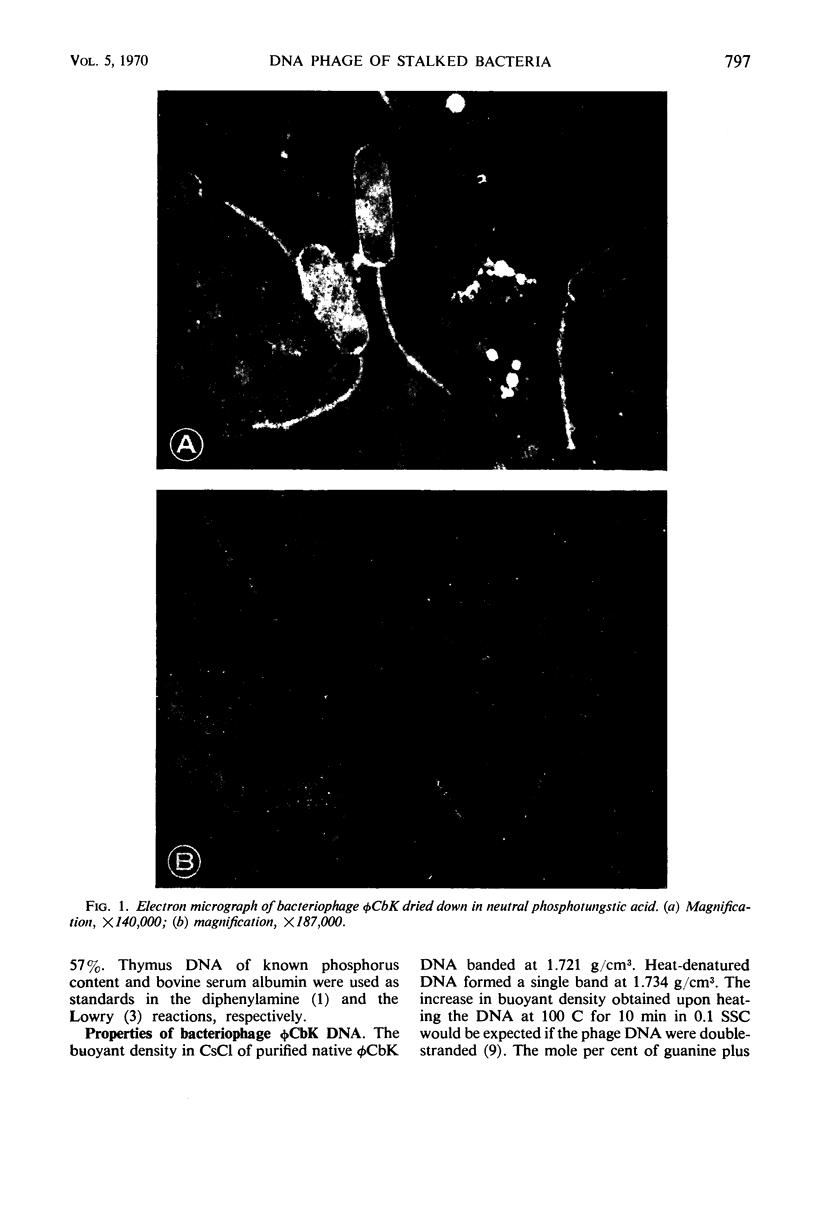
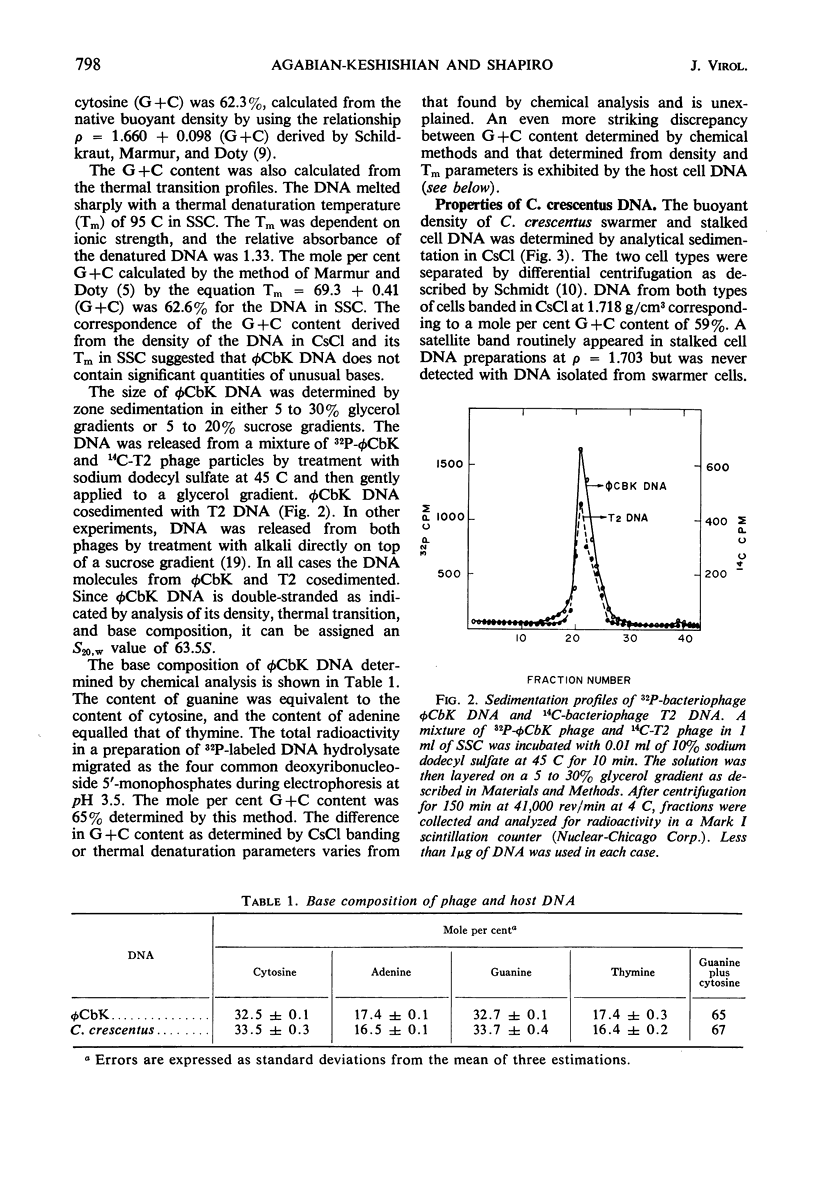
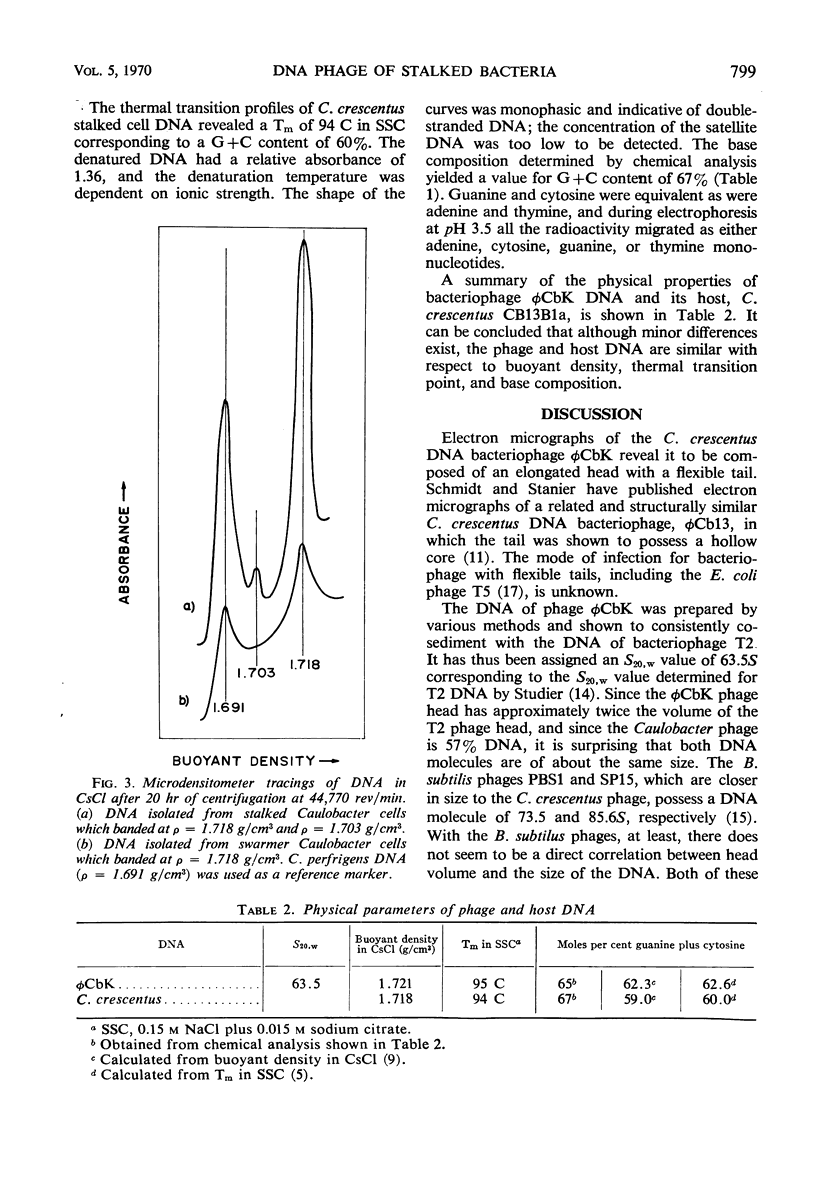
The Caulobacter crescentus bacteriophage φCbK was studied with respect to the physical and chemical properties of both the phage and its deoxyribonucleic acid (DNA). Electron micrographs reveal the phage to be among the largest DNA bacteriophages reported, with head dimensions of 64 by 195 nm and a flexible tail 275 nm in length. The phage is composed of 57% DNA. This DNA is double-stranded as indicated by (i) the sharp increase in extinction coefficient over a narrow range of temperature increase, (ii) an increase in density in CsCl upon thermal denaturation, and (iii) the equivalence of guanine and cytosine as well as adenine and thymine, determined by chemical analysis. φCbK DNA cosediments with Escherichia coli phage T2 DNA and has therefore been assigned an S20,w value of 63.5S. The size of the phage and its DNA and the percentage of DNA indicate that the φCbK phage head is relatively loosely packed. The properties of the DNA from bacteriophage φCbK are similar to those of host C. crescentus DNA with respect to buoyant density, thermal transition point, and guanine plus cytosine content.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W., Vinograd J. EQUILIBRIUM SEDIMENTATION OF MACROMOLECULES IN DENSITY GRADIENTS. Proc Natl Acad Sci U S A. 1957 Jul 15;43(7):581–588. doi: 10.1073/pnas.43.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POINDEXTER J. S. BIOLOGICAL PROPERTIES AND CLASSIFICATION OF THE CAULOBACTER GROUP. Bacteriol Rev. 1964 Sep;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- SCHMIDT J. M., STANIER R. Y. ISOLATION AND CHARACTERIZATION OF BACTERIOPHAGES ACTIVE AGAINST STALKED BACTERIA. J Gen Microbiol. 1965 Apr;39:95–107. doi: 10.1099/00221287-39-1-95. [DOI] [PubMed] [Google Scholar]
- STOVEPOINDEXTER J. L., COHEN-BAZIRE G. THE FINE STRUCTURE OF STALKED BACTERIA BELONGING TO THE FAMILY CAULOBACTERACEAE. J Cell Biol. 1964 Dec;23:587–607. doi: 10.1083/jcb.23.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Schmidt J. M. Observations on the adsorption of Caulobacter bacteriophages containing ribonucleic acid. J Gen Microbiol. 1966 Nov;45(2):347–353. doi: 10.1099/00221287-45-2-347. [DOI] [PubMed] [Google Scholar]
- Tyeryar F. J., Jr, Taylor M. J., Lawton W. D., Goldberg I. D. Cotransduction and cotransformation of genetic markers in Bacillus subtilis and Bacillus licheniformis. J Bacteriol. 1969 Nov;100(2):1027–1036. doi: 10.1128/jb.100.2.1027-1036.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS R. C., FRASER D. Morphology of the seven T-bacteriophages. J Bacteriol. 1953 Oct;66(4):458–464. doi: 10.1128/jb.66.4.458-464.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Kaiser A. D. Mapping the 5'-terminal nucleotides of the DNA of bacteriophage lambda and related phages. Proc Natl Acad Sci U S A. 1967 Jan;57(1):170–177. doi: 10.1073/pnas.57.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi H. Release of DNA from phage on the top of sucrose gradients. Biochim Biophys Acta. 1968 Oct 29;166(3):738–740. doi: 10.1016/0005-2787(68)90390-0. [DOI] [PubMed] [Google Scholar]