Abstract
Background:
Health-related quality-of-life (HRQOL) measures have been used as patient-reported outcomes in clinical trials in cystic fibrosis (CF), but there are limited data on HRQOL changes over time in adults with CF.
Methods:
The Project on Adult Care in Cystic Fibrosis, a prospective, longitudinal panel study of 333 adults with CF at 10 CF centers in the United States, administered a disease-specific HRQOL measure, the Cystic Fibrosis Questionnaire-Revised (CFQ-R), seven times over 21 months. The CFQ-R assesses both physical and psychosocial domains of health. Growth curve regression models were developed for each CFQ-R domain, adjusting for demographic and clinical characteristics.
Results:
Between 205 and 303 adults completed surveys (response rate, 70%-93%). Mean age at baseline was 33 years (range, 19-64 years); mean FEV1 % predicted was 59.8% (SD, 22%). Over the 21 months of follow-up, lung function, frequency of pulmonary exacerbations, and nutritional indices were associated with physical CFQ-R domain scores. There were no significant population trends over time in the physical domain scores; however, there were population time trends in three psychosocial domains: treatment burden (+8.9 points/y), emotional functioning (+3.2 points/y), and social functioning (−2.4 points/y). Individual variation in both physical and psychosocial subscales was seen over 21 months.
Conclusions:
In a longitudinal multicenter population of adults with CF, clinical variables such as FEV1, exacerbation frequency, and weight were correlated with related CFQ-R subscales. For the population as a whole, the physical domains of CFQ-R, such as respiratory symptoms, were stable. In contrast, population changes in several psychosocial domains of CFQ-R suggest that differentiating between the physical and the psychosocial trajectories in health among adults with CF is critical in evaluating patient-reported outcomes.
More than 40% of the current US cystic fibrosis (CF) patient population is over the age of 18, and the median predicted survival has now reached > 36 years.1 As CF has become a chronic, though life-limiting, illness in young adults, there is increasing interest in assessing and improving the quality of life for adults with CF. Assessment of health-related quality of life (HRQOL) is seen as an adjunct to the standard measures of disease severity, such as FEV1, BMI, and survival. The emphasis on patient-reported outcomes has led to an increased use of measures of HRQOL as outcomes in clinical trials.2,3
The Cystic Fibrosis Questionnaire-Revised (CFQ-R) is a validated, disease-specific HRQOL measure of both the physical and the psychosocial aspects of health.4‐6 The physical HRQOL domains include patient-reported assessments of respiratory and digestive symptoms, global health perceptions, and physical functioning, whereas the psychosocial domains include areas such as perceived burden of treatment and emotional and social functioning. Prior cross-sectional studies have focused on the relationship of sex, lung function, or frequency of pulmonary exacerbations with the different CFQ-R domains.7‐10 HRQOL scores have also been associated with survival in CF.11
Few studies have examined the change in the physical and psychosocial domains of HRQOL over longer periods of time. Longitudinal assessments of CFQ-R scores, particularly those in the respiratory domains, have been used as patient-reported outcomes for several CF therapeutic clinical trials.12‐15 A longitudinal study spanning 18 months using a non-CF specific HRQOL instrument demonstrated stability over time in both general and health-related domains.16 In contrast, longer-term observational studies of CFQ-R scores in CF populations have not been reported. An analysis of the Epidemiologic Study of Cystic Fibrosis showed that CFQ-R scores were stable over a 12-month, two-time-point assessment.17 To address this gap, as part of the Project on Adult Care in Cystic Fibrosis (PAC-CF), we collected longitudinal data on HRQOL using the CFQ-R in a large cohort of adults with CF at 10 geographically diverse CF care centers in the United States. Our overarching goal was to describe the trajectories of CFQ-R scores over a longer time frame in a nonclinical trial population of adults with CF. By extending the observation time and the frequency of administration of the CFQ-R in our cohort, our specific aims were to identify factors associated with baseline CFQ-R scores and to examine trends over time among each of the CFQ-R domains. We hypothesized that both clinical and sociodemographic factors would influence baseline CFQ-R scores and that changes in CFQ-R scores over time would be variable, based on the type of HRQOL assessed by each domain.
Materials and Methods
Participants
Participants in the current study were enrolled in PAC-CF, a prospective, longitudinal panel study of adults with CF. Adults 18 years of age or older receiving care at one of 10 participating CF centers were eligible for the study (e-Appendix 1 (437.3KB, pdf) ). The study coordinator at each participating center provided, without any identifying information, age, sex, and the following clinical variables from 2003: maximum FEV1 % predicted, weight-for-age z score, frequency of pulmonary exacerbations, presence of diabetes, colonization with Burkholderia cepacia complex and Staphylococcus aureus, and pancreatic sufficiency. These data were used to calculate each individual’s predicted probability of surviving 5 years, using the validated prognostic model of Liou et al.18 All adults with a predicted probability of 5-year survival of less than 0.975 (n = 515) and a randomly selected 25% of adults with a predicted probability of 5-year survival of 0.975 or higher (n = 60) were recruited for the study beginning in fall 2004. This stratified sampling design was adopted because adults with a very high predicted probability of survival were the least informative with respect to the overall PAC-CF goal of examining trends over time in quality of life as CF progresses. In other words, adults whose CF was least advanced were sampled at a lower rate because their CF was less likely to progress to moderate or severe during the 3-year time frame of the study. A total of 333 adults with CF enrolled in the study (301 with predicted survival ≤ 0.975 and 32 with predicted survival > 0.975). Compared with adults with CF who were asked to participate in PAC-CF but either refused or could not be contacted (n = 242), the 333 adults who enrolled in PAC-CF were more likely to be white, women, and older. Clinically, participants had, on average, better weight-for-age z scores and a higher number of exacerbations. By the seventh survey wave of the study (21 months after the first survey wave), a total of 46 participants were dropped from data collection because of death (n = 17), transplant (n = 18), personal decision (n = 9), or administrative difficulty (n = 2). The study protocol was approved by the institutional review boards at Education Development Center, Inc and by the 10 participating CF centers.
Measures
The data presented in this study were collected over a period of seven waves of PAC-CF. Participants were mailed surveys in April 2005, July 2005, October 2005, January 2006, May 2006, October 2006, and January 2007. The CFQ-R5,6 was included in each survey; it consists of 50 questions generating standardized scores from 0 to 100 in the following domains: physical functioning, body image, eating disturbances, digestive symptoms, respiratory symptoms, weight, health perceptions, vitality, treatment burden, emotional functioning, social functioning, and role. Higher scores in each domain of the CFQ-R indicate better HRQOL. Clinical information was provided by the CF centers. Because the clinical data were collected at routine clinical visits, they were not necessarily coordinated with the mail-in survey collection time points. Because most adults with CF are seen on a quarterly basis in their respective CF care centers, the clinical data collected were generally within 3 months of the surveys. For the first survey wave, 98% of subjects had clinical data within the 12-week window, and by the sixth survey wave of the study, > 75% of survey data remained within the 12-week window of clinical data from the Cystic Fibrosis Foundation patient registry. Allowing response to the survey waves outside the clinical setting was also thought to reduce the possibility of response bias, particularly due to social desirability.
For this analysis, the number of pulmonary exacerbations (defined as episodes requiring IV antibiotics either inpatient or at home) was calculated as the number of exacerbations since the previous survey wave. For the first wave, the number of exacerbations between January and April 2005 was used as a baseline. The FEV1 (% predicted) and weight percentile used in the analysis were the values that were closest to the survey completion date and within 12 weeks of survey completion. If an individual did not have a recorded FEV1 % predicted or weight percentile within 12 weeks of the survey completion date, the mean level of the clinical variable was calculated for that individual over the previous year. For each survey, data on CF-related complications and airway microbiology for the current survey year were used. Sociodemographic data (age, sex, ethnicity, race, marital status, education, employment, and income) were collected during the first survey. Educational attainment was dichotomized, with a higher education level coded for individuals with a post-high school degree. To account for the stratified sampling design of PAC-CF, a dichotomous design variable was coded so that the higher value indicated a predicted probability of 5-year survival of 0.975 or higher.
Statistical Methods
Descriptive statistics were calculated at all seven waves for the HRQOL and clinical measures by using sample selection weights to adjust for the disproportionate stratified sampling design. Individual growth curve models were fit to each HRQOL scale. Both linear and quadratic terms were tested (with time calculated in years). A design variable (to account for the stratified sampling design) and demographic variables were forced into the model. Clinical variables were tested as predictors (both main effect and interaction terms) using a stepwise procedure. Nonsignificant clinical variables were removed from the final models. An effect size measure for each regression model (pseudo-R2 value) was computed as an overall summary of the proportion of variance of the HRQOL scale that was explained by the time, design, demographic, and clinical variables. These pseudo-R2 values were calculated by squaring the correlation between the observed and predicted HRQOL values.19 For all tests, a P value of < .05 was considered statistically significant. All analyses were performed with SAS software, version 9.1 (SAS Institute Inc).
Results
Study Demographics
Completed surveys were received from between 205 and 303 adults during various survey waves (response rate between 70% and 93%). The mean age of respondents at wave 1 was 33 years old (median, 31 years; range, 19-64 years). Fifty-five percent of respondents were female; 55% had completed a post-high school degree. Additional demographic data are provided in Table 1. Tables 1 and 2 also provide descriptive statistics for clinical measures and HRQOL scales, respectively, at all seven waves.
Table 1.
—Descriptive Statistics for Demographic and Clinical Variables for Each PAC-CF Survey
| Variables | Time, mo |
||||||
| Baseline | 3 | 6 | 9 | 13 | 18 | 21 | |
| Age, y | 32.52 (10.65) | … | … | … | … | … | … |
| Female | 55 | … | … | … | … | … | … |
| White | 94 | … | … | … | … | … | … |
| Hispanic/Latino | 4 | … | … | … | … | … | … |
| Currently married | 43 | … | … | … | … | … | … |
| Completed post-high school degree | 55 | … | … | … | … | … | … |
| Employed or student | 59 | … | … | … | … | … | … |
| Annual household income | |||||||
| ≤ $25,000 | 23 | … | … | … | … | … | … |
| $25,001-$49,999 | 14 | … | … | … | … | … | … |
| $50,000-$74,999 | 16 | … | … | … | … | … | … |
| ≥ $75,000 | 29 | … | … | … | ... | … | … |
| No response | 18 | … | … | … | … | … | … |
| Weight percentile | 42.04 (29.81) | 41.29 (29.13) | 42.22 (29.63) | 42.10 (29.54) | 42.35 (29.70) | 43.12 (28.88) | 42.86 (29.16) |
| FEV1 % predicted | 59.76 (22.40) | 60.33 (22.90) | 59.44 (23.61) | 58.93 (21.73) | 59.06 (22.56) | 57.93 (22.20) | 57.89 (22.46) |
| Exacerbations, No. | 0.65 (0.99) | 0.36 (0.77) | 0.40 (0.71) | 0.34 (0.66) | 0.66 (1.22) | 0.42 (0.86) | 0.35 (0.86) |
| Pancreatic sufficient | 12 | 13 | 12 | 11 | 11 | 12 | 14 |
| With CF-related diabetes | 15 | 15 | 15 | 23 | 21 | 23 | 22 |
| With Staphylococcus aureus | 35 | 38 | 41 | 40 | 44 | 44 | 45 |
| With Burkholderia cepacia | 8 | 9 | 9 | 10 | 10 | 11 | 10 |
| With Pseudomonas aeruginosa | 77 | 79 | 80 | 83 | 83 | 82 | 82 |
Data are presented as mean (SD) or %. Descriptive statistics were calculated using sample selection weights to adjust for the disproportionate stratified sampling design. CF = cystic fibrosis; PAC-CF = Project on Adult Care in Cystic Fibrosis.
Table 2.
—Descriptive Statistics for HRQOL Scores at Each PAC-CF Survey
| CFQ-R Domain | Time, mo |
||||||
| Baseline | 3 | 6 | 9 | 13 | 18 | 21 | |
| Physical domains | |||||||
| Physical functioning | 57.63 (26.81) | 59.44 (27.03) | 56.37 (28.19) | 56.24 (27.86) | 56.77 (28.21) | 54.71 (28.50) | 55.62 (28.04) |
| Body image | 63.85 (26.57) | 66.74 (25.49) | 64.35 (24.16) | 66.21 (25.37) | 68.04 (25.52) | 67.05 (25.79) | 67.16 (25.74) |
| Eating disturbances | 86.16 (20.07) | 84.48 (21.05) | 83.57 (20.95) | 83.60 (21.29) | 84.27 (20.52) | 83.78 (21.11) | 81.68 (24.12) |
| Digestive symptoms | 72.30 (20.28) | 75.39 (19.14) | 76.10 (18.89) | 74.93 (19.36) | 74.42 (19.09) | 74.33 (18.68) | 75.50 (16.81) |
| Respiratory symptoms | 55.69 (20.66) | 55.82 (21.45) | 54.40 (21.50) | 54.81 (21.59) | 55.83 (20.89) | 54.61 (20.81) | 56.34 (20.30) |
| Weight | 68.92 (38.81) | 69.56 (37.43) | 73.00 (35.04) | 71.97 (37.45) | 73.14 (35.64) | 71.54 (36.89) | 72.64 (35.58) |
| Health perceptions | 56.02 (23.77) | 59.06 (23.55) | 55.85 (22.68) | 55.42 (24.44) | 58.25 (23.07) | 56.85 (23.27) | 56.25 (21.55) |
| Vitality | 50.56 (19.05) | 50.32 (18.85) | 47.51 (21.19) | 47.15 (20.61) | 49.86 (20.01) | 45.69 (18.87) | 47.92 (18.81) |
| Psychosocial domains | |||||||
| Treatment burden | 55.64 (22.50) | 56.00 (22.92) | 54.03 (22.62) | 51.93 (22.83) | 51.84 (22.76) | 52.36 (22.09) | 51.06 (20.62) |
| Emotional functioning | 67.23 (20.29) | 69.63 (20.13) | 67.07 (20.57) | 67.00 (21.18) | 70.83 (19.26) | 67.94 (18.11) | 69.51 (20.13) |
| Social functioning | 64.52 (19.27) | 63.82 (16.41) | 62.08 (18.19) | 60.19 (18.48) | 62.52 (18.38) | 59.69 (18.60) | 57.78 (18.84) |
| Role functioning | 73.25 (23.74) | 75.28 (23.11) | 72.53 (22.64) | 70.40 (26.11) | 73.84 (22.33) | 72.12 (21.62) | 72.49 (22.13) |
Data are presented as mean (SD). Descriptive statistics were calculated using sample selection weights to adjust for the disproportionate stratified sampling design. CFQ-R = Cystic Fibrosis Questionnaire-Revised; HRQOL = health-related quality of life. See Table 1 legend for expansion of other abbreviation.
Population Trends in Physical HRQOL Domains
Table 3 presents the population-level results of the individual growth curve models for the physical HRQOL domains. On average (across subjects), there were no significant linear or quadratic growth trends for any of the physical HRQOL domains. Subgroup analyses within sex, age, and FEV1 categories also supported a lack of growth trends in the physical domains. However, there was considerable individual variation in trends over time regarding these domains of HRQOL. Table 4 presents the fifth and 95th percentiles of the distribution of individual linear slopes for the physical HRQOL domains that did not have significant linear slopes at the population level.
Table 3.
—Population Trends for Physical HRQOL Domains
| Variables | Physical Functioning | Body Image | Eating Disturbances | Digestive Symptoms | Respiratory Symptoms | Weight | Health Perception | Vitality |
| Subjects, No. | 278 | 278 | 277 | 277 | 277 | 277 | 278 | 278 |
| Observations, No. | 1,444 | 1,447 | 1,443 | 1,442 | 1,440 | 1,435 | 1,442 | 1,449 |
| Intercept | 53.67a | 42.45a | 76.99a | 77.82a | 41.62a | 20.26b | 43.48a | 47.47a |
| Time, y | … | … | … | … | … | … | … | … |
| Design | −3.05 | −2.67 | 0.52 | −6.48 | −2.61 | −6.01 | −3.25 | −6.37 |
| Sociodemographic | ||||||||
| Age | −0.52a | −0.06 | −0.11 | −0.12 | 0.05 | −0.03 | −0.20 | −0.12 |
| Female | −12.69a | 9.80a | −4.26 | −2.25 | −3.98 | 10.17b | 0.43 | −4.26c |
| Higher education | 2.50 | 3.92 | 2.18 | 4.95c | 0.29 | 5.52 | 1.76 | 1.99 |
| Clinical | ||||||||
| FEV1 % predicted | 0.46a | 0.10c | 0.14b | … | 0.26a | 0.32a | 0.27a | 0.16a |
| Weight % | … | 0.25a | 0.10b | … | … | 0.62a | 0.08c | … |
| Pancreatic sufficient | … | 13.18c | … | 9.00c | … | … | … | … |
| No. exacerbations | −3.10a | −1.56a | −2.22a | … | −1.32b | −3.07a | −1.66a | −2.11a |
| Pseudo-R2 | 0.25 | 0.19 | 0.05 | 0.03 | 0.08 | 0.26 | 0.10 | 0.04 |
Models were adjusted for a design variable (a dichotomous indicator based on predicted probability of 5-y survival of 0.975 or higher). The presence of CF-related diabetes and the presence of Staphylococcus aureus, Burkholderia cepacia, and Pseudomonas aeruginosa were not statistically significant predictors of any of the physical HRQOL domains; therefore, these variables were deleted from all the models using a stepwise procedure. Other clinical variables were also deleted from the final models when nonsignificant. No interaction terms with time were included in the models for the HRQOL physical domains because there were no significant linear trends for any of these variables. See Table 1 and 2 legends for expansion of abbreviations.
P < .001.
P < .01.
P < .05.
Table 4.
—Individual Variability in Longitudinal Changes for Physical HRQOL Domains
| CFQ-R Domain | 5th Percentile | 95th Percentile |
| Physical functioning | −5.37 | 5.43 |
| Body image | −7.78 | 8.12 |
| Eating disturbances | −5.82 | 2.86 |
| Digestive symptoms | −3.62 | 4.07 |
| Respiratory symptoms | −3.01 | 3.82 |
| Weight | −6.44 | 8.92 |
| Health perception | −4.52 | 4.17 |
| Vitality | −3.12 | 2.12 |
See Table 2 legend for expansion of abbreviations.
Despite the absence of population-level trends, certain demographic and clinical variables were associated with an individual’s mean HRQOL over time (Table 3). Demographic results showed that older age was associated with decreased physical functioning. Females had significantly lower scores in physical functioning, vitality, and respiratory symptoms, and higher scores in body image and weight. Having received a post-high school degree was related to fewer reported digestive symptoms.
Several consistent patterns were noted regarding the effect of clinical variables on physical HRQOL. Both FEV1 and the number of pulmonary exacerbations were significantly related to all respiratory-related physical domains of HRQOL. Higher FEV1 was associated with higher quality of life scores in all cases (ie, a change of 5% in FEV1 was associated with a change ranging from 0.5 to 2.3 points in HRQOL). Conversely, pulmonary exacerbations were associated with lower quality-of-life scores in all cases (ie, each exacerbation was associated with a decrease in HRQOL ranging from 1.3 to 3.1 points). An individual’s weight was positively associated with the HRQOL domains of body image, eating disturbances, weight, and health perceptions. Finally, pancreatic sufficiency was associated with improved scores in the body image and digestive symptoms domains. Pseudo-R2 values reported in Table 3 indicate that the overall proportion of variance of the physical HRQOL variables that was accounted for by the inclusion of the design, demographic, and clinical variables ranged from 3% to 26%.
Population Trends in Psychosocial HRQOL Domains
Table 5 presents the population-level results of the growth curve models for psychosocial HRQOL domains. There were significant linear time trends (over 21 months) for emotional functioning, social functioning, and treatment burden. No significant time trend was observed in role functioning. No domain showed significant quadratic trends.
Table 5.
—Population Trends for Psychosocial HRQOL Domains
| Variables | Role Functioning | Treatment Burden | Emotional Functioning | Social Functioning |
| Subjects, No. | 278 | 278 | 278 | 277 |
| Observations, No. | 1,441 | 1,443 | 1,448 | 1,441 |
| Intercept | 67.76a | 37.52a | 62.06a | 74.97a |
| Time, y | … | 8.89b | 3.22b | −2.42a |
| Design | −6.13 | −0.93 | −1.00 | 1.71 |
| Sociodemographic | ||||
| Age | −0.20 | 0.30c | 0.06 | −0.35a |
| Female | −2.90 | −2.31 | 1.30 | −2.97 |
| Higher education | 5.13c | −1.76 | 6.26c | 1.41 |
| Clinical | ||||
| FEV1 % predicted | 0.22a | 0.17b | … | … |
| Weight % | … | … | … | … |
| Pancreatic sufficient | … | … | … | 9.66b |
| No. exacerbations | −4.16a | −1.14c | −1.27b | −1.03b |
| Interaction terms | ||||
| Age × time | … | −0.15c | … | … |
| Female × time | … | … | … | … |
| Post-high school degree × time | … | … | −3.28c | … |
| FEV1 % predicted × time | … | −0.11b | … | … |
| Pseudo-R2 | 0.12 | 0.04 | 0.03 | 0.03 |
Models were adjusted for a design variable (a dichotomous indicator based on predicted probability of 5-y survival of 0.975 or higher). The presence of CF-related diabetes and the presence of Staphylococcus aureus, Burkholderia cepacia, and Pseudomonas aeruginosa were not statistically significant predictors of any of the psychosocial HRQOL domains; therefore, these variables were deleted from all the models using a stepwise procedure. Other clinical variables were also deleted from the final models when nonsignificant. Interaction terms with time were included in the models for which a significant time trend was found. Only statistically significant interaction terms are included in the table, because nonsignificant interaction terms were removed from the models using a stepwise procedure. See Table 1 and 2 legends for expansion of abbreviations.
P < .001.
P < .01.
P < .05.
The main effects for the demographic and clinical variables (top half of Table 5) are interpreted as predictors of initial status (as measured at baseline). For these domains, there were no differences in the initial reporting of psychosocial HRQOL for individuals with differing probabilities of 5-year survival. In addition, there were no sex effects. Having received a post-high school degree was related to higher initial emotional functioning, and being older was related to lower initial treatment burden and decreased social functioning. Higher FEV1 was associated with lower initial treatment burden. On the other hand, pulmonary exacerbations were associated with higher initial treatment burden and with lower initial scores in emotional functioning, role functioning, and social functioning. Pancreatic sufficiency was associated with higher initial social functioning.
The lower portion of Table 5 reports three significant interaction terms between predictor variables and time. In these cases, age, education, and FEV1 were significant predictors of both initial status and the trend over time in HRQOL. Pseudo-R2 values reported in Table 5 indicate that the overall proportion of variance of the psychosocial HRQOL variables that was accounted for by the inclusion of the time, design, demographic, and clinical variables ranged from 3% to 12%.
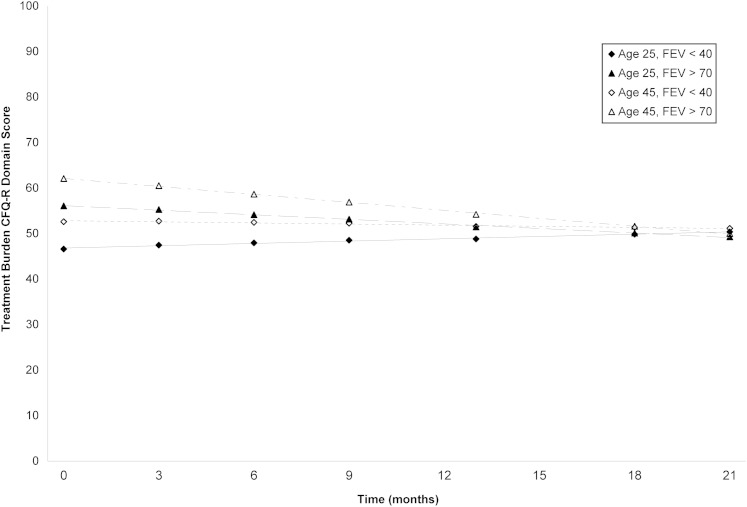
In our final models, the treatment burden domain had two significant interaction terms: age and lung function. Figure 1 presents the four trend lines for the treatment burden domain, stratified by age and FEV1, and shows that both the direction and magnitude of the trend depended on these variables. Younger individuals tended to report higher treatment burden initially. Furthermore, individuals who began the study with higher levels of FEV1 tended to experience an increase in treatment burden over the course of the study, whereas individuals who began the study with lower levels of FEV1 either remained steady or slightly decreased regarding treatment burden.
Figure 1.
Average linear trend lines for the treatment burden health-related quality of life domain, stratified according to age and FEV1. CFQ-R = Cystic Fibrosis Questionnaire-Revised.
Discussion
Monitoring HRQOL adds additional information to routine assessments in chronic conditions such as CF. Understanding the natural history of HRQOL in a CF population allows for a more accurate interpretation of such assessments over time. In this study, we prospectively evaluated HRQOL in a large observational cohort of adults with CF. We found that although individual variation exists, at the population level there is no significant change over time in physical HRQOL. In contrast, several measures of psychosocial HRQOL do change over time.
We found that lung function, sex, pulmonary exacerbations, and weight all impacted baseline HRQOL, similar to the findings of prior published studies.6,8‐10 Not surprisingly, baseline lung function was correlated with all measures of physical HRQOL related to respiratory health. Similarly, higher weight was associated with improved scores in the domains related to nutritional health (body image and weight) but also to improved scores in health perceptions. Female sex was associated with higher scores in body image and weight scales; such sex differences may result from men with CF identifying body dysmorphisms related to nutritional deficits rather than women perceiving improved health status related to nutritional outcomes. Prior literature suggests worse health outcomes in women with CF, and, outside the body image and weight domains, we found that women reported lower physical functioning and vitality scores. Future work examining the reasons underlying such sex differences in outcomes in CF is still warranted. It was also particularly striking that female sex, lower lung function, and more pulmonary exacerbations were associated with reductions in physical functioning CFQ-R scores, particularly because a physical functioning scale on a similar HRQOL instrument (the CF-QOL) was predictive of mortality.11 A HRQOL domain such as respiratory symptoms may be critically important in relation to more current functioning, but physical functioning assessments may reflect a longer-term progression of disease. Future studies should pay closer attention to the impact of interventions on changes in physical functioning scores.
Although longitudinal assessment of HRQOL in CF has been evaluated previously using non-disease-specific QOL scales,16 ours is the first, to our knowledge, to evaluate a CF-specific HRQOL measure, the well-validated, reliable CFQ-R,6 in a multicenter observational cohort using multiple assessments over a long time period. Similar to a 12-month, two-time-point analysis of CFQ-R scores in a large observational study in CF,17 our longitudinal data show no population trends over 21 months of repeated assessments in physical CFQ-R domains. This suggests that adults with CF have an established baseline physical HRQOL that does not vary significantly over 21 months. The stability of HRQOL over time at a population level may also reflect adaptive behavior employed by adults with CF, including coping strategies to reduce disease and treatment-related distress, to maintain a subjective well-being and functioning. The stability of HRQOL we observed was present in adults, suggesting that a baseline HRQOL is established before adulthood, and future studies need to extend longitudinal assessments of HRQOL into the adolescent population to evaluate when such a population baseline is established. It must be noted that despite population-level stability in HRQOL over time, we did observe significant variability at the patient level. Further studies are needed to evaluate the differences in individuals with stable HRQOL compared with those with either improving or worsening physical HRQOL over time.
The stability of population-level physical CFQ-R scores over time demonstrates little response shift in this HRQOL instrument. Therefore, our data suggest that detecting population changes in HRQOL reflects a meaningful shift in patient outcomes above and beyond what would be expected based on secular trends over time. The respiratory symptom subscale of the CFQ-R, for example, has been used as a clinical patient-reported end point in multiple trials,12‐15 with a minimal clinically important difference established at four points.20 The lack of change in the respiratory symptom subscale over 21 months in our non-clinical-trial-based population lends additional support to the use of this subscale as a patient-reported outcome when assessing therapeutic or programmatic and clinical interventions.
In recent years, the psychosocial impact of CF has garnered increasing attention. Adult CF care guidelines have advocated careful attention to mental health,21 and a large, multicenter, international observational CF study demonstrated depressive and anxious symptoms at rates far higher than in the general population.22 Cross-sectional studies have shown that the psychologic symptom burden is high in adults with CF and is associated with lower HRQOL and worse adherence to chronic therapies.23 Measures of coping have also been linked previously to HRQOL in CF.24 In this study, we found that pulmonary exacerbations were associated with lower scores in all psychosocial CFQ-R domains at baseline, suggesting that exacerbations not only impact physical health but also appear to have a very important influence on an adult’s ability to perform daily roles, such as working, developing family and peer relationships, or attending school. We also found that, in contrast to physical CFQ-R domains, the psychosocial CFQ-R domains showed significant changes over time in our population. Specifically, we found that, over time, adults with CF reported decreased scores in social functioning, yet improved emotional functioning. Although we are not able to identify other factors that could have led to changes in social functioning, one possibility could be increased application of infection control policies throughout CF care centers, leading to increased social isolation among adults with CF. Similarly, increases in emotional functioning over time could reflect adaptation to chronic disease. Future studies should aim to identify the factors that impact such changes in psychosocial health over time among adults with CF.
Treatment burden is becoming an increasingly important issue with the introduction of new therapies and the increasing survival of individuals with CF.25 Treatment burden in adults with CF is high, with most adults reporting > 2 h per day of routine maintenance therapies.26 Therapies recommended by evidence-based guidelines often are initiated well before adulthood and are generally additive throughout the lifespan of an individual with CF.27 In this context, therefore, it is interesting that we identified significant time trends in the treatment burden domain of the CFQ-R. In our study, younger adults with lower lung function reported the highest treatment burden at baseline, reflecting the higher demands of chronic therapy in a sicker population. It is interesting, therefore, that we found a significant decrease in reported treatment burden over time. We assume that most adults did not substantially change their treatment regimen over this time frame. It is likely that this improvement in perceived treatment burden reflects adaptation, or perhaps scale recalibration,25 to complex treatment demands, regardless of baseline severity or age. Further studies are needed to more fully understand individual determinants of perceived treatment burden in CF.
Our study has several limitations. Because of the mechanism of clinical data collection via participating CF centers, our survey data are not necessarily synchronous to clinical changes. However, because adults with CF are typically seen at their care centers on a quarterly basis, the lag time between clinical data and survey data was generally within 12 weeks, a short period of time in which the majority of individuals would maintain clinical stability. We also believed that allowing HRQOL assessments to occur outside routine clinical visits would reduce potential response bias by exerting less social desirability pressure among respondents. In addition, the recruitment strategy for PAC-CF was skewed by design toward adults with more severe disease and, thus, our results may not be generalizable to all adults with CF. However, the average lung function of our cohort was only slightly lower than the US average based on the Cystic Fibrosis Foundation Registry report.1 Finally, as with most observational studies, the R2 values of most of our models are quite low, and there are likely other variables not included in our data collection that impact HRQOL in adults with CF over time.
Conclusions
In summary, this longitudinal multicenter study of adults with CF demonstrated that physical HRQOL domains such as respiratory symptoms remain stable over time, whereas the psychosocial domains of HRQOL such as treatment burden and social functioning change over 21 months. Differentiating between the physical and psychosocial trajectories in health among adults with CF is, therefore, critical in designing interventions to improve patient outcomes. Given the stability of HRQOL over time, the assessment of HRQOL in a clinical setting would be valuable if conducted over longer intervals, such as annually, or with significant changes in individual health status, but likely would not be of clinical use if assessed more frequently. Such assessments could be used to inform discussions with individual patients with CF to identify specific needs and points for intervention. At the population level, assessing for changes in HRQOL over time would augment existing clinical assessments and population-based outcome tools and help direct management decisions for the care of adults with CF.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Dr Sawicki had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Dill: contributed to the concept and design of the study, data interpretation and analysis, drafting of the manuscript, and final approval of the version to be published.
Dr Dawson: contributed to the concept and design of the study, data interpretation and analysis, revision of the manuscript, and final approval of the version to be published.
Dr Sellers: contributed to the concept and design of the study, data interpretation and analysis, revision of the manuscript, and final approval of the version to be published.
Dr Robinson: contributed to the concept and design of the study, data interpretation, drafting of the manuscript, and final approval of the version to be published.
Dr Sawicki: contributed to the concept and design of the study, data interpretation, drafting of the manuscript, and final approval of the version to be published.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendix can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- CF
cystic fibrosis
- CFQ-R
Cystic Fibrosis Questionnaire-Revised
- HRQOL
health-related quality of life
- PAC-CF
Project on Adult Care in Cystic Fibrosis
Footnotes
Funding/Support: This work was supported by a grant from the National Heart, Lung, and Blood Institute [R01 HL72938].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Cystic Fibrosis Foundation Cystic Fibrosis Foundation Patient Registry Annual Data Report for 2009. Bethesda, MD: Cystic Fibrosis Foundation; 2010 [Google Scholar]
- 2.Abbott J, Hart A. Measuring and reporting quality of life outcomes in clinical trials in cystic fibrosis: a critical review. Health Qual Life Outcomes. 2005;3(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goss CH, Quittner AL. Patient-reported outcomes in cystic fibrosis. Proc Am Thorac Soc. 2007;4(4):378-386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Modi AC, Quittner AL. Validation of a disease-specific measure of health-related quality of life for children with cystic fibrosis. J Pediatr Psychol. 2003;28(8):535-545 [DOI] [PubMed] [Google Scholar]
- 5.Quittner AL, Buu A, Messer MA, Modi AC, Watrous M. Development and validation of The Cystic Fibrosis Questionnaire in the United States: a health-related quality-of-life measure for cystic fibrosis. Chest. 2005;128(4):2347-2354 [DOI] [PubMed] [Google Scholar]
- 6.Quittner AL, Sawicki GS, McMullen A, et al. Erratum to: Psychometric evaluation of the Cystic Fibrosis Questionnaire-Revised in a national, US sample. Qual Life Res. 2012;21(7):1279-1290 [DOI] [PubMed] [Google Scholar]
- 7.Arrington-Sanders R, Yi MS, Tsevat J, Wilmott RW, Mrus JM, Britto MT. Gender differences in health-related quality of life of adolescents with cystic fibrosis. Health Qual Life Outcomes. 2006;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Britto MT, Kotagal UR, Hornung RW, Atherton HD, Tsevat J, Wilmott RW. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest. 2002;121(1):64-72 [DOI] [PubMed] [Google Scholar]
- 9.Gee L, Abbott J, Conway SP, Etherington C, Webb AK. Quality of life in cystic fibrosis: the impact of gender, general health perceptions and disease severity. J Cyst Fibros. 2003;2(4):206-213 [DOI] [PubMed] [Google Scholar]
- 10.Yi MS, Tsevat J, Wilmott RW, Kotagal UR, Britto MT. The impact of treatment of pulmonary exacerbations on the health-related quality of life of patients with cystic fibrosis: does hospitalization make a difference? J Pediatr. 2004;144(6):711-718 [DOI] [PubMed] [Google Scholar]
- 11.Abbott J, Hart A, Morton AM, Dey P, Conway SP, Webb AK. Can health-related quality of life predict survival in adults with cystic fibrosis? Am J Respir Crit Care Med. 2009;179(1):54-58 [DOI] [PubMed] [Google Scholar]
- 12.Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;354(3):241-250 [DOI] [PubMed] [Google Scholar]
- 13.McCoy KS, Quittner AL, Oermann CM, Gibson RL, Retsch-Bogart GZ, Montgomery AB. Inhaled aztreonam lysine for chronic airway Pseudomonas aeruginosa in cystic fibrosis. Am J Respir Crit Care Med. 2008;178(9):921-928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramsey BW, Davies J, McElvaney NG, et al. ; VX08-770-102 Study Group A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365(18):1663-1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Retsch-Bogart GZ, Quittner AL, Gibson RL, et al. Efficacy and safety of inhaled aztreonam lysine for airway pseudomonas in cystic fibrosis. Chest. 2009;135(5):1223-1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldbeck L, Zerrer S, Schmitz TG. Monitoring quality of life in outpatients with cystic fibrosis: feasibility and longitudinal results. J Cyst Fibros. 2007;6(3):171-178 [DOI] [PubMed] [Google Scholar]
- 17.Sawicki GS, Rasouliyan L, McMullen AH, et al. Longitudinal assessment of health-related quality of life in an observational cohort of patients with cystic fibrosis. Pediatr Pulmonol. 2011;46(1):36-44 [DOI] [PubMed] [Google Scholar]
- 18.Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153(4):345-352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singer JDW, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York, NY: Oxford University Press; 2003 [Google Scholar]
- 20.Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire-Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest. 2009;135(6):1610-1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yankaskas JR, Marshall BC, Sufian B, Simon RH, Rodman D. Cystic fibrosis adult care: consensus conference report. Chest. 2004;125(1)(suppl):1S-39S [DOI] [PubMed] [Google Scholar]
- 22.Goldbeck L, Besier T, Hinz A, Singer S, Quittner AL; TIDES Group Prevalence of symptoms of anxiety and depression in German patients with cystic fibrosis. Chest. 2010;138(4):929-936 [DOI] [PubMed] [Google Scholar]
- 23.Sawicki GS, Sellers DE, Robinson WM. Self-reported physical and psychological symptom burden in adults with cystic fibrosis. J Pain Symptom Manage. 2008;35(4):372-380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abbott J, Hart A, Morton A, Gee L, Conway S. Health-related quality of life in adults with cystic fibrosis: the role of coping. J Psychosom Res. 2008;64(2):149-157 [DOI] [PubMed] [Google Scholar]
- 25.Sawicki GS, Tiddens H. Managing treatment complexity in cystic fibrosis: challenges and opportunities. Pediatr Pulmonol. 2012;47(6):523-533 [DOI] [PubMed] [Google Scholar]
- 26.Sawicki GS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis: challenges to disease self-management. J Cyst Fibros. 2009;8(2):91-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flume PA, O’Sullivan BP, Robinson KA, et al. ; Cystic Fibrosis Foundation, Pulmonary Therapies Committee Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176(10):957-969 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement