Abstract
Modern neurostimulation approaches in humans provide controlled inputs into the operations of cortical regions, with highly specific behavioral consequences. This enables causal structure–function inferences, and in combination with neuroimaging, has provided novel insights into the basic mechanisms of action of neurostimulation on distributed networks. For example, more recent work has established the capacity of transcranial magnetic stimulation (TMS) to probe causal interregional influences, and their interaction with cognitive state changes. Combinations of neurostimulation and neuroimaging now face the challenge of integrating the known physiological effects of neurostimulation with theoretical and biological models of cognition, for example, when theoretical stalemates between opposing cognitive theories need to be resolved. This will be driven by novel developments, including biologically informed computational network analyses for predicting the impact of neurostimulation on brain networks, as well as novel neuroimaging and neurostimulation techniques. Such future developments may offer an expanded set of tools with which to investigate structure–function relationships, and to formulate and reconceptualize testable hypotheses about complex neural network interactions and their causal roles in cognition.
Keywords: state-dependence, effective connectivity, transcranial magnetic stimulation, causal inference, EEG, fMRI, MRS, computational neurostimulation
Introduction
Over the past two decades, neurostimulation approaches have become successful tools for noninvasively studying the basic physiology of, and cognitive processes in, the human brain. This review discusses how the use of neurostimulation in combination with neuroimaging has answered questions about the relationship between the physiological impact of transcranial magnetic stimulation (TMS) and its behavioral consequences, the distributed impact TMS can have on functional brain networks, and how this impact can be exploited to address novel questions about causal network interactions underlying cognition.
The format of this paper prevents an exhaustive treatment of combinations of neurostimulation and neuroimaging, and therefore the focus here is on TMS and its concurrent combinations with functional magnetic resonance imaging (fMRI) and electroencephalography (EEG). Other neurostimulation techniques provide equally powerful means for transiently interacting with neural processing. Of these, transcranial direct current stimulation (tDCS) stands out in its capacity to interact with ongoing neuronal activity.1–3 Although the relative paucity of combined tDCS and neuroimaging studies prevents an in-depth review of the technique,4–8 many of the arguments presented here equally apply to tDCS. We furthermore focus on concurrent combinations, and for elegant offline work, recent reviews and examples are referred to.9–19 Focusing on fMRI and EEG, the examples discussed in this review establish principles and ideas that similarly apply to other imaging modalities. Finally, for safety considerations,14,20,21 technical and methodological details, and requirements for combining TMS with neuroimaging, the reader may refer to previous publications.14,22–36
The below discussion is divided into three parts, the past, present, and potential future contributions to the field. First, early work, particularly in the motor system, is reviewed that provided evidence for the anatomically distributed effects of TMS. This in turn led to the combination with neuroimaging to study such effects with high anatomical and temporal precision throughout the brain. Since its implementation, the combination of TMS with neuroimaging has established several, now widely accepted insights into the basic mechanism of action of TMS and its distributed impact on brain networks. Next reviewed are more recent examples of combined TMS–fMRI and TMS–EEG that demonstrate how interregional interactions depend not only on anatomical connectivity, but also on the activation state of network constituents. A key question when evaluating successful combinations of TMS and neuroimaging concerns their ability to formulate novel and testable hypotheses about the role of distributed brain networks for cognition. As argued in the final section, the field is at a segue: the potential of multimodal neurostimulation approaches for understanding human cognition has not been fully exploited, but recent developments provide an ever increasing arsenal of possibilities for their use in establishing network accounts of human cognition.
The past: resting state studies and perturb-and-measure approaches
TMS stimulates cortical tissue through electromagnetic induction by discharging a short (∼1 ms) but strong (several kA) electrical current through an induction coil, which is placed over a cortical region of interest. The electric pulse induces a time-varying magnetic field perpendicular to the stimulation coil that passes through the scalp without attenuation, and is therefore painless and well tolerated. The induced electrical current may directly interact with ongoing neural processing at the site of stimulation, but also in remote and connected brain regions, as discussed in further detail below. By having such direct input into a cortical operation, one can study its behavioral consequences and thereby ask questions about the requirement of ongoing activity for a cognitive operation. Early seminal studies by Amassian et al.37,38 in the visual system provided the first compelling examples of how the possibility to directly and noninvasively interact with cortical processing can be hedged to infer causal structure–function relationships. In brief, these studies demonstrated, with a high degree of both temporal and anatomical specificity, that stimulation of early visual cortex can transiently interfere with perception.37,39,40 Early studies on human motor cortex, on the other hand, have provided the first evidence that TMS is capable of impacting distal sites. For example, a single pulse applied to the primary motor cortex (M1) hand representation can elicit motor-evoked potentials (MEPs) in muscles of the contralateral hand.41–46 The generation of MEPs involves (at least) three stages where signals are relayed first via synapses onto corticospinal neurons, then via synapses onto motor neurons located in the spinal cord, and finally, by the neuromuscular synapses that generate the evoked potentials recorded from peripheral muscles.47 TMS to M1 also significantly influences activity in the contralateral homologue,48–50 with functional relevance to motor output.51
Behaviorally, the first double-coil TMS studies provided a similar picture. For example, stimulation of visual area V5/MT with one TMS coil was reported to influence the excitability of primary visual cortex, as assessed with subsequent TMS pulses applied with a second stimulation coil.52 Functionally, this remote influence significantly affected visual awareness,52 thus providing additional evidence that even single TMS pulses can elicit remote responses strong enough to shape behavior. Although the relationship between the physiological and behavioral consequences resulting from TMS are more complex than simply that of transient interference,53–55 it is clear that TMS has the capacity to provide focal and temporally precise inputs into the operation of a cortical region,53 with highly specific behavioral consequences.56
But real progress in our understanding of local and remote TMS effects came with the near-simultaneous advent of combinations of TMS combined with fMRI,57 EEG,58 and positron emission tomography (PET).59 Early studies employed perturb-and-measure approaches60 in which TMS is used at rest to cause activity in one brain region while concurrent neuroimaging characterizes the distributed impact of this intervention. Two findings of these early resting-state TMS–neuroimaging studies are emphasized here, as they have particular relevance for later studies on cognition. First, the early combinations, using both single pulses or short bursts of TMS to motor cortical regions, established the now accepted view that TMS can interact with distal sites, including subcortical structures (Fig. 1),61–68 in a dose-dependent manner.61,63,68–74 Such interactions might be predicted given the strong anatomical projections across different corticobasal ganglia–thalamic loops,75–77 but direct quantification of such interactions had not been possible until the combination with neuroimaging. Even more specific cortico–subcortical interactions were demonstrated with combinations of TMS and ligand-PET (albeit conducted with offline TMS). These showed, for example, that stimulation of the dorsolateral prefrontal cortex (DLPFC) can elicit changes in dopamine release in the caudate nucleus (Fig. 1),78 or in the putamen after M1 stimulation.79 Similarly, concurrent TMS–fMRI studies showed that changes in subcortical activity during M1 stimulation can be distinguished from those evoked by stimulation of the dorsal premotor cortex (PMd), despite considerable and expected overlap of their anatomical footprints.61,68
Figure 1.
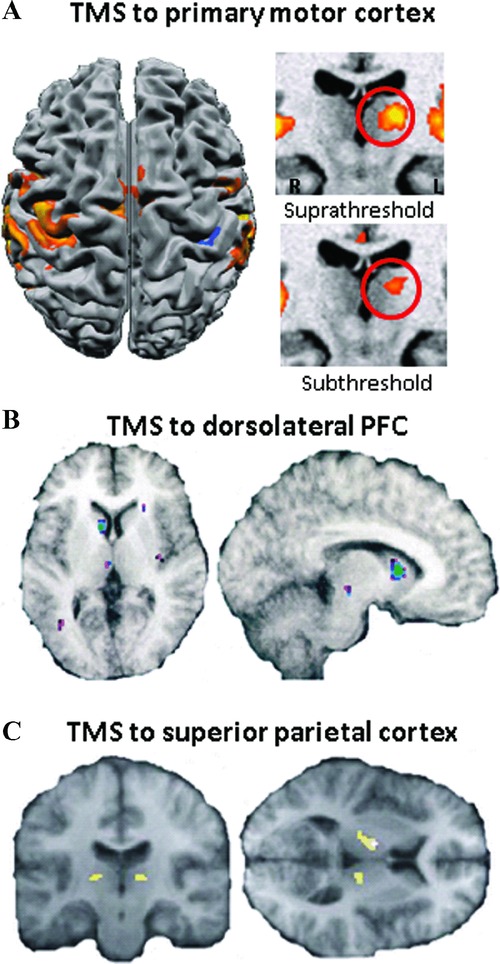
Subcortical activity changes evoked by TMS as measured using fMRI and PET. (A) Brief bursts of rTMS to the left primary motor cortex (M1) evoke BOLD signal changes not only in the vicinity of the stimulation site but additionally in the ipsilateral motor thalamic nuclei (note: left and right reversal). These subcortical effects occur even during subthreshold TMS and therefore in the absence of evoked movements.68 (B) Changes in extracellular dopamine concentration measured in vivo using [(11)C]raclopride and positron emission tomography. Repetitive TMS of the left dorsolateral prefrontal cortex caused a reduction in [(11)C]raclopride binding in the left dorsal caudate nucleus compared with rTMS of the left occipital cortex.78 (C) Interaction of TMS (high and low intensity) and median nerve stimulation (ON and OFF) within the thalamus. BOLD signal in the thalamus was highest during combined right-hand somatosensory stimulation and high-intensity TMS over the right parietal cortex.110
Second, resting-state TMS–EEG studies have provided clear demonstrations that the impact of TMS on remote regions is rapid and can spread over relatively widespread areas of cortex even with administration of single pulses or short stimulation bursts.71,80–87 Indeed, TMS-evoked activity spreads within tens of milliseconds to directly adjacent cortical regions, but then quickly disperses to more distal sites in a reverberating pattern.58,82,88 The magnitude of this spread is dose dependent,71,89 and can also be observed even after single pulses of stimulation.58,90 The changes in network activity observed with neuroimaging in response to single pulses or short bursts of TMS are thus not compensatory or plastic adjustments (as those likely induced by offline repetitive TMS protocols), but instead reflect the immediate propagation or broadcasting of the induced activity to distal, but connected, sites.
Together, this early combined resting-state neuroimaging–TMS work provided empirical confirmation that the distributed impact of TMS is spatially and temporally specific and occurs within distinct anatomical networks.a It is important to remember that this approach departs from the use of TMS in many behavioral applications, where it is aimed at transiently and reversibly disrupting behavior to induce virtual lesions. Instead, TMS is now used to cause activity in a cortical region, and measure with neuroimaging how this change is broadcast to distal regions, including subcortical sites. Without such combinations, our inferences would largely be confined to the cortex directly underneath the stimulating coil (although we note that double-coil TMS approaches also provide powerful ways for looking at interregional influences on, for example, M1 or V152,91–97).
Two key issues arise: first, earlier electrophysiological and behavioral work has shown that the impact of TMS depends on the excitability of connections (and/or the current level of activity) at the time of stimulation. Put simply, the more excitable a given region or its connections are, the more likely it is that TMS will influence activity in local and distant brain regions. For example, applying TMS to M1 during voluntary contraction affects the size and number of descending corticospinal volleys.98–100 This state-dependence of TMS is also supported by studies in the visual cortex demonstrating that the intensity of TMS-evoked perceived light flashes (phosphenes) changes during migraine,101 spatial attention,102 or neural adaptation paradigms.103–105 The next section will address how combined TMS and neuroimaging has helped to understand state-dependent network interactions.
The second issue is to what degree combined TMS and neuroimaging has not just enriched our understanding about the mechanisms of action of TMS itself, but has also provided novel views on the role of network interactions in human cognition, which is addressed in the final section.
The present: state-dependent network effects and oscillatory changes
Recently published studies combining TMS with neuroimaging extend the earlier work described, now asking about changes in the influence of TMS-targeted brain regions on anatomically remote regions that also change as a function of the behavioral requirements. The spread of TMS-induced activity to functionally connected areas is therefore a means for probing the varying states of connectivity, as stimulation can be applied during different behavioral states, at different times, to different areas. This approach is briefly illustrated using examples in which TMS was combined online with fMRI or EEG. We particularly acknowledge here the significant contribution Jon Driver made to the combined TMS–neuroimaging field; he was very much at the forefront of applying this approach to investigate a variety of cognitive domains, and his success in doing so is made apparent in this section.
State-dependent interhemispheric interactions
One striking result from early resting-state TMS–neuroimaging combinations was the rapid and reliable spread of effects to the hemisphere contralateral to the stimulation site.58,61–63,68,106 The nature of such interhemispheric interactions among homologous premotor and M1 areas was the focus of a concurrent TMS–fMRI investigation of simple force production.107 Specifically, this study addressed the hypothesis that the PMd might increase its influence with the contralateral PMd and M1 when a voluntary motor action is performed, as opposed to when at rest. By applying a short burst of TMS to the left PMd, its influences on contralateral motor regions during isometric force production or rest were measured. TMS produced a differential effect on blood oxygenation level–dependent (BOLD) signal changes both in the stimulated area, and in the contralateral right PMd and M1. Critically, these effects depended on the behavioral state (voluntary grip force production versus rest); at rest, TMS led to a relative BOLD signal decrease in these regions, whereas during grip, a relative increase was observed (Fig. 2). The remote influences of TMS on a known motor network therefore depend on the trial-by-trial changes in the state of the targeted region at the time of stimulation, and can even reverse with changes in state. This finding suggests that specific brain regions within a functional network only influence one another when a specific behavioral context is present, shown here as influences among contralateral and homologous brain regions.
Figure 2.
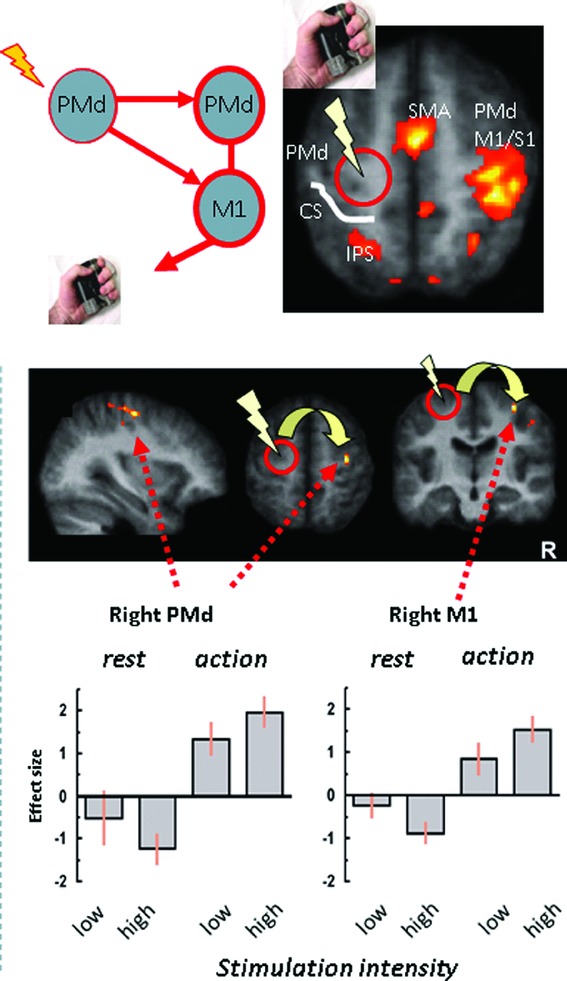
State-dependence of interhemispheric influences of TMS in the motor system. Short bursts of TMS (high vs. low intensity) were applied to the left PMd during left-hand isometric force production (or nonmotor rest), concurrently with fMRI. Within the task-related right PMd and M1 (i.e., contralateral to TMS, top), TMS at high intensity leads to a relative activity increase (bottom), compared to low-intensity control TMS. However, this effect is reversed during nonmotor rest, with high TMS now leading to a relative activity decrease in contralateral motor regions.108
This work was extended using TMS in combination with arterial spin labeling,108 an MRI approach that measures cerebral blood flow,28 now asking about the role of the PMd in the mapping of prelearned associations to motor responses. TMS produced regional cerebral blood flow changes (rCBF) on a task requiring sequences of previously learned key presses in response to visual stimuli. Compared to free selection of key presses, TMS increased rCBF in the contralateral PMd and other regions of the motor network, indicating a role for the PMd in the mapping of external cues to motor movements, via the formation of a transient functional network.
Finally, in another concurrent TMS–fMRI study, TMS applied to the right parietal cortex during wrist nerve stimulation (compared to no stimulation) produced increased activity in the contralateral primary somatosensory cortex and subcortically in the thalamus. This provides another example for causal interhemispheric interactions, now in the somatosensory system.109 The state-dependent effects support a role for the stimulated parietal region in response enhancement via a corticothalamic circuit when somatosensory inputs are present. Collectively, these examples all show the utility of combining online TMS with fMRI to address specific questions related to the interplay between regions in a functional network that can involve brain regions contralateral to the point of stimulation.
Anatomically distributed state-dependent and top-down influences
More recent work has started to use concurrent TMS–fMRI to investigate state-dependent interregional interactions outside the motor system. Note, however, that recent examples in the visual domain are discussed here only in passing; for an in-depth discussion of top-down visual influences, readers can refer to the contribution by Ruff (this volume).
To investigate top-down influences in the domain of multisensory integration, TMS has been applied to an association area (intraparietal sulcus; IPS) to examine TMS-modulated feedback to primary sensory cortex during auditory, visual, or no external stimulation.110 Participants were presented with different sensory stimulation during which effective, ineffective, or no TMS was applied to the right IPS. State-dependent effects resulting from visual stimulation were produced in primary sensory areas; IPS-TMS increased BOLD response in visual areas, again supporting a role for this area in response amplification/enhancement. When auditory or no external stimulation was present, IPS-TMS instead revealed cross-modal effects, in which early visual cortex activity decreased, in addition to expected increases in auditory cortex activations. These results suggested a role for the IPS in sensory gain control or modulation of interactions between different sensory cortices.
The role of the parietal cortex in attention has also been studied with concurrent TMS–fMRI.111,112 In these investigations, the top-down influence of parietal sites over visual cortex was shown, in which TMS applied during the directing of covert attention toward one hemifield increased BOLD signal in early visual areas. These findings show the causal influence of parietal over visual areas during varying states of attention, and fit with models in which modulatory effects of spatial attention on visual cortex occur via effective connectivity with parietal regions. More detailed descriptions of these studies, and more examples of using TMS–fMRI to study top-down effects in the visual system, are provided by Ruff (this volume).
In another investigation of how a top-down control region exerts control over anatomically remote visual areas, concurrent TMS–fMRI was used to resolve the question of whether the DLPFC mitigates distraction during working memory (WM), through an enhancement of relevant memory targets or suppression of irrelevant distractors.113 To this end, during fMRI scanning, TMS was applied to the right DLPFC to coincide with distractors, and the resultant effects of TMS on the BOLD response were measured in remote visual areas responding to either memory targets or distractors; in this way, the recipient of the DLPFC control signals (memory targets or distractors) would be revealed. TMS increased BOLD signal in memory target regions only, providing support for an enhancement mechanism of top-down DLPFC-mediated control (Fig. 3). This result fits with previous findings in which relevant (over irrelevant) information for the task is preferentially targeted by DLPFC signals.114 Moreover, there was no effect of TMS on memory target regions in the absence of distractors, providing further constraints on the role of the DLPFC during WM (i.e., only when the contents of WM need protection from external distraction). In sum, these results provide a strong line of causal evidence on the conditions under which the DLPFC is effectively connected to visual regions representing memory targets during WM, with additional conclusions able to be made on the mechanism by which a control region protects memory targets (i.e., via their enhancement). More generally, this study is an elegant example of the inferential power provided by concurrent TMS–fMRI, which has moved beyond simply assessing which regions are functionally coupled during a task. The nature of the TMS effect (i.e., an increase in BOLD signal in target-relevant regions) suggests a mechanistic action of DLPFC-based control, providing a unique form of evidence that is consistent with those observed in single unit recordings in monkeys, previous fMRI studies,115 and models of (prefrontal-based) cognitive control.116,117
Figure 3.
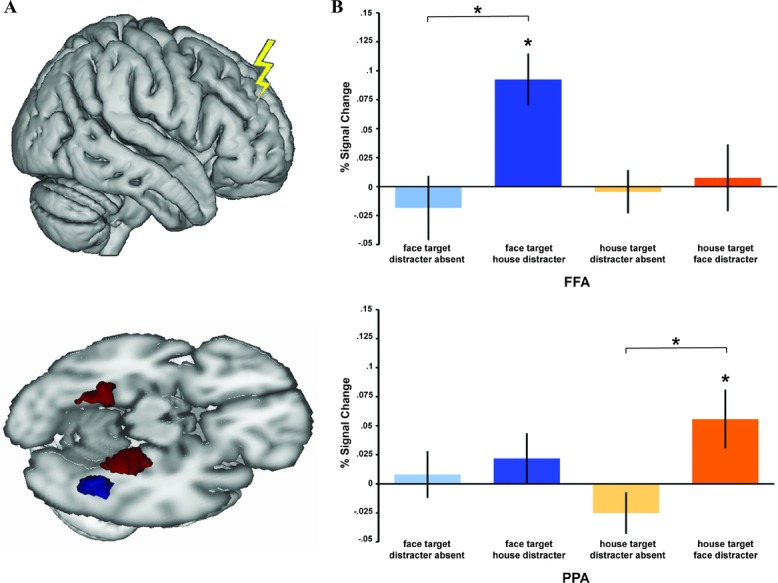
Anatomically remote effects of TMS to reveal the mechanisms of DLPFC-based control on WM representations. (A) Schematic of the right DLPFC stimulation site (upper), a region involved in distractor mitigation during WM. The impact of stimulation was assessed in posterior visual category-specific areas (lower; fusiform face area (FFA) in red, parahippocampal place area (PPA) in blue)). (B) Interparticipant mean BOLD percent signal changes due to effective vs. ineffective DLPFC-TMS are shown in FFA (upper) and PPA (lower). Effective TMS increased BOLD in FFA specifically when faces were memory targets, in the presence of house distractors. Analogously, effective TMS increased activity in PPA when houses were memory targets and faces were distractors. Thus, DLPFC stimulation has an impact on the posterior region representing the current memory target (rather than the current distractor), but only in the presence of distraction.114
Of note for the majority of the studies described above is the use of TMS during fMRI in physiological probe mode (i.e., without interfering with behavior). The reasons for this are often practical; for most behaviors, more TMS than permitted by the constrained TMS–fMRI environment must be given to significantly interfere with behavior; but by not disturbing behavior, networks can be investigated under comparable behavioral conditions. At the same time, the absence of behavioral consequences of TMS may instead, or at least under certain conditions, be due to rapid compensatory changes that counteract the stimulation. This now opens the possibility to ask about the specific brain regions that may enable such rapid compensatory adjustments, and at which point in the information processing stage such adjustments may occur.118 Furthermore, by not modulating behavior, one of the strengths of TMS is lost: the causal conclusions that can be drawn when a disrupted brain region impairs performance of the cognitive operation of interest. In a rare example of TMS interference during concurrent fMRI119 (see also Ref. 112 for a similar approach), right (but not left) parietal TMS during visuospatial task performance increased reaction times, with concomitant decreases in BOLD signal in the stimulated region and also in the right medial frontal gyrus. The task-specific (i.e., state-dependent) effect of parietal TMS on the frontal cortex raised the question of whether this disruption contributed to the behavioral impairments observed. One indication that this may have been the case was that TMS-evoked activity changes in the right frontal and parietal cortex correlated with the behavioral impairments.
Another approach for disrupting behavior that circumvents fMRI-imposed constraints is to use an offline TMS protocol, applied immediately before scanning. Such offline approaches to investigate compensatory or adaptive processes are not discussed here, but for recent examples using this approach to investigate the connectivity and mechanistic actions of the prefrontal cortex on posterior visual perception–related regions, see Refs. 12 and 120.
TMS-induced oscillatory changes
TMS pulses can be applied rhythmically at frequencies to match those of endogenous oscillations, and this capacity offers the potential to causally unlock the cognitive role of oscillations. Of particular interest has been the role of α band oscillations. Traditionally known as the idling rhythm, α has been associated more with a resting-type state than with active cognitive operations.121 This view has undergone a recent shift, of which evidence using combined TMS–EEG has contributed. For example, a recent study demonstrated a dependence of the preceding α phase on the efficacy of phosphene production by a single TMS pulse applied over the occipital cortex.122 For frontal and occipital electrodes, the phase of oscillations in the α range was systematically coupled with the probability of phosphene report by participants for a period of up to 400 ms prior to phosphene induction, a result that provides causal evidence for ongoing α oscillations in sensory perception (see also Ref. 123). This idea has been further consolidated in recent combined TMS–EEG work on α oscillations and perception, now applying TMS to the IPS during a Posner cueing task to investigate the interaction between α and endogenous allocation of attention.124 Specifically, both the left and right IPS were targeted during the presentation of a cue indicating the location of an upcoming visual target. TMS interfered with subsequent target detection and also disrupted occipito-parietal α desynchronization in the hemisphere contralateral to the cue, supporting a role for this region in the allocation of spatial attention to one hemifield. However, right IPS TMS had an additional effect of synchronizing α rhythms bilaterally, suggesting a spatially nonselective role for α as well, possibly to control visual cortex excitability prior to an upcoming target, a result that is also in agreement with parietal asymmetries revealed by concurrent TMS–fMRI.73,125
It has been suggested, however, that α oscillations might not just be related to processing in the visual domain, but may also play an important role in decision making. Recent work has elegantly addressed the question regarding specific roles for α and β oscillations in a probabilistic reasoning task.126 Using EEG, a recent study demonstrated that the arrival and accumulation of evidence about an upcoming decision, which was indicated via left or right hand button press, was indexed by α and β oscillations in sensorimotor cortex.126 To causally establish the role of these oscillatory signatures for evidence accumulation and decision making, a subsequent experiment applied short trains of 10 Hz TMS, during EEG, over a region of the left IPS where sensorimotor implementation of the decision was expected to occur (Fig. 4). TMS now biased decision responses toward the left hand, indicating disruption of evidence integration for responses that were to be given from the contralateral-to-TMS hand. TMS also affected β band power, which, after TMS, showed a positive correlation with an individual's decision threshold bias. Moreover, the largest β power increases occurred when TMS was applied at a specific phase of the β cycle. No effects of TMS were observed on α, however. These results thus provide a novel form of evidence for the causal role of β oscillations in the processes underlying an upcoming decision, perhaps reflecting the incoming signals tracking probabilistic information that goes toward response selection, with the IPS playing a critical role.
Figure 4.
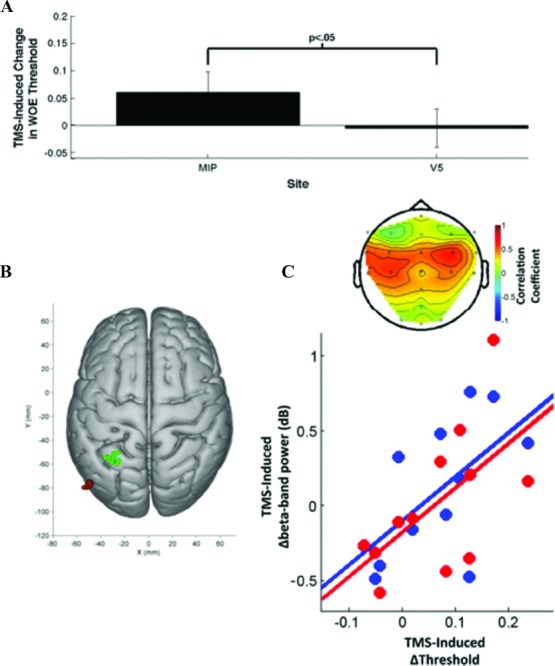
Effects of intraparietal TMS during perceptual decision making on sensorimotor oscillatory activity. (A) TMS-induced change in psychometric function during a perceptual decision-making task, for the left medial intraparietal area (MIP; green) vs. V5 (red) control stimulation (B) revealed a specific correlation between TMS-induced behavioral changes during a perceptual decision task, and concurrently measured β-band changes for electrode positions overlying sensorimotor cortex in both hemispheres (C).127
Turning once again to WM, a role for oscillations in WM storage has also been proposed,127–129 which was directly tested using combined TMS–EEG.130 During the maintenance period of a spatial or object WM task, α frequency (10 Hz) repetitive TMS was applied over the superior parietal lobule (SPL), which was previously shown to be affected by TMS during spatial WM.131 Correlations between TMS-induced changes in task performance and α band power were identified in several (source-localized) brain areas related to spatial WM, with TMS-induced impairment in spatial WM accuracy corresponding specifically to decreases in α band power. Cross-frequency effects between α and γ were also modulated by TMS, adding causal support for a proposed relationship between these frequency bands.132 In addition to demonstrating specificity for certain frequencies in WM, these results also demonstrated the biasing of endogenous oscillations by rhythmic TMS. Another study also used combined TMS–EEG to help clarify the interaction between endogenous behavioral states of WM and exogenously induced electrophysiological effects by TMS, producing effects on a range of EEG measures, including event-related potentials and oscillations, that were consistent with both behavioral state and the underlying physiological state of the cortical target at the time of stimulation.133
Several studies have used TMS to interact more explicitly with endogenous oscillations related to cognition to show the ability of TMS to bias, or entrain, specific frequencies. An excellent example is provided by a study134 that used concurrent TMS–EEG to entrain and measure a cortical generator of α oscillations related to shifts of attention and target detection. TMS pulse trains were applied at a frequency corresponding to each subject's individual α, with resultant effects on endogenous α from each pulse, measured concurrently with EEG. Against a series of control conditions, their results showed that α-frequency trains of TMS started off with broadband, and topographically broad, increases in oscillatory power that narrowed to the α range as the TMS train progressed in time. The phase of an individual's intrinsic α cycle immediately prior to the start of a TMS train also influenced TMS-induced entrainment, with the strongest entrainment observed when TMS pulses were phase locked to a specific period in the α cycle. This phase locking increased as, again, the number of TMS pulses applied in a train increased. These results complement earlier work,127,129 and provide yet more evidence of the causal effect that rhythmic TMS has on intrinsic oscillations; TMS can synchronize oscillations and, as shown previously in a visual detection task, this entrainment can have behavioral consequences.135 More recent developments in this area are discussed in the following section.
The future: can neurostimulation be an arbitrator for network accounts of cognition and their physiological underpinnings?
Next considered is how the field of cognition with neurostimulation perhaps can and should progress. Combined neurostimulation and neuroimaging applications for studies of cognition must be judged by their ability to refine or reshape cognitive models by exploring their implementation via neuroanatomical and neurophysiological underpinnings. Despite the success of multimodal neurostimulation approaches, we argue that the impact of combined neurostimulation and neuroimaging has yet to reach its full potential when it comes to informing neural models of cognition.
TMS as arbitrator for neural models of cognition
Perhaps owing to the complexity of the TMS-evoked physiology and the methodological prowess required to conduct combined studies, much of the work to date has been geared toward the understanding and characterization of the basic physiology and state-dependence of TMS effects in distributed networks, all prerequisites for an in-depth understanding of the impact of TMS (and indeed all forms of neurostimulation) on cognition. One highly constructive role for TMS–neuroimaging in particular may be that of an arbitrator, in which the interventional capacity of TMS can be used to distinguish between competing accounts about how specific cognitive processes arise neurally. Specifically, the question is: To what extent can these approaches provide novel insight about cognition beyond established uses of TMS for structure–function relationships? Can TMS also be used to test for the requirement of specific regional interactions in cognition, and can TMS-evoked changes in such interactions allow for disambiguating competing cognitive theories?
One such example has already been provided, in which concurrent TMS–fMRI was used to adjudicate between two competing mechanisms (of prefrontal-based control) in working memory.113 The physiological predictions (in terms of BOLD signal increase in memory target areas) matched the expected outcome corresponding to one of two theoretical functions for top-down control (enhancement versus suppression). With the causal conclusions permitted by TMS, this study provides a strong line of evidence favoring a specific (theoretically described) implementation of cognitive control. Furthermore, other combined offline TMS–fMRI12 and TMS–EEG studies120 revealed other prefrontal, but anatomically distinct, regions also involved in the control of task relevant versus irrelevant information. This raises the question for future studies about the specific roles of these three prefrontal regions, and whether they indeed fulfill essentially the same function. Alternatively, enhancement and suppression could be differentially invoked under different conditions, by different brain regions (e.g., when items are already in WM but face exogenous distractors versus when items among distractors are being encoded, or need to be selected from among already encoded items that have become distractors).
Note that an experiment utilizing concurrent TMS–fMRI does not automatically confer an informative contribution to a (neuro)cognitive model. The danger of simply applying an anatomical label to a function, albeit a causal one, remains (although, to some degree, this problem applies to all TMS approaches). However, concurrent TMS-neuroimaging does hold a unique position through its ability to produce specific context-based modulations of physiological signals with direct and immediate effects able to be observed. Another strength of concurrent TMS–neuroimaging is that it provides strong evidence about network accounts of cognition (i.e., how cognitive behaviors arise through concerted communication between brain regions, some of which may enact control over others). As addressed below, the possible directionality of signals can be explored with formal models of effective connectivity. However, we caution against the fallacies of reverse inference. It will not always be possible to unambiguously link TMS-induced changes in neural activity and cognitive processing, particularly in cases where behavioral perturbations do not occur. While it may be safe to conclude that the TMS-induced impact on region A and its interconnected regions B and C varies as a function of cognitive state (e.g., attention), one cannot conclude that attention is enabled because of the interactions between A, B, and C.
Combined TMS–neuroimaging and multivoxel pattern analyses
Multivoxel pattern analysis (MVPA) is a recently developed approach to the analysis of neuroimaging data that shows the information content of signals in high dimensional data sets, such as those of fMRI, EEG, and magnetoencephalography (MEG). Using machine learning, MVPA can decode the information contained in a signal, revealing the nature of how a brain region or an electrophysiological signal represents that information, and how it can change over different stages of processing.136 For example, returning to the experimental question of whether the DLPFC enhances memory targets or suppresses distractors during working memory,113 disrupting the DLPFC might result in the representation of targets being affected such that the (MVPA-identified) representations are decreased as a result of the disturbance of top-down enhancement. Similarly, if distractor suppression is also a DLPFC-based control mechanism, disruption of this might result in the improvement of distractor decoding, due to the lifting of suppression (unpublished data, Feredoes et al.). Given that the technological requirements for combining TMS with MVPA are already in place, the experimenter is limited merely by their ability to formulate questions appropriate for this approach; the ability to observe TMS-induced modulation of information representation may significantly increase the inferential power afforded by combined TMS–neuroimaging.
Computational neurostimulation
Given the causal inferences afforded by TMS, it may be integrated with emerging classes of connectivity models, as a way to test their parameters and/or validity as a whole. Recent appraisals of neuroimaging highlight the shift from establishing functional specialization of regions toward establishing the connections between regions instead, and thus functional integration;137,138 with this has come the desire to establish causal links between such connected regions via neurobiologically informed models that provide intermediate levels of description about the basic physiology of TMS and its behavioral consequences.
For example, biophysical models, such as those instantiated in dynamic causal models (DCMs), can in principle help to test whether remote stimulation effects predominantly arise from ortho- and/or antidromic stimulation of connections, and/or via pyramidal projections or intra-regional interneurons.53,55,139–141 In principle, DCMs for electrophysiological data, such as provided by MEG or EEG, are best equipped to address such mechanistic questions, for example, by forming deterministic generative models of ensemble or population dynamics.142 DCMs now exist for evoked responses,143 steady state responses,144 cross-spectral densities,145 phase coupling,146 and induced responses,147 thus offering complementary ways for investigating how TMS elicits observed population responses, but also how such responses affect cognitive processing.
One can furthermore proceed to ask about competing candidate mechanisms by which cognitive states might be maximally expressed in effective connectivity changes, and how these changes then interact with neurostimulation. For example, DCMs also test for TMS-induced changes in physiological responses, and their interaction with cognition. By estimating parameters of the neural model, one might expect that the most accurately predicted physiological signals would correspond closely to the observed fMRI/EEG/MEG signals. Put simply, a realistic biophysical model should also be able to explain an additional input into the system in the form of a TMS-induced perturbation, and in this way, a causal intervention dimension can be added to modelling. In other words, the combination of in vivo perturbation through neurostimulation, neuroimaging, and realistic brain models provides a computational neurostimulation approach with which to investigate the impact on cognition of physiological mechanisms of neurostimulation-induced effects.148,149
In what could be considered a precursor to such an approach, one study150 investigated how TMS-evoked interregional influences within motor and corticolimbic circuits change with drug-induced global state changes. Using DCMs to investigate TMS-evoked changes in effective connectivity and their interaction with drug treatment, drug-specific changes were demonstrated in the brain networks targeted by TMS. This provides an example of how concurrent TMS and neuroimaging can quantify drug-induced changes in interregional interactions (Fig. 4A). More significantly, it illustrates how concurrent neurostimulation and neuroimaging can be used together with neurobiologically informed analyses of effective connectivity to ask questions about the rapid and flexible causal network interactions during cognition.
TMS and entrainment
The previous sections described how TMS can entrain α oscillations and how this entrainment relates to endogenous attention134 (Fig. 4B). This application of TMS promises a direct and therefore powerful way in which to investigate how oscillations might be integral to cognition. The seemingly fortuitous ability of TMS to be applied rhythmically to match natural oscillations of various frequencies means a variety of questions are ready to be answered using this approach. For example, the proposed long-range communication role for the lower frequencies151 can be tested by applying TMS over one region and measuring the resultant oscillatory changes in distant, connected regions. Moreover, improvements in behaviors can also be produced, via the boosting of frequency-specific amplitudes with TMS.152 However, for this latter point, transcranial alternating current stimulation techniques may be an alternative and potentially more powerful way for inducing behaviorally relevant entrainment,153–158 mainly because of their potential to entrain at high (e.g., γ range) frequencies.159 Recent developments in high-definition tDCS for increased focality of stimulation may also enable more selective targeting of cortical regions.160–164 Moreover, recent advances in forward modelling of induced currents now allow, in principle, precise directing of induced currents onto the desired neural target structures.162,165 Together, these developments hold promise for the use of tDCS in locally constrained neural entrainment.
Thus, we predict a rapid growth in studies using neurostimulation to understand the role of specific frequencies of oscillations for specific cognitive processes and for entrainment to facilitate their function.
Neurostimulation and neurotransmitters
The burgeoning development of novel imaging and neurostimulation approaches is likely to continue to fuel studies that address hitherto inaccessible questions. For example, the abovementioned DCMs empower one to use anatomically constrained computational perturbations of specific neurotransmitters (i.e., testing hypotheses regarding neurotransmitter changes throughout the brain evoked by neurostimulation). Importantly, such changes can also be quantified both directly at the stimulation site and throughout the brain with magnetic resonance spectroscopy (MRS).166 MRS allows quantification of neurotransmitter concentrations within a defined region of interest in the brain and has recently been used to measure both TMS- and tDCS-induced neurotransmitter changes (albeit in offline approaches)167–171 (Fig. 5C). Moreover, it has become clear that interindividual variation in, for example, MRS-measured GABA levels relate to variation in task performance in a number of regions.172–176 This now opens up possibilities to address questions about causal neuropharmacology by changing neurotransmitter concentrations in a defined cortical network through neurostimulation, and relating such changes to task performance. One crucial advantage of this approach is that, in principle, neurotransmitter concentrations can be modified in a more spatially selective way than otherwise possible with pharmacological interventions, although it remains to be seen whether MRS can be usefully combined with concurrent neurostimulation. Currently, the technology requires a priori focus on specific neurotransmitters (e.g., GABA and glutamate/glutamine (Glx)), despite the known impact of both TMS and tDCS on a larger variety of neurotransmitters,2,177–179 and additionally provides a relatively poor spatial resolution (compared to fMRI and the focality of TMS), with standard voxel-sizes at field strengths of 3T of around 30 mm3. This inevitable sampling-bias currently constrains inferences about causal neurotransmitter–function relationships, but advances in multivoxel MRS180 (particularly at higher field strengths) are likely to provide increasingly detailed mappings of stimulation-evoked changes in neurotransmitter concentration with relevance for cognition.
Figure 5.
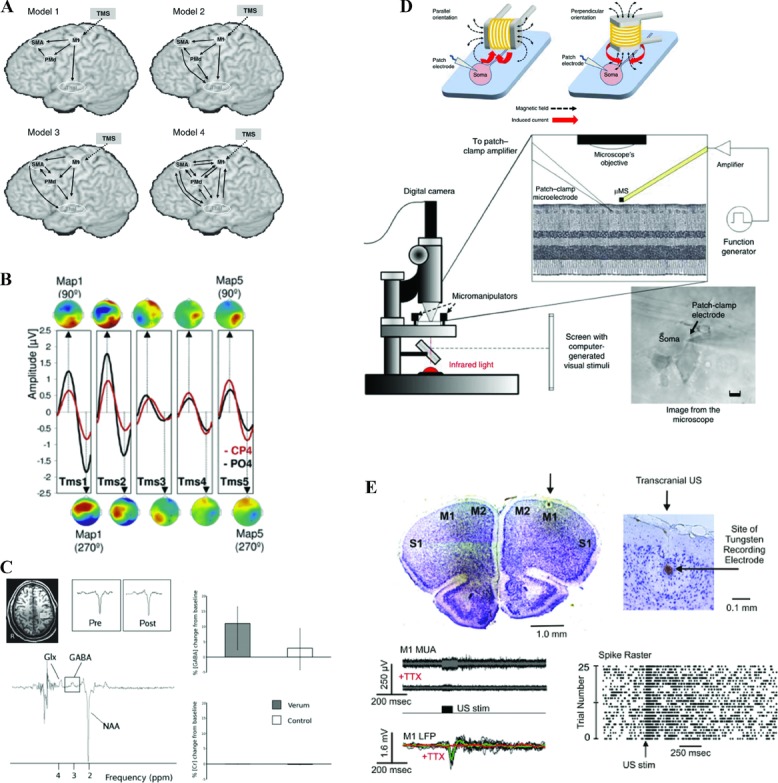
The future of concurrent neurostimulation and neuroimaging in humans for studies of cognition. (A) Network analyses such as dynamic causal modeling provide intermediate levels of description that link the physiological impact of neurostimulation and its behavioral consequences, and allow for generating testable hypotheses about resulting network connectivity changes. Adapted from Li et al. 150 with permission. (B) Neurostimulation-induced entrainment of intrinsic cortical rhythms can have direct consequences for perception, depending on the frequency of stimulation. This allows for identifying the causal role of oscillatory activity for behavior, and holds the possibility to shape behavior through the selective entrainment of local and distributed oscillatory activity. Adapted from Thut et al.134 with permission. (C) Combined magnetic resonance spectroscopy can quantify the specific neurotransmitter changes induced by neurostimulation, potentially including online neurostimulation protocols. Moreover, the changes can be directly related to behavior, and thereby provide causal links between stimulation-evoked neurotransmitter changes in focal and defined parts of the brain, and cognition. Adapted from Stagg et al.167 with permission. (D) Micro-stimulation coils in principle allow for selective stimulation of cortical micro-circuits, and include the possibility for application during neuroimaging Adapted from Bonmassar et al.181 with permission. (E) Ultrasound stimulation holds promise to allow for targeted and selective stimulation of neural tissue throughout the brain, including subcortical structures. Adapted from Tufail et al.184 with permission. Note that for the examples in D–E, demonstration of their applicability in humans is pending.
Novel ways for neurostimulation
Other, potentially more powerful and more focal neurostimulation techniques may be in the offing and invite more speculative projections. Recent work demonstrates the ability of micro-magnetic stimulation (μMS) coils with dimensions of around 500 μm to stimulate retinal ganglion cells181 (Fig. 4D). Such coils can be used in vivo and in vitro with high focality, and are inherently compatible with neuroimaging. Although it remains to be shown that they can be usefully applied in human studies, the possibility of selective stimulation of small neural circuits appears achievable. Other developments hint at the possibility of low-intensity–focused ultrasound pulsation to interact with neural processing,182,183 at a spatial scale of 2 mm or less184 (Fig. 4E). Pending demonstration that this technique can be safely applied in humans to stimulate brain tissue, focal stimulation beyond the range of present technology during neuroimaging may become a possibility, again being potentially compatible with neuroimaging, including MR-based techniques.182 The increasing feasibility of optogenetic approaches for studies of behavior185 may, in the future, provide yet another powerful avenue for neurostimulation. Optogenetic stimulation is the excitation or inhibition of specific cell types and neural pathways through light pulses. This provides a causal, time-resolved assay in which to test for the contribution of specific neural circuit elements that participate or contribute to the computations required for emergent behavior; optogentically controlled stimulation has recently been used in conjunction with fMRI to investigate the foundations of the BOLD fMRI signal.186 Any transfer of this technique to humans is likely to be at least a decade away, though recent work suggests that translational applications could become feasible within such a time frame,187 and, if that is the case, such applications could provide auxiliary insights into the neural underpinnings of human cognition. It is not clear whether and/or when some of these techniques will be safely transferrable to humans, but some of the rapid developments will, in some form, make the transition to human studies of cognition and the neural networks that underpin behavior. This review does not cover an exhaustive list of possibilities in which neurostimulation and neuroimaging might be applied in the future, but illustrates how extant combinations of these methods already offer us an expanded set of tools and a rich vein of information with which to interrogate and reconceptualize how complex neural network interactions can give rise to behavior.
Conclusions
This review has illustrated the increasing sophistication of studies combining neurostimulation with neuroimaging, as well as an exciting future outlook, for studies of human cognition. As cognitive neuroscience integrates with more fundamental aspects of neurophysiology, we suggest that neurostimulation will have an important role to play, given that it modulates neurophysiology at a fundamental level. The shift in the use of neurostimulation to study cognition is already apparent, via the more complex combinations and experimental questions and designs using combined neurostimulation-neuroimaging, some of which we have described above. By continuing in this direction, along with developments in the field of neurostimulation, the current possibilities and future developments hold promise to establish a causal neurocognition account in the human brain.
Acknowledgments
This review was written in memory of Professor Jon Driver. S.B. is funded by the Biotechnology and Biological Research council (BBSRC) and European Research Council (ActSelectContext; 260424). We thank Christopher Chambers for feedback and advice.
Footnotes
Perhaps we owe the strength of such distal effects to the fact that TMS interacts with the activity of relatively large populations of neurons, compared to combined neuroimaging and microstimulation experiments in nonhuman primates.188–194 The exact extent of the functional impact depends on many parameters, but can be as little as 0.5–1 cm2,195 particularly when combined with neuronavigation.196,197 But, as opposed to microstimulation in nonhuman primates, the stimulation of much larger populations of neurons may in fact be a prerequisite for substantial distal effects elicited by TMS.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nitsche MA, Paulus W. Transcranial direct current stimulation—update 2011. Restor. Neurol. Neurosci. 2011;29:463–492. doi: 10.3233/RNN-2011-0618. [DOI] [PubMed] [Google Scholar]
- 3.Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- 4.Holland R, Leff AP, Josephs O, et al. Speech facilitation by left inferior frontal cortex stimulation. Curr. Biol. 2011;21:1403–1407. doi: 10.1016/j.cub.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antal A, Polania R, Schmidt-Samoa C, et al. Transcranial direct current stimulation over the primary motor cortex during fMRI. Neuroimage. 2011;55:590–596. doi: 10.1016/j.neuroimage.2010.11.085. [DOI] [PubMed] [Google Scholar]
- 6.Antal A, Bikson M, Datta A, et al. Imaging artifacts induced by electrical stimulation during conventional fMRI of the brain. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2012.10.026. DOI: 10.1016/j.neuroimage.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turi Z, Paulus W, Antal A. Functional neuroimaging and transcranial electrical stimulation. Clin. EEG Neurosci. 2012;43:200–208. doi: 10.1177/1550059412444978. [DOI] [PubMed] [Google Scholar]
- 8.Baudewig J, Nitsche MA, Paulus W, Frahm J. Regional modulation of BOLD MRI responses to human sensorimotor activation by transcranial direct current stimulation. Magn. Reson. Med. 2001;45:196–201. doi: 10.1002/1522-2594(200102)45:2<196::aid-mrm1026>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Hartwigsen G, Bestmann S, Ward NS, et al. Left dorsal premotor cortex and supramarginal gyrus complement each other during rapid action reprogramming. J. Neurosci. 2012;32:16162–16171. doi: 10.1523/JNEUROSCI.1010-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee L, Siebner H, Bestmann S. Rapid modulation of distributed brain activity by transcranial magnetic stimulation of human motor cortex. Behav. Neurol. 2006;17:135–148. doi: 10.1155/2006/287276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reithler J, Peters JC, Sack AT. Multimodal transcranial magnetic stimulation: using concurrent neuroimaging to reveal the neural network dynamics of noninvasive brain stimulation. Prog. Neurobiol. 2011;94:149–165. doi: 10.1016/j.pneurobio.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Higo T, Mars RB, Boorman ED, Buch ER, Rushworth MF. Distributed and causal influence of frontal operculum in task control. Proc. Natl. Acad. Sci. USA. 2011;108:4230–4235. doi: 10.1073/pnas.1013361108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'shea J, Johansen-Berg H, Trief D, et al. Functionally specific reorganization in human premotor cortex. Neuron. 2007;54:479–490. doi: 10.1016/j.neuron.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Siebner HR, Bergmann TO, Bestmann S, et al. Consensus paper: combining transcranial stimulation with neuroimaging. Brain Stimul. 2009;2:58–80. doi: 10.1016/j.brs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Lee TG, D'esposito M. The dynamic nature of top-down signals originating from prefrontal cortex: a combined fMRI-TMS study. J. Neurosci. 2012;32:15458–15466. doi: 10.1523/JNEUROSCI.0627-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller BT, Vytlacil J, Fegen D, et al. The prefrontal cortex modulates category selectivity in human extrastriate cortex. J. Cogn Neurosci. 2011;23:1–10. doi: 10.1162/jocn.2010.21516. [DOI] [PubMed] [Google Scholar]
- 17.Pleger B, Blankenburg F, Bestmann S, et al. Repetitive transcranial magnetic stimulation-induced changes in sensorimotor coupling parallel improvements of somatosensation in humans. J. Neurosci. 2006;26:1945–1952. doi: 10.1523/JNEUROSCI.4097-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pleger B, Ruff CC, Blankenburg F, et al. Neural coding of tactile decisions in the human prefrontal cortex. J. Neurosci. 2006;26:12596–12601. doi: 10.1523/JNEUROSCI.4275-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward NS, Bestmann S, Hartwigsen G, et al. Low-frequency transcranial magnetic stimulation over left dorsal premotor cortex improves the dynamic control of visuospatially cued actions. J. Neurosci. 2010;30:9216–9223. doi: 10.1523/JNEUROSCI.4499-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossi S, Hallett M, Rossini PM, Pascual-Leone A and the Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr. Clin. Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 22.Bestmann S, Baudewig J, Frahm J. On the synchronization of transcranial magnetic stimulation and functional echo-planar imaging. J. Magn Reson. Imaging. 2003;17:309–316. doi: 10.1002/jmri.10260. [DOI] [PubMed] [Google Scholar]
- 23.Ilmoniemi RJ, Kicic D. Methodology for combined TMS and EEG. Brain Topogr. 2010;22:233–248. doi: 10.1007/s10548-009-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korhonen RJ, Hernandez-Pavon JC, Metsomaa J, et al. Removal of large muscle artifacts from transcranial magnetic stimulation-evoked EEG by independent component analysis. Med. Biol. Eng Comput. 2011;49:397–407. doi: 10.1007/s11517-011-0748-9. [DOI] [PubMed] [Google Scholar]
- 25.Mutanen T, Maki H, Ilmoniemi RJ. The effect of stimulus parameters on TMS-EEG muscle artifacts. Brain Stimul. 2012 doi: 10.1016/j.brs.2012.07.005. DOI: 10.1016/j.brs.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Weiskopf N, Josephs O, Ruff CC, et al. Image artifacts in concurrent transcranial magnetic stimulation (TMS) and fMRI caused by leakage currents: modeling and compensation. J. Magn Reson. Imaging. 2009;29:1211–1217. doi: 10.1002/jmri.21749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bungert A, Chambers CD, Phillips M, Evans CJ. Reducing image artefacts in concurrent TMS/fMRI by passive shimming. Neuroimage. 2011;59:2167–2174. doi: 10.1016/j.neuroimage.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Moisa M, Pohmann R, Uludag K, Thielscher A. Interleaved TMS/CASL: comparison of different rTMS protocols. Neuroimage. 2010;49:612–620. doi: 10.1016/j.neuroimage.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Moisa M, Pohmann R, Ewald L, Thielscher A. New coil positioning method for interleaved transcranial magnetic stimulation (TMS)/functional MRI (fMRI) and its validation in a motor cortex study. J. Magn Reson. Imaging. 2009;29:189–197. doi: 10.1002/jmri.21611. [DOI] [PubMed] [Google Scholar]
- 30.Bohning DE, Denslow S, Bohning PA, et al. A TMS coil positioning/holding system for MR image-guided TMS interleaved with fMRI. Clin. Neurophysiol. 2003;114:2210–2219. doi: 10.1016/s1388-2457(03)00232-3. [DOI] [PubMed] [Google Scholar]
- 31.Thut G, Ives JR, Kampmann F, et al. A new device and protocol for combining TMS and online recordings of EEG and evoked potentials. J. Neurosci. Methods. 2005;141:207–217. doi: 10.1016/j.jneumeth.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 32.Litvak V, Komssi S, Scherg M, et al. Artifact correction and source analysis of early electroencephalographic responses evoked by transcranial magnetic stimulation over primary motor cortex. Neuroimage. 2007;37:56–70. doi: 10.1016/j.neuroimage.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 33.Bestmann S, Ruff CC, Driver J. "Concurrent TMS and functional magnetic resonance imaging: methods and current advances". In: Wasserman EA, Epstein CM, Ziemann U, Walsh V, Paus T, Lisanby SH, Blankenburg F, editors. Oxford Handbook of Transcranial Stimulation. Oxford: Oxford University Press; 2008. pp. 569–592. [Google Scholar]
- 34.Bestmann S, Ruff CC, Blankenburg F, et al. Mapping causal interregional influences with concurrent TMS-fMRI. Exp. Brain Res. 2008;191:383–402. doi: 10.1007/s00221-008-1601-8. [DOI] [PubMed] [Google Scholar]
- 35.Driver J, Blankenburg F, Bestmann S, et al. Concurrent brain-stimulation and neuroimaging for studies of cognition. Trends Cogn Sci. 2009;13:319–327. doi: 10.1016/j.tics.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Ruff CC, Driver J, Bestmann S. Combining TMS and fMRI: from ‘virtual lesions’ to functional-network accounts of cognition. Cortex. 2009;45:1043–1049. doi: 10.1016/j.cortex.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amassian VE, Cracco RQ, Maccabee PJ, et al. Suppression of visual perception by magnetic coil stimulation of human occipital cortex. Electroencephalogr. Clin. Neurophysiol. 1989;74:458–462. doi: 10.1016/0168-5597(89)90036-1. [DOI] [PubMed] [Google Scholar]
- 38.Amassian VE, Maccabee PJ, Cracco RQ, et al. Measurement of information processing delays in human visual cortex with repetitive magnetic coil stimulation. Brain Res. 1993;605:317–321. doi: 10.1016/0006-8993(93)91758-k. [DOI] [PubMed] [Google Scholar]
- 39.Amassian VE, Cracco RQ, Maccabee PJ, et al. Unmasking human visual perception with the magnetic coil and its relationship to hemispheric asymmetry. Brain Res. 1993;605:312–316. doi: 10.1016/0006-8993(93)91757-j. [DOI] [PubMed] [Google Scholar]
- 40.Maccabee PJ, Amassian VE, Cracco RQ, et al. Magnetic coil stimulation of human visual cortex: studies of perception. Electroencephalogr. Clin. Neurophysiol. 1991;43:111–120. Suppl. [PubMed] [Google Scholar]
- 41.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 42.Amassian VE, Cracco RQ. Human cerebral cortical responses to contralateral transcranial stimulation. Neurosurgery. 1987;20:148–155. [PubMed] [Google Scholar]
- 43.Mills KR, Murray NM, Hess CW. Magnetic and electrical transcranial brain stimulation: physiological mechanisms and clinical applications. Neurosurgery. 1987;20:164–168. doi: 10.1097/00006123-198701000-00033. [DOI] [PubMed] [Google Scholar]
- 44.Day BL, Rothwell JC, Thompson PD, et al. Motor cortex stimulation in intact man. 2: multiple descending volleys. Brain. 1987;110:1191–1209. doi: 10.1093/brain/110.5.1191. [DOI] [PubMed] [Google Scholar]
- 45.Rothwell JC, Thompson PD, Day BL, et al. Motor cortex stimulation in intact man. 1: general characteristics of EMG responses in different muscles. Brain. 1987;110:1173–1190. doi: 10.1093/brain/110.5.1173. [DOI] [PubMed] [Google Scholar]
- 46.Rothwell JC, Day BL, Thompson PD, et al. Some experiences of techniques for stimulation of the human cerebral motor cortex through the scalp. Neurosurgery. 1987;20:156–163. doi: 10.1097/00006123-198701000-00032. [DOI] [PubMed] [Google Scholar]
- 47.Huerta PT, Volpe BT. Transcranial magnetic stimulation, synaptic plasticity and network oscillations. J. Neuroeng. Rehabil. 2009;6:7. doi: 10.1186/1743-0003-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferbert A, Priori A, Rothwell JC, et al. Interhemispheric inhibition of the human motor cortex. J. Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyer BU, Roricht S, Grafin VE, et al. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 1995;118:429–440. doi: 10.1093/brain/118.2.429. [DOI] [PubMed] [Google Scholar]
- 50.Cracco RQ, Amassian VE, Maccabee PJ, Cracco JB. Comparison of human transcallosal responses evoked by magnetic coil and electrical stimulation. Electroencephalogr. Clin. Neurophysiol. 1989;74:417–424. doi: 10.1016/0168-5597(89)90030-0. [DOI] [PubMed] [Google Scholar]
- 51.Irlbacher K, Voss M, Meyer BU, Rothwell JC. Influence of ipsilateral transcranial magnetic stimulation on the triphasic EMG-pattern accompanying fast ballistic movements. J. Physiol. 2006;574:917–928. doi: 10.1113/jphysiol.2006.108563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pascual-Leone A, Walsh V. Fast backprojections from the motion to the primary visual area necessary for visual awareness. Science. 2001;292:510–512. doi: 10.1126/science.1057099. [DOI] [PubMed] [Google Scholar]
- 53.Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp. Brain Res. 2003;148:1–16. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- 54.Di Lazzaro V, Oliviero A, Pilato F, et al. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin. Neurophysiol. 2004;115:255–266. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 55.Di L, Ziemann VU, Lemon RN. State of the art: physiology of transcranial motor cortex stimulation. Brain Stimul. 2008;1:345–362. doi: 10.1016/j.brs.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 56.Pascual-Leone A, Walsh V, Rothwell J. Transcranial magnetic stimulation in cognitive neuroscience—virtual lesion, chronometry, and functional connectivity. Curr. Opin. Neurobiol. 2000;10:232–237. doi: 10.1016/s0959-4388(00)00081-7. [DOI] [PubMed] [Google Scholar]
- 57.Bohning DE, Pecheny AP, Epstein CM, Speer AM, et al. Mapping transcranial magnetic stimulation (TMS) fields in vivo with MRI. Neuroreport. 1997;8:2535–2538. doi: 10.1097/00001756-199707280-00023. [DOI] [PubMed] [Google Scholar]
- 58.Ilmoniemi RJ, Virtanen J, Ruohonen J, et al. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport. 1997;8:3537–3540. doi: 10.1097/00001756-199711100-00024. [DOI] [PubMed] [Google Scholar]
- 59.Paus T, Jech R, Thompson CJ, et al. Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J. Neurosci. 1997;17:3178–3184. doi: 10.1523/JNEUROSCI.17-09-03178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paus T. Inferring causality in brain images: a perturbation approach. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:1109–1114. doi: 10.1098/rstb.2005.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bestmann S, Baudewig J, Siebner HR, et al. BOLD MRI responses to repetitive TMS over human dorsal premotor cortex. Neuroimage. 2005;28:22–29. doi: 10.1016/j.neuroimage.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 62.Baudewig J, Siebner HR, Bestmann S, et al. Functional MRI of cortical activations induced by transcranial magnetic stimulation (TMS) Neuroreport. 2001;12:3543–3548. doi: 10.1097/00001756-200111160-00034. [DOI] [PubMed] [Google Scholar]
- 63.Bestmann S, Baudewig J, Siebner HR, et al. Subthreshold high-frequency TMS of human primary motor cortex modulates interconnected frontal motor areas as detected by interleaved fMRI-TMS. Neuroimage. 2003;20:1685–1696. doi: 10.1016/j.neuroimage.2003.07.028. [DOI] [PubMed] [Google Scholar]
- 64.Bestmann S, Oliviero A, Voss M, et al. Cortical correlates of TMS-induced phantom hand movements revealed with concurrent TMS-fMRI. Neuropsychologia. 2006;44:2959–2971. doi: 10.1016/j.neuropsychologia.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 65.Hanakawa T, Mima T, Matsumoto R, Abe M, et al. Stimulus–response profile during single-pulse transcranial magnetic stimulation to the primary motor cortex. Cereb. Cortex. 2009;19:2605–2615. doi: 10.1093/cercor/bhp013. [DOI] [PubMed] [Google Scholar]
- 66.Shitara H, Shinozaki T, Takagishi K, et al. Time course and spatial distribution of fMRI signal changes during single-pulse transcranial magnetic stimulation to the primary motor cortex. Neuroimage. 2011;56:1469–1479. doi: 10.1016/j.neuroimage.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 67.Denslow S, Lomarev M, George MS, Bohning DE. Cortical and subcortical brain effects of transcranial magnetic stimulation (TMS)-induced movement: an interleaved TMS/functional magnetic resonance imaging study. Biol. Psychiatr. 2005;57:752–760. doi: 10.1016/j.biopsych.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 68.Bestmann S, Baudewig J, Siebner HR, et al. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur. J. Neurosci. 2004;19:1950–1962. doi: 10.1111/j.1460-9568.2004.03277.x. [DOI] [PubMed] [Google Scholar]
- 69.Bohning DE, Shastri A, Lomarev MP, et al. BOLD-fMRI response vs. transcranial magnetic stimulation (TMS) pulse-train length: testing for linearity. J. Magn. Reson. Imaging. 2003;17:279–290. doi: 10.1002/jmri.10271. [DOI] [PubMed] [Google Scholar]
- 70.Ruff CC, Blankenburg F, Bjoertomt O, S, et al. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr. Biol. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 71.Kahkonen S, Komssi S, Wilenius J, Ilmoniemi RJ. Prefrontal transcranial magnetic stimulation produces intensity-dependent EEG responses in humans. Neuroimage. 2005;24:955–960. doi: 10.1016/j.neuroimage.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 72.Veniero D, Brignani D, Thut G, Miniussi C. Alpha-generation as basic response-signature to transcranial magnetic stimulation (TMS) targeting the human resting motor cortex: a TMS/EEG co-registration study. Psychophysiology. 2011;48:1381–1389. doi: 10.1111/j.1469-8986.2011.01218.x. [DOI] [PubMed] [Google Scholar]
- 73.Ruff CC, Bestmann S, Blankenburg F, et al. Distinct causal influences of parietal versus frontal areas on human visual cortex: evidence from concurrent TMS fMRI. Cereb. Cortex. 2008;18:817–827. doi: 10.1093/cercor/bhm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bohning DE, Shastri A, Mcconnell KA, et al. A combined TMS/fMRI study of intensity-dependent TMS over motor cortex. Biol. Psychiatr. 1999;45:385–394. doi: 10.1016/s0006-3223(98)00368-0. [DOI] [PubMed] [Google Scholar]
- 75.Draganski B, Kherif F, Kloppel S, et al. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J. Neurosci. 2008;28:7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alexander GE, Crutcher MD, Delong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog. Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- 77.Haber SN. The primate basal ganglia: parallel and integrative networks. J. Chem. Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 78.Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J. Neurosci. 2001;21:RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Strafella AP, Paus T, Fraraccio M, Dagher A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain. 2003;126:2609–2615. doi: 10.1093/brain/awg268. [DOI] [PubMed] [Google Scholar]
- 80.Ilmoniemi R. "TMS and electroencephalography: methods and current advances". In: Wasserman EM, Epstein CM, Ziemann U, Walsh V, Paus T, Lisanby SH, Karhu J, editors. The Oxford Handbook of Transcranial Magnetic Stimulation. Oxford: Oxford University Press; 2008. pp. 593–608. [Google Scholar]
- 81.Esser SK, Huber R, Massimini M, et al. A direct demonstration of cortical LTP in humans: a combined TMS/EEG study. Brain Res. Bull. 2006;69:86–94. doi: 10.1016/j.brainresbull.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 82.Massimini M, Ferrarelli F, Huber R, et al. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- 83.Nikulin VV, Linkenkaer-Hansen K, Nolte G, et al. A novel mechanism for evoked responses in the human brain. Eur. J. Neurosci. 2007;25:3146–3154. doi: 10.1111/j.1460-9568.2007.05553.x. [DOI] [PubMed] [Google Scholar]
- 84.Paus T, Sipila PK, Strafella AP. Synchronization of neuronal activity in the human primary motor cortex by transcranial magnetic stimulation: an EEG study. J. Neurophysiol. 2001;86:1983–1990. doi: 10.1152/jn.2001.86.4.1983. [DOI] [PubMed] [Google Scholar]
- 85.Taylor PC, Nobre AC, Rushworth MF. FEF TMS affects visual cortical activity. Cereb. Cortex. 2007;17:391–399. doi: 10.1093/cercor/bhj156. [DOI] [PubMed] [Google Scholar]
- 86.Taylor PC, Nobre AC, Rushworth MF. Subsecond changes in top down control exerted by human medial frontal cortex during conflict and action selection: a combined transcranial magnetic stimulation electroencephalography study. J. Neurosci. 2007;27:11343–11353. doi: 10.1523/JNEUROSCI.2877-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Komssi S, Aronen HJ, Huttunen J, et al. Ipsi- and contralateral EEG reactions to transcranial magnetic stimulation. Clin. Neurophysiol. 2002;113:175–184. doi: 10.1016/s1388-2457(01)00721-0. [DOI] [PubMed] [Google Scholar]
- 88.Iwahashi M, Arimatsu T, Ueno S, Iramina K. Differences in evoked EEG by transcranial magnetic stimulation at various stimulus points on the head. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2008;2008:2570–2573. doi: 10.1109/IEMBS.2008.4649725. [DOI] [PubMed] [Google Scholar]
- 89.Komssi S, Kahkonen S, Ilmoniemi RJ. The effect of stimulus intensity on brain responses evoked by transcranial magnetic stimulation. Hum. Brain Mapp. 2004;21:154–164. doi: 10.1002/hbm.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ilmoniemi RJ, Ruohonen J, Karhu J. Transcranial magnetic stimulation—a new tool for functional imaging of the brain. Crit. Rev. Biomed. Eng. 1999;27:241–284. [PubMed] [Google Scholar]
- 91.Koch G, Franca M, Albrecht UV, et al. Effects of paired pulse TMS of primary somatosensory cortex on perception of a peripheral electrical stimulus. Exp. Brain Res. 2006;172:416–424. doi: 10.1007/s00221-006-0359-0. [DOI] [PubMed] [Google Scholar]
- 92.Hasan A, Galea JM, Casula EP, et al. Muscle and Timing-specific Functional Connectivity between the Dorsolateral Prefrontal Cortex and the Primary Motor Cortex. J. Cogn Neurosci. 2013;25:558–570. doi: 10.1162/jocn_a_00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Davare M, Montague K, Olivier E, JC, et al. Ventral premotor to primary motor cortical interactions during object-driven grasp in humans. Cortex. 2009;45:1050–1057. doi: 10.1016/j.cortex.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mochizuki H, Huang YZ, Rothwell JC. Interhemispheric interaction between human dorsal premotor and contralateral primary motor cortex. J. Physiol. 2004;561:331–338. doi: 10.1113/jphysiol.2004.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Civardi C, Cantello R, Asselman P, Rothwell JC. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage. 2001;14:1444–1453. doi: 10.1006/nimg.2001.0918. [DOI] [PubMed] [Google Scholar]
- 96.Koch G, Franca M, Del Olmo MF, et al. Time course of functional connectivity between dorsal premotor and contralateral motor cortex during movement selection. J. Neurosci. 2006;26:7452–7459. doi: 10.1523/JNEUROSCI.1158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koch G, Fernandez DO, Cheeran B, et al. Functional interplay between posterior parietal and ipsilateral motor cortex revealed by twin-coil transcranial magnetic stimulation during reach planning toward contralateral space. J. Neurosci. 2008;28:5944–5953. doi: 10.1523/JNEUROSCI.0957-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fujiwara T, Rothwell JC. The after effects of motor cortex rTMS depend on the state of contraction when rTMS is applied. Clin. Neurophysiol. 2004;115:1514–1518. doi: 10.1016/j.clinph.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 99.Mazzocchio R, Rothwell JC, Day BL, Thompson PD. Effect of tonic voluntary activity on the excitability of human motor cortex. J. Physiol. 1994;474:261–267. doi: 10.1113/jphysiol.1994.sp020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J. Physiol. 1995;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aurora SK, Welch KM. Brain excitability in migraine: evidence from transcranial magnetic stimulation studies. Curr. Opin. Neurol. 1998;11:205–209. doi: 10.1097/00019052-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 102.Bestmann S, Ruff CC, Blakemore C, et al. Spatial attention changes excitability of human visual cortex to direct stimulation. Curr. Biol. 2007;17:134–139. doi: 10.1016/j.cub.2006.11.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cattaneo Z, Mattavelli G, Papagno C, et al. The role of the human extrastriate visual cortex in mirror symmetry discrimination: a TMS-adaptation study. Brain Cogn. 2011;77:120–127. doi: 10.1016/j.bandc.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 104.Cattaneo Z, Silvanto J. Investigating visual motion perception using the transcranial magnetic stimulation-adaptation paradigm. Neuroreport. 2008;19:1423–1427. doi: 10.1097/WNR.0b013e32830e0025. [DOI] [PubMed] [Google Scholar]
- 105.Silvanto J, Muggleton NG, Cowey A, Walsh V. Neural adaptation reveals state-dependent effects of transcranial magnetic stimulation. Eur. J. Neurosci. 2007;25:1874–1881. doi: 10.1111/j.1460-9568.2007.05440.x. [DOI] [PubMed] [Google Scholar]
- 106.Ilmoniemi RJ, Ruohonen J, Virtanen J, et al. EEG responses evoked by transcranial magnetic stimulation. Electroencephalogr. Clin. Neurophysiol. 1999;Suppl 51:22–29. [PubMed] [Google Scholar]
- 107.Bestmann S, Swayne O, Blankenburg F, et al. Dorsal premotor cortex exerts state-dependent causal influences on activity in contralateral primary motor and dorsal premotor cortex. Cereb. Cortex. 2008;18:1281–1291. doi: 10.1093/cercor/bhm159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moisa M, Siebner HR, Pohmann R, Thielscher A. Uncovering a context-specific connectional fingerprint of human dorsal premotor cortex. J. Neurosci. 2012;32:7244–7252. doi: 10.1523/JNEUROSCI.2757-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blankenburg F, Ruff CC, Bestmann S, et al. Interhemispheric effect of parietal TMS on somatosensory response confirmed directly with concurrent TMS-fMRI. J. Neurosci. 2008;28:13202–13208. doi: 10.1523/JNEUROSCI.3043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leitao J, Thielscher A, Werner S, et al. Effects of parietal TMS on visual and auditory processing at the primary cortical level—a concurrent TMS-fMRI study. Cereb. Cortex. 2012 doi: 10.1093/cercor/bhs078. DOI: 10.1093/cercor/bhs078. [DOI] [PubMed] [Google Scholar]
- 111.Blankenburg F, Ruff CC, Bestmann S, et al. Studying the role of human parietal cortex in visuospatial attention with concurrent TMS-fMRI. Cereb. Cortex. 2010;20:2711. doi: 10.1093/cercor/bhq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Heinen K, Ruff CC, Bjoertomt O, et al. Concurrent TMS-fMRI reveals dynamic interhemispheric influences of the right parietal cortex during exogenously cued visuospatial attention. Eur. J. Neurosci. 2011;33:991–1000. doi: 10.1111/j.1460-9568.2010.07580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Feredoes E, Heinen K, Weiskopf N, Ruff C, Driver J. Causal evidence for frontal involvement in memory target maintenance by posterior brain areas during distracter interference of visual working memory. Proc. Natl. Acad. Sci. USA. 2011;108:17510–17515. doi: 10.1073/pnas.1106439108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miller EK, Desimone R. Parallel neuronal mechanisms for short-term memory. Science. 1994;263:520–522. doi: 10.1126/science.8290960. [DOI] [PubMed] [Google Scholar]
- 115.Sakai K, Rowe JB, Passingham RE. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nat. Neurosci. 2002;5:479–484. doi: 10.1038/nn846. [DOI] [PubMed] [Google Scholar]
- 116.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 117.Duncan J. An adaptive coding model of neural function in prefrontal cortex. Nat. Rev. Neurosci. 2001;2:820–829. doi: 10.1038/35097575. [DOI] [PubMed] [Google Scholar]
- 118.O'shea J, Thut G. "Noninvasive brain stimulation and neuorimaging: novel ways of assessing causal relationships in brain networks". In: Miniussi C, Paulus W, Rossini PM, Bestmann S, editors. Transcranial Magnetic Stimulation. Boca Raton: CRC Press; 2013. pp. 307–334. [Google Scholar]
- 119.Sack AT, Kohler A, Bestmann S, et al. Imaging the brain activity changes underlying impaired visuospatial judgments: simultaneous FMRI, TMS, and behavioral studies. Cereb. Cortex. 2007;17:2841–2852. doi: 10.1093/cercor/bhm013. [DOI] [PubMed] [Google Scholar]
- 120.Zanto TP, Rubens MT, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat. Neurosci. 2011;14:656–661. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pfurtscheller G, Stancak A, Jr, Neuper C. Event-related synchronization (ERS) in the alpha band—an electrophysiological correlate of cortical idling: a review. Int. J. Psychophysiol. 1996;24:39–46. doi: 10.1016/s0167-8760(96)00066-9. [DOI] [PubMed] [Google Scholar]
- 122.Dugue L, Marque P, Vanrullen R. The phase of ongoing oscillations mediates the causal relation between brain excitation and visual perception. J. Neurosci. 2011;31:11889–11893. doi: 10.1523/JNEUROSCI.1161-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Romei V, Brodbeck V, Michel C, et al. Spontaneous fluctuations in posterior {alpha}-and EEG activity reflect variability in excitability of human visual areas. Cereb. Cortex. 2008;18:2010–2018. doi: 10.1093/cercor/bhm229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Capotosto P, Babiloni C, Romani GL, Corbetta M. Differential contribution of right and left parietal cortex to the control of spatial attention: a simultaneous EEG-rTMS study. Cereb. Cortex. 2012;22:446–454. doi: 10.1093/cercor/bhr127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ruff CC, Blankenburg F, Bjoertomt O, et al. Hemispheric differences in frontal and parietal influences on human occipital cortex: direct confirmation with concurrent TMS-fMRI. J. Cogn. Neurosci. 2009;21:1146–1161. doi: 10.1162/jocn.2009.21097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gould IC, Nobre AC, Wyart V, Rushworth MF. Effects of decision variables and intraparietal stimulation on sensorimotor oscillatory activity in the human brain. J. Neurosci. 2012;32:13805–13818. doi: 10.1523/JNEUROSCI.2200-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sauseng P, Klimesch W, Heise KF, et al. Brain oscillatory substrates of visual short-term memory capacity. Curr. Biol. 2009;19:1846–1852. doi: 10.1016/j.cub.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 128.Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cereb. Cortex. 2002;12:877–882. doi: 10.1093/cercor/12.8.877. [DOI] [PubMed] [Google Scholar]
- 129.Klimesch W, Sauseng P, Gerloff C. Enhancing cognitive performance with repetitive transcranial magnetic stimulation at human individual alpha frequency. Eur. J. Neurosci. 2003;17:1129–1133. doi: 10.1046/j.1460-9568.2003.02517.x. [DOI] [PubMed] [Google Scholar]
- 130.Hamidi M, Slagter HA, tononi G, Postle BR. Repetitive transcranial magnetic stimulation affects behavior by biasing endogenous cortical oscillations. Front. Integr. Neurosci. 2009;3:14. doi: 10.3389/neuro.07.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hamidi M, Tononi G, Postle BR. Evaluating the role of prefrontal and parietal cortices in memory-guided response with repetitive transcranial magnetic stimulation. Neuropsychologia. 2009;47:295–302. doi: 10.1016/j.neuropsychologia.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Palva S, Palva JM. New vistas for alpha-frequency band oscillations. Trends Neurosci. 2007;30:150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 133.Johnson JS, Kundu B, Casali AG, Postle BR. Task-dependent changes in cortical excitability and effective connectivity: a combined TMS-EEG study. J. Neurophysiol. 2012;107:2383–2392. doi: 10.1152/jn.00707.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Thut G, Veniero D, Romei V, et al. Rhythmic TMS causes local entrainment of natural oscillatory signatures. Curr. Biol. 2011;21:1176–1185. doi: 10.1016/j.cub.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Romei V, Gross J, Thut G. On the role of prestimulus alpha rhythms over occipito-parietal areas in visual input regulation: correlation or causation? J. Neurosci. 2010;30:8692–8697. doi: 10.1523/JNEUROSCI.0160-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kriegeskorte N. Relating Population-Code Representations between Man, Monkey, and Computational Models. Front. Neurosci. 2009;3:363–373. doi: 10.3389/neuro.01.035.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Smith SM. The future of FMRI connectivity. Neuroimage. 2012;62:1257–1266. doi: 10.1016/j.neuroimage.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 138.Friston KJ. Modalities, modes, and models in functional neuroimaging. Science. 2009;326:399–403. doi: 10.1126/science.1174521. [DOI] [PubMed] [Google Scholar]
- 139.Kujirai T, Caramia MD, Rothwell JC, et al. Corticocortical inhibition in human motor cortex. J. Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J. Physiol. 1996;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ziemann U, Rothwell JC. I-waves in motor cortex. J. Clin. Neurophysiol. 2000;17:397–405. doi: 10.1097/00004691-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 142.Marreiros AC, Kiebel SJ, Daunizeau J, et al. Population dynamics under the Laplace assumption. Neuroimage. 2009;44:701–714. doi: 10.1016/j.neuroimage.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 143.Kiebel SJ, Garrido MI, Friston KJ. Dynamic causal modelling of evoked responses: the role of intrinsic connections. Neuroimage. 2007;36:332–345. doi: 10.1016/j.neuroimage.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 144.Moran RJ, Stephan KE, Dolan RJ, Friston KJ. Consistent spectral predictors for dynamic causal models of steady-state responses. Neuroimage. 2011;55:1694–1708. doi: 10.1016/j.neuroimage.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Moran RJ, Mallet N, Litvak V, et al. Alterations in brain connectivity underlying beta oscillations in Parkinsonism. PLoS Comput. Biol. 2011;7:e1002124. doi: 10.1371/journal.pcbi.1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Penny WD, Litvak V, Fuentemilla L, et al. Dynamic Causal Models for phase coupling. J. Neurosci. Methods. 2009;183:19–30. doi: 10.1016/j.jneumeth.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen CC, Kiebel SJ, Friston KJ. Dynamic causal modelling of induced responses. Neuroimage. 2008;41:1293–1312. doi: 10.1016/j.neuroimage.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 148.Esser SK, Hill SL, Tononi G. Modeling the effects of transcranial magnetic stimulation on cortical circuits. J. Neurophysiol. 2005;94:622–639. doi: 10.1152/jn.01230.2004. [DOI] [PubMed] [Google Scholar]
- 149.Cona F, Zavaglia M, Massimini M, et al. A neural mass model of interconnected regions simulates rhythm propagation observed via TMS-EEG. Neuroimage. 2011;57:1045–1058. doi: 10.1016/j.neuroimage.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 150.Li X, Large CH, Ricci R, et al. Using interleaved transcranial magnetic stimulation/functional magnetic resonance imaging (fMRI) and dynamic causal modeling to understand the discrete circuit specific changes of medications: lamotrigine and valproic acid changes in motor or prefrontal effective connectivity. Psychiatry Res. 2011;194:141–148. doi: 10.1016/j.pscychresns.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 151.Donner TH, Siegel M. A framework for local cortical oscillation patterns. Trends Cogn. Sci. 2011;15:191–199. doi: 10.1016/j.tics.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 152.Romei V, Murray MM, Cappe C, Thut G. Preperceptual and stimulus-selective enhancement of low-level human visual cortex excitability by sounds. Curr. Biol. 2009;19:1799–1805. doi: 10.1016/j.cub.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 153.Zaehle T, Rach S, Herrmann CS. Transcranial alternating current stimulation enhances individual alpha activity in human EEG. PLoS One. 2010;5:e13766. doi: 10.1371/journal.pone.0013766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Polania R, Nitsche MA, Korman C, et al. The importance of timing in segregated theta phase-coupling for cognitive performance. Curr. Biol. 2012;22:1314–1318. doi: 10.1016/j.cub.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 155.Antal A, Paulus W. Investigating neuroplastic changes in the human brain induced by transcranial direct (tDCS) and alternating current (tACS) stimulation methods. Clin. EEG. Neurosci. 2012;43:175. doi: 10.1177/1550059412448030. [DOI] [PubMed] [Google Scholar]
- 156.Antal A, Boros K, Poreisz C, L, et al. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimul. 2008;1:97–105. doi: 10.1016/j.brs.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 157.Chaieb L, Antal A, Paulus W. Transcranial alternating current stimulation in the low kHz range increases motor cortex excitability. Restor. Neurol. Neurosci. 2011;29:167–175. doi: 10.3233/RNN-2011-0589. [DOI] [PubMed] [Google Scholar]
- 158.Laczo B, Antal A, Niebergall R, et al. Transcranial alternating stimulation in a high gamma frequency range applied over V1 improves contrast perception but does not modulate spatial attention. Brain Stimul. 2012;5:484–491. doi: 10.1016/j.brs.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 159.Moliadze V, Antal A, Paulus W. Boosting brain excitability by transcranial high frequency stimulation in the ripple range. J. Physiol. 2010;588:4891–4904. doi: 10.1113/jphysiol.2010.196998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bikson M, Rahman A, Datta A, et al. High-resolution modeling assisted design of customized and individualized transcranial direct current stimulation protocols. Neuromodulation. 2012;15:306–315. doi: 10.1111/j.1525-1403.2012.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bikson M, Datta A, Rahman A, Scaturro J. Electrode montages for tDCS and weak transcranial electrical stimulation: role of “return” electrode's position and size. Clin. Neurophysiol. 2010;121:1976–1978. doi: 10.1016/j.clinph.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bikson M, Rahman A, Datta A. Computational models of transcranial direct current stimulation. Clin. EEG. Neurosci. 2012;43:176–183. doi: 10.1177/1550059412445138. [DOI] [PubMed] [Google Scholar]
- 163.Kuo HI, Bikson M, Datta A, et al. Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: a neurophysiological study. Brain Stimul. 2012 doi: 10.1016/j.brs.2012.09.010. DOI: 10.1016/j.brs.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 164.Minhas P, Bansal V, Patel J, et al. Electrodes for high-definition transcutaneous DC stimulation for applications in drug delivery and electrotherapy, including tDCS. J. Neurosci. Methods. 2010;190:188–197. doi: 10.1016/j.jneumeth.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Datta A, Truong D, Minhas P, et al. Inter-individual variation during transcranial direct current stimulation and normalization of dose using MRI-derived computational models. Front Psychiatr. 2012;3:91. doi: 10.3389/fpsyt.2012.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Stagg CJ, Bachtiar V, Johansen-Berg H. What are we measuring with GABA magnetic resonance spectroscopy? Commun. Integr. Biol. 2011;4:573–575. doi: 10.4161/cib.4.5.16213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Stagg CJ, Wylezinska M, Matthews PM, et al. The Neurochemical Effects of Theta Burst Stimulation as assessed by Magnetic Resonance Spectroscopy. J. Neurophysiol. 2009;101:2872–2877. doi: 10.1152/jn.91060.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Stagg CJ, O'shea J, Johansen-Berg H. Imaging the effects of rTMS-induced cortical plasticity. Restor. Neurol. Neurosci. 2010;28:425–436. doi: 10.3233/RNN-2010-0553. [DOI] [PubMed] [Google Scholar]
- 169.Stagg CJ, Best JG, Stephenson MC, et al. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J. Neurosci. 2009;29:5202–5206. doi: 10.1523/JNEUROSCI.4432-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stagg CJ, Jayaram G, Pastor D, et al. Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia. 2011;49:800–804. doi: 10.1016/j.neuropsychologia.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Stagg CJ, Bestmann S, Constantinescu AO, et al. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J. Physiol. 2011;589:5845–5855. doi: 10.1113/jphysiol.2011.216978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Boy F, Evans CJ, Edden RA, et al. Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr. Biol. 2010;20:1779–1785. doi: 10.1016/j.cub.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Stagg CJ, Bachtiar V, Johansen-Berg H. The role of GABA in human motor learning. Curr. Biol. 2011;21:480–484. doi: 10.1016/j.cub.2011.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Floyer-Lea A, Wylezinska M, Kincses T, Matthews PM. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J. Neurophysiol. 2006;95:1639–1644. doi: 10.1152/jn.00346.2005. [DOI] [PubMed] [Google Scholar]
- 175.Jocham G, Hunt LT, Near J, Behrens TE. A mechanism for value-guided choice based on the excitation-inhibition balance in prefrontal cortex. Nat. Neurosci. 2012;15:960–961. doi: 10.1038/nn.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sumner P, Edden RA, Bompas A, et al. More GABA, less distraction: a neurochemical predictor of motor decision speed. Nat. Neurosci. 2010;13:825–827. doi: 10.1038/nn.2559. [DOI] [PubMed] [Google Scholar]
- 177.Ziemann U. TMS and drugs. Clin. Neurophysiol. 2004;115:1717–1729. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 178.Paulus W, Classen J, Cohen LG, et al. State of the art: pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimul. 2008;1:151–163. doi: 10.1016/j.brs.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 179.Nitsche MA, Monte-Silva K, Kuo MF, Paulus W. Dopaminergic impact on cortical excitability in humans. Rev. Neurosci. 2010;21:289–298. doi: 10.1515/revneuro.2010.21.4.289. [DOI] [PubMed] [Google Scholar]
- 180.Rosen Y, Lenkinski RE. Recent advances in magnetic resonance neurospectroscopy. Neurotherapeutics. 2007;4:330–345. doi: 10.1016/j.nurt.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bonmassar G, Lee SW, Freeman DK, et al. Microscopic magnetic stimulation of neural tissue. Nat. Commun. 2012;3:921. doi: 10.1038/ncomms1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bystritsky A, Korb AS, Douglas PK, et al. A review of low-intensity focused ultrasound pulsation. Brain Stimul. 2011;4:125–136. doi: 10.1016/j.brs.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 183.Tufail Y, Yoshihiro A, Pati S, et al. Ultrasonic neuromodulation by brain stimulation with transcranial ultrasound. Nat. Protoc. 2011;6:1453–1470. doi: 10.1038/nprot.2011.371. [DOI] [PubMed] [Google Scholar]
- 184.Tufail Y, Matyushov A, Baldwin N, et al. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 2010;66:681–694. doi: 10.1016/j.neuron.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 185.Bernstein JG, Boyden ES. Optogenetic tools for analyzing the neural circuits of behavior. Trends Cogn. Sci. 2011;15:592–600. doi: 10.1016/j.tics.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lee JH, Durand R, Gradinaru V, et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature. 2010;465:788–792. doi: 10.1038/nature09108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pina-Crespo JC, Talantova M, Cho EG, et al. High-frequency hippocampal oscillations activated by optogenetic stimulation of transplanted human ESC-derived neurons. J. Neurosci. 2012;32:15837–15842. doi: 10.1523/JNEUROSCI.3735-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Armstrong KM, Fitzgerald JK, Moore T. Changes in visual receptive fields with microstimulation of frontal cortex. Neuron. 2006;50:791–798. doi: 10.1016/j.neuron.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 189.Allen EA, Pasley BN, Duong T, Freeman RD. Transcranial magnetic stimulation elicits coupled neural and hemodynamic consequences. Science. 2007;317:1918–1921. doi: 10.1126/science.1146426. [DOI] [PubMed] [Google Scholar]
- 190.Logothetis NK, Augath M, Murayama Y, et al. The effects of electrical microstimulation on cortical signal propagation. Nat. Neurosci. 2010;13:1283–1291. doi: 10.1038/nn.2631. [DOI] [PubMed] [Google Scholar]
- 191.Armstrong KM, Moore T. Rapid enhancement of visual cortical response discriminability by microstimulation of the frontal eye field. Proc. Natl. Acad. Sci. USA. 2007;104:9499–9504. doi: 10.1073/pnas.0701104104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- 193.Tehovnik EJ, Tolias AS, Sultan F, et al. Direct and indirect activation of cortical neurons by electrical microstimulation. J. Neurophysiol. 2006;96:512–521. doi: 10.1152/jn.00126.2006. [DOI] [PubMed] [Google Scholar]
- 194.Tolias AS, Sultan F, Augath M, et al. Mapping cortical activity elicited with electrical microstimulation using FMRI in the macaque. Neuron. 2005;48:901–911. doi: 10.1016/j.neuron.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 195.Walsh V, Rushworth M. A primer of magnetic stimulation as a tool for neuropsychology. Neuropsychologia. 1999;37:125–135. [PubMed] [Google Scholar]
- 196.Thielscher A, Wichmann FA. Determining the cortical target of transcranial magnetic stimulation. Neuroimage. 2009;47:1319–1330. doi: 10.1016/j.neuroimage.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 197.Freund P, Rothwell J, Craggs M, et al. Corticomotor representation to a human forearm muscle changes following cervical spinal cord injury. Eur. J. Neurosci. 2011;34:1839–1846. doi: 10.1111/j.1460-9568.2011.07895.x. [DOI] [PubMed] [Google Scholar]


