Abstract
Objective
To determine whether the combination of triiodothyronine (T3) plus sertraline at treatment initiation confers greater antidepressant efficacy than sertraline plus placebo in patients with major depressive disorder.
Method
Eight-week, double blind, randomized placebo controlled clinical trial of 153 adult outpatients between 18 and 60 years of age, with DSM-IV defined major depressive disorder. Patients were treated with sertraline flexibly adjusted for tolerability and in a double blind fashion with placebo or T3 (25 μg/day in week 1 and increasing to 50 μg/day in week 2). Response was defined categorically as 50% reduction and total score less than 15 in 21-item Hamilton Rating Scale for Depression (HRSD-21) at week 8 and remission as HRSD-21 less than 8.
Results
There was no difference between treatment groups at final assessment; 65% of placebo and 61.8% of T3 treated subjects achieved response and 50.6% of placebo and 40.8% of T3 treated patients achieved remission. The mean daily dose at final assessment of sertraline and T3, respectively was 144.7 mg (±48.7 mg) and 48.2 μg (±7 μg). Median time to response did not differ between treatment groups. Baseline thyroid function tests did not predict response to sertraline treatment or T3 augmentation.
Conclusions
These results do not support the routine use of T3 to enhance or accelerate onset of antidepressant response in patients with major depressive disorder.
Keywords: Depression, Triiodothyronine, Augmentation, Response, Remission, Antidepressant
1. Introduction
The goal of acute treatment of unipolar depressive illness is full remission (Rush et al., 2006a). The long-term objective of treatment is a protracted period of remission, which is referred to as recovery. Remission is generally defined as a period during which the patient does not meet syndromal criteria for a depressive episode and has only negligible residual symptoms (Frank et al., 1991; Nierenberg and Wright, 1999; Rush et al., 2006a). Practically, the definition of remission is based on a minimal score on one of the instruments utilized to quantify severity of depressive symptoms such as the Hamilton Rating Scale for Depression (HRDS), Montgomery– Asberg Depression Rating Scale (MADRS) or Quick Inventory of Depressive Symptoms (QIDS). Unfortunately, remission is only achieved by 30–45% of subjects during the first course of treatment. The common definition of response is a 50% reduction in baseline depression severity scores. Response has been reported to occur in 40–65% of subjects during the first course of treatment with antidepressant agents, in a variety of different treatment settings (Rush et al., 2004; Seemuller et al., 2010; Thase et al., 2001; Trivedi et al., 2006b).
A variety of strategies have been utilized to increase the disappointingly low numbers of patients treated with antidepressants who attain remission and ultimately recover. The main approach to increasing the therapeutic yield of antidepressants has been to augment their action with other agents and modalities. Combination therapies have been studied as augmentation agents in subjects who have had an inadequate response to antidepressant monotherapy and in coinitiation formats to attempt to enhance antidepressant benefit from the very beginning of treatment. Agents that have been scrutinized as follow-on augmentation agents include thyroid hormones, lithium, second antidepressants, atypical antipsychotics, pindolol, electroconvulsive therapy (ECT) and psychotherapies (Aronson et al., 1996; Bauer et al., 2003; Keitner et al., 2009; Maes et al., 1999; Nierenberg et al., 2006; Papkostas and Shelton, 2008; Rush et al., 2006b; Trivedi et al., 2006a). Coinitiation strategies involving combinations of two antidepressants have been investigated with equivocal results. There is at least some evidence in support of these interventions and simultaneously considerable evidence for lack of benefit for this approach (Blier et al., 2010; Rush et al., 2011; Stewart et al., 2009). And for some common augmentation strategies there is virtually no supporting data (Fava and Rush, 2006). Despite the lack of clear evidence based guidance, augmentation strategies are remarkably common in clinical practice, with as many as 20% or more of antidepressant treated patients receiving a second medication, although the sequence of addition is not reported in this survey (Valenstein et al., 2006).
Thyroid hormone augmentation of antidepressant action, usually with triiodothyronine (T3) has been studied for the past five decades (Feldmesser-Reiss, 1958; Flach et al., 1958). Thyroid hormone addition to antidepressants has been investigated for a number of different possible uses. These include the addition of T3 to augment the response in patients with treatment resistant depression and to accelerate or potentiate the antidepressant action at treatment initiation in subjects who are not treatment resistant. Initial reports were of a positive benefit to the addition of T3 to tricyclic antidepressants (TCA) in patients who were partial responders, but these results have not been consistently replicated in large controlled clinical trials (Aronson et al., 1996). The combination of T3 with TCAs has also been reported to accelerate the onset of antidepressant action in a limited number of studies. This effect seemed to be more prominent in female patients (Altshuler et al., 2001). These results need to be interpreted with significant caution though, as the studies included in this meta-analysis included only 125 total patients.
The data on the use of thyroid hormone augmentation with newer classes of antidepressant (SSRI, SNRI, bupropion, mirtazapine) appears even less clear than that for the TCAs (Abraham et al., 2006; Appelhof et al., 2004; Cooper-Kazaz et al., 2007; Iosifescu et al., 2005; Nierenberg et al., 2006; Papkostas et al., 2009; Posternak et al., 2007). One relatively large placebo-controlled clinical trial (124 subjects) in which T3 was added to sertraline reported a higher remission rate for the combination with 58% of T3 versus 38% of placebo patients attaining this result (Cooper-Kazaz et al., 2007). A second very similar placebo-controlled study of 106 subjects utilizing paroxetine and two doses of T3 (25 and 50 ug/d) found no benefit to the combination (Appelhof et al., 2004). However, the patients in the second study who received T3 did experience more adverse events than those on placebo. A smaller study (50 subjects) utilizing an acceleration design and a mixture of antidepressants (SSRIs, SNRI, bupropion and mirtazapine) demonstrated a numerically superior but statistically non-significant benefit to the combination of 25 ug T3 plus antidepressant on overall response rate; 61% of T3 versus 52% of placebo and remission rate, 41% of T3 versus 37% of placebo treated patients (Posternak et al., 2007). Meta-analysis of 4 trials in which T3 was initiated simultaneously with an SSRI in patients who were not treatment resistant (444 total patients) revealed no added benefit to the combination in terms of achieving response or remission (Papkostas et al., 2009).
Augmentation of antidepressant action with T3 in treatment resistant patients was investigated in the recently published STAR*D trial (Nierenberg et al., 2006). Subjects entering Level 3 of the protocol, who had previously failed two different regimens were randomly assigned to receive either T3 or lithium augmentation. In this group of treatment resistant patients, the addition of T3 resulted in 24.7% reaching remission whereas 15.9% of lithium treated patients met this goal.
We present the single largest, prospective, randomized, placebo-controlled study of T3 added to an SSRI (sertraline) at treatment initiation (so called enhancement or acceleration design) in patients with major depressive disorder, specifically in subjects who were not treatment resistant. The primary hypothesis tested was that the addition of T3 to sertraline would significantly increase response and remission rates at study end point compared to placebo.
2. Methods
The Institutional Review Board of Emory University reviewed and approved of the conduct of this clinical investigation. All subjects were provided complete description of the study and signed written informed consent to participate. This study was conducted at the Mood and Anxiety Disorders Research Clinic in the Department of Psychiatry and Behavioral Sciences at Emory University School of Medicine between 1998 and 2003.
2.1. Patients
Male and female patients between the ages of 18 and 60 were included in this trial. Diagnostic inclusion criteria were a DSM-IV defined diagnosis of major depressive disorder (MDD), without psychotic features, as identified with the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1998). There was no exclusion based on length of current depressive episode, number of previous episodes, chronicity or treatment failure. Symptom severity inclusion criteria were of 21-item HRSD of 18 or greater at screening and baseline assessment one week later. Patients with stable medical conditions (excluding thyroid disease) were included if treatment with sertraline and T3 was not contraindicated. Suicide risk did not exclude subjects a priori, but was assessed on an individual basis by experienced psychiatrists to determine advisability of study participation, with the guiding principle of including patients who could be safely maintained in a frequently monitored outpatient setting. Psychiatric exclusion criteria included any other DSM-IV axis I or II diagnosis including Bipolar disorder, Schizophrenia, Schizoaffective disorder, substance abuse or dependence in the past year, with the exception of nicotine dependence, and personality disorders. Patients with depression who were in active treatment and met criteria for remission were excluded. Patients who had previously failed treatment with sertraline and those who had ECT in the past 6 months were excluded. Patients with unstable medical conditions and with known thyroid disease were excluded. Women who were pregnant or breast-feeding were excluded and all subjects had to utilize effective methods of contraception. Patients were not permitted to participate in any form of depression specific psychotherapy during the time they were enrolled in this trial.
Recruitment was from within the various clinical services maintained by the Department of Psychiatry at Emory, through contacts with various providers in the region and through advertisements in local media (print, radio). Therefore a mixture of spontaneously care seeking individuals and those responding to advertisements was recruited. Potential subjects underwent a structured telephone interview conducted by an experienced research associate and those who seemed likely candidates were invited in for assessment.
2.2. Study design
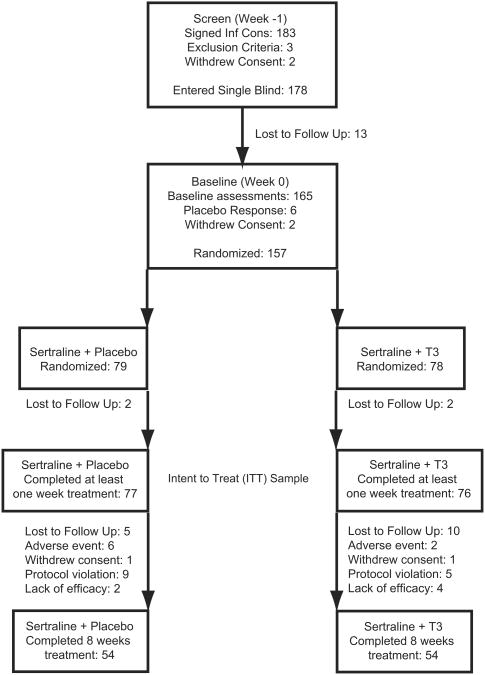
There were a total of eight study visits in a nine-week interval, with screening followed at 1 week by baseline assessments and then six clinical visits in the ensuing eight weeks (Fig. 1). During the screening visit, the patients signed informed consent and then received a comprehensive psychiatric assessment including Structured Clinical Interview for DSM-IV (SCID), 21-item Hamilton Depression Rating Scale for Depression (HRSD-21), Montgomery–Asberg Depression Rating Scale (MADRS), Hamilton Rating Scale for Anxiety (HRSA), Clinical Global Impression-Severity (CGI-S), and semi structured interview to capture other relevant patient characteristics/demographics and treatment history (Guy, 1976; Hamilton, 1960; Montgomery and Asberg, 1979). Psychiatric inclusion and exclusion criteria were established with SCID and HRSD-21. Those subjects that met psychiatric inclusion criteria had a comprehensive medical history taken, physical examination, electrocardiogram, and laboratory evaluation including blood chemistries, hematology, urinalysis, urine drug screen, pregnancy test (for female subjects), and thyroid function. The Emory Clinical Laboratory performed the basic thyroid function tests including TSH, T3, total T4, and free T4 with standard immunoassay methods (A subset of subjects (n = 111) was admitted to the General Clinical Research Center (GCRC) at Emory University Hospital for more comprehensive thyroid function testing and these results are reported elsewhere). Subjects who fulfilled all inclusion criteria at screening received one week of single blind placebo treatment.
Fig. 1.
Flowchart of study with disposition of all subjects who signed informed consent and completed screening visit.
Subjects returned one week later for baseline assessments that included all of the previously described symptom severity scales with the addition of the CGI-Improvement (CGI-I) scale. Those subjects that continued to have HRSD-21 scores of 18 or greater entered the active treatment phase of the study and the early placebo responsive patients did not. All patients who entered active treatment received sertraline at an initial dose of 50 mg per day, which was titrated to therapeutic effect and tolerability in 50 mg increments each week to maximal daily dose of 200 mg. Patients were randomly assigned to receive T3 (as 25 μg pill) or identical pill placebo starting at 25 μg per day, with mandatory increase to 50 μg per day in the second week of treatment and adjustable downward thereafter based on tolerability. Randomization was by a block schedule without stratification, prepared by a statistician. Patients returned for clinical assessment and medication adjustment 1, 2, 3, 4, 6, and 8 weeks after initiation of active treatment. Those patients who took at least one dose of study medication (T3) in combination with sertraline and returned for their first clinical assessment were included in the intent to treat (ITT) sample and in the final data analysis. The full battery of symptom severity scales was administered to subjects at each clinical visit. Adverse events were elicited by open-ended interview. Treatment adherence was determined with pill counts of the returned medication packages and new medications were dispensed at each clinical visit. At study completion, T3 was discontinued and patients were given a month supply of sertraline and referral to an appropriate treatment provider.
2.3. Statistical analysis
The primary outcome was response, which was defined as a 50% reduction in baseline HRSD-21 score and a total less than 15 at study end point. The secondary outcome was remission, which was defined as HRSD-21 less than 8 at study end point. A last observation carried forward (LOCF) design was utilized and all comparisons were made to baseline symptom severity scores. Categorical variables were compared with contingency tables with χ2 and Fishers exact statistics. Continuous variables were compared with t tests, ANOVA and repeated measures ANOVA as appropriate. Kaplan–Meier survival analysis was utilized to scrutinize time to achieving response criteria between treatment groups. All statistical significance was defined as two-tailed α < 0.05. Statistical analysis was conducted with JMP 8 (SAS Institute Inc).
3. Results
A total of 183 subjects signed informed consent and completed the screening visit (Fig. 1). Of these, three did not meet inclusion criteria and two withdrew consent, leaving 178 that entered the single-blind placebo phase. Thirteen of the subjects that entered the single-blind phase did not return for their next visit, with a resultant 165 subjects receiving baseline assessments. There were six subjects who responded to placebo who were removed from the study after baseline assessments and an additional two subjects withdrew consent at that time. The remaining 157 subjects were initiated on open label sertraline and randomly assigned to treatment with placebo or triiodothyronine (T3). Of the subjects who entered the double-blind portion of the study, 153 returned for at least one post randomization assessment and are included in the modified ITT efficacy analysis. There were 32 (20.9%) minority subjects in the sample, with 28 (18.3%) African American, 1 Hispanic, and 3 Asian/Pacific patients. More minority subjects were assigned to placebo (p < 0.03, two-tailed), but otherwise there were no baseline differences in demographic or clinical characteristics between the two treatment groups (Table 1). There were 108 subjects that completed all of the study visits and there were no significant differences between treatment groups in the numbers of subjects or reasons for early discontinuation (Table 2).
Table 1.
Baseline characteristics of all subjects included in ITT sample.
| Placebo (n = 77) | Triiodothyronine (n = 76) | Total (n = 153) | |
|---|---|---|---|
| Patient characteristics | |||
| Age (SD) | 41.4 (11.5) | 43 (10.5) | 42.2 (11) |
| Female No. (%) | 51 (68) | 44 (57.9) | 95 (62.1) |
| Education yrs (SD) | 14.9 (2) | 15.5 (1.7) | 15.1 (1.9) |
| Racea | |||
| White (%) | 55 (71.4) | 66 (86.8) | 121 (79.1) |
| African American (%) | 19 (12.4) | 9 (5.9) | 28 (18.3) |
| Other minority (%) | 3 (2) | 1 (0.65) | 4 (2.6) |
| Disease characteristics | |||
| Past depressive episodes (SD) | 1.1(1) | 1.5 (1.4) | 1.3 (1.2) |
| Any past treatment (%) | 47 (62.7) | 53 (69.7) | 102 (66) |
| Past SSRI treatment (%) | 42 (56) | 45 (59.2) | 89 (58.1) |
| Past response SSRI (%) | 20 (26.6) | 29 (38.1) | 51 (33.3) |
| Baseline HSRD-21 (SD) | 22.8 (4.6) | 22.8 (4.3) | 22.8 (4.4) |
| Baseline MADRS (SD) | 26.6 (5.9) | 27.7 (5.4) | 27.2 (5.7) |
| Thyroid function | |||
| Baseline TSH mIU/ml (SD) | 1.6 (1.2) | 1.8 (2) | 1.7 (1.6) |
| Baseline total T3 ng/dl (SD) | 115.8 (27.6) | 110.5 (26.6) | 113.2 (27) |
| Baseline free T4 ng/dl (SD) | 0.91 (0.3) | 0.83 (0.22) | 0.87 (0.27) |
(p < 0.03, two tailed) otherwise no statistically significant differences between treatment groups in any other variable.
Table 2.
Final disposition of all subjects included in the ITT sample; no statistically significant differences between treatment groups.
| Placebo n (%) | Triiodothyronine n (%) | Total n (%) | |
|---|---|---|---|
| Study completers | 54 (70.1) | 54 (71) | 108 (70.5) |
| Adverse event | 6 (7.8) | 2 (2.6) | 8 (5.2) |
| Lack of efficacy | 2 (2.6) | 4 (5.3) | 6 (3.9) |
| Lost to follow up | 5 (6.5) | 10 (13.2) | 15 (9.8) |
| Protocol violation | 9 (11.7) | 5 (6.7) | 14 (9.2) |
| Withdrew consent | 1 (0.65) | 1 (1.3) | 2 (1.3) |
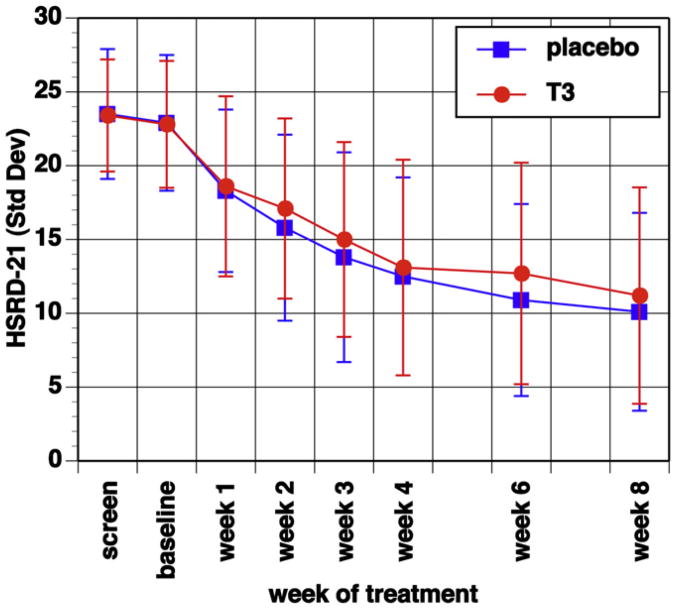
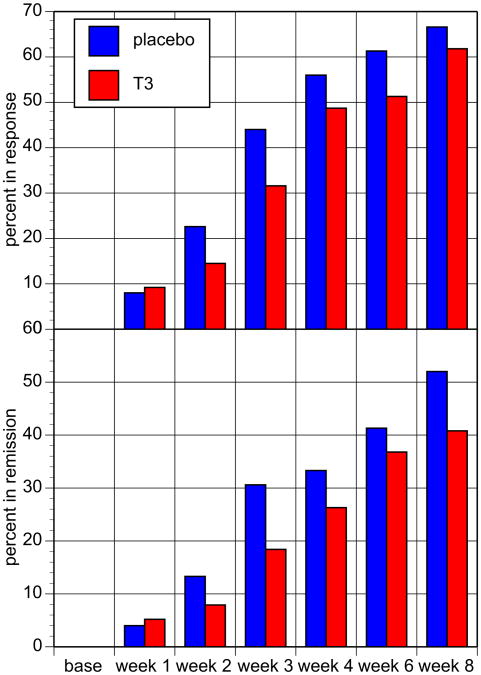
Overall 97 (63.4%) of all subjects met response and 70 (45.7%) met remission criteria at final study visit. There were no differences between the two treatment groups in the primary or secondary outcome measures (Table 3, Figs. 2 and 3). Categorically, equal numbers and percentages of subjects from each treatment group met response and remission criteria at week 8. There was no difference in total HRSD-21 score at last assessment between placebo- and T3-treated patients. The change in HRSD-21 scores between treatment groups across the study did not differ significantly as determined with MANOVA with time in treatment as the only significant effect (f = 66.6; df = 7, 145; p < 0.0001). Median time to response was 4 weeks in the placebo plus sertraline and 6 weeks in T3 plus sertraline groups, with the 25th percentile of 3 weeks for both groups and this difference was not statistically significant.
Table 3.
Outcome characteristics of all subjects included in the ITT sample; no statistically significant differences between treatment groups in any variable.
| Placebo (n = 77) | Triiodothyronine (n = 76) | Total (n = 153) | |
|---|---|---|---|
| Study completers (%) | 54 (70.1) | 54 (71) | 108 (70.6) |
| Baseline HSRD-21 (SD) | 22.8 (4.6) | 22.8 (4.3) | 22.8 (4.4) |
| Week 8 HSRD-21 (SD) | 10.4 (6.9) | 11.2 (7.3) | 10.6 (7) |
| Baseline MADRS (SD) | 26.6 (5.9) | 27.7 (5.4) | 27.2 (5.7) |
| Week 8 MADRS | 11.9 (8.2) | 12.7 (9.3) | 12.2 (8.8) |
| Response by HSRD-21 (%) | 50 (65) | 47 (61.8) | 97 (63.4) |
| Remission by HSRD-21 (%) | 39 (50.1) | 31 (40.8) | 70 (45.75) |
| Week 8 CGI-I 1 or 2 (%) | 56 (72.7) | 52 (68.4) | 108 (70.6) |
| Week 8 CGI-S 1 or 2 (%) | 38 (49.3) | 31 (40.8) | 69 (45.1) |
| Week 8 sertraline dose mg (SD) | 141.6 (52.2) | 148 (45) | 146 (47.8) |
| Week 8 full dose T3/placebo (%) | 70 (90.9) | 73 (96) | 143 (93.5) |
| Week 8 T3 dose μg (SD) | 0 | 48.7 (6.9) | NA |
Fig. 2.
Change in mean HRSD-21 total scores for each study visit and treatment group, with no statistically significant difference between treatment groups, but statistically significant effect of time in treatment (f = 66.6; df = 7, 145; p < 0.0001); error bars are standard deviations.
Fig. 3.
Proportion of subjects that met response criteria (top panel) and remission criteria (bottom panel) by study visit and treatment group, no statistically significant difference between treatment groups.
Essentially identical results were obtained when outcome was assessed for each gender separately, although fewer males met response and remission criteria in both treatment groups, these differences were not statistically significant. Overall 66.6% of female and 58.6% of male subjects met response criteria and 50.5% of female and 38% of males remitted. There was no interaction of treatment group by gender, with 32 (62.75%) placebo- and 31 (70.5%) T3-treated female subjects as responders and 18 (69.2%) of placebo- and 16 (50%) T3-treated males in this category. Gender specific results for remission paralleled those for response with 28 (54.9%) placebo- and 20 (45.5%) T3-treated females and 11 (42.3%) placebo- and 11 (34.4%) T3-treated males achieving remission and these differences were not statistically significant. Baseline serum TSH concentration did not impact treatment outcome. Baseline serum TSH values where analyzed as a continuous variable and categorically as high and low via median split or by quartiles. Similarly, none of the other baseline thyroid function measures had an impact on treatment outcome. There was no interaction between baseline thyroid function values and exposure to T3 in terms of achieving response or remission. Subjects in their first course of treatment for depression were more likely to attain remission than those who had received any previous treatment, with 32 of 51 (62.75%) treatment-naive versus 38 of 102 (37.3%) subjects with past treatment reaching this goal (p < 0.003; two-tailed), regardless of treatment group assignment. Identical results were obtained with MADRS and CGI and therefore only HRSD-21 are reported here.
The majority of subjects, 54 (70.1%) placebo and 54 (71%) T3 treated subjects successfully completed the entire protocol. There were no differences in the rates of study discontinuation between treatment groups or in the causes for early withdrawal (Table 2). Likewise there were no differences in the timing of termination between groups, with equal numbers dropping out at each post randomization visit. There were no differences between the treatment groups in the numbers of patients who experienced treatment emergent adverse events or in the types of side effects reported (Table 4). There were 10 subjects [3 (4%) placebo and 7 (9.2%) T3 treated] who reported no adverse events at any study visit. Overall, the adverse events were consistent with those reported for sertraline in the prescribing information for this medication. Symptoms that might be referable to T3 including tremulousness, sweating, feeling jittery, palpitations, chest pain and feeling hot occurred equally in both treatment groups. Subjects that terminated early due to adverse events did so in response to multiple symptoms with no single common event reported as causal. Side effects associated with early discontinuation were again referable to sertraline treatment and included diarrhea and GI disturbances, sexual dysfunction, headaches and sleep and energy disturbances. There was no relationship between type of reported adverse event and visit at which participation was terminated.
Table 4.
Numbers and percentages of patients with spontaneously reported adverse events, reported by 5% or more of subjects; no statistically significant difference between treatment groups.
| Placebo n (%) | Triiodothyronine n (%) | Total n (%) | |
|---|---|---|---|
| Headache | 40 (53.3) | 42 (55.2) | 82 (54.3) |
| Diarrhea | 23 (30.6) | 18 (23.7) | 41 (27.2) |
| Nausea | 22 (29.3) | 13 (17.1) | 35 (23.2) |
| Insomnia | 21 (28) | 13 (17.1) | 34 (22.5) |
| Dry Mouth | 15 (20) | 12 (15.8) | 27 (17.9) |
| Stomach Discomfort | 15 (20) | 10 (13.2) | 25 (16.6) |
| Fatigue | 12 (16) | 11 (14.5) | 23 (15.2) |
| Tremulousness | 13 (17.3) | 5 (6.6) | 18 (11.9) |
| Sweating | 10 (13.3) | 7 (9.2) | 17 (11.3) |
| Sedation | 6 (8) | 9 (11.8) | 15 (9.9) |
| Constipation | 4 (5.3) | 9 (11.8) | 13 (8.6) |
| Feeling Jittery | 8 (10.6) | 5 (6.6) | 13 (8.6) |
| Urination Increased | 7 (9.3) | 6 (7.9) | 13 (8.6) |
| Palpitations | 7 (9.3) | 5 (6.6) | 12 (7.9) |
| Libido Decreased | 3 (4) | 8 (10.5) | 11 (7.3) |
| URI | 7 (9.3) | 4 (5.3) | 11 (7.3) |
| Change in Attention | 3 (4) | 7 (9.2) | 10 (6.6) |
| Chest Pain | 7 (9.3) | 2 (2.6) | 9 (6) |
| Flatulence | 5 (6.6) | 4 (5.3) | 9 (6) |
| Anxiety | 8 (10.6) | 0 (0) | 8 (5.3) |
| Feeling Hot | 5 (6.6) | 3 (3.9) | 8 (5.3) |
| Muscle Cramps | 3 (4) | 5 (6.6) | 8 (5.3) |
4. Discussion
The results of this trial, the largest prospective, placebocontrolled study of the addition of T3 to an SSRI antidepressant in an enhancement design, demonstrated no benefit to combining T3 with sertraline in terms of achieving response or remission or in accelerating onset of antidepressant action. Contrary to previous reports there was no added benefit of the combination to female patients. There was no evidence that baseline thyroid function was related to overall antidepressant response or response to T3 augmentation. The patients included in this study were not specifically treatment resistant and overall were relatively early in the course of disease and treatment. Fully one third of the subjects had no past treatment for depression. The mean number of previous depressive episodes was 1.3 (±1.2), again indicating that these subjects on average did not have chronic, longstanding or highly recurrent disease. The negative finding in this trial may in part be due to the high overall responsiveness to SSRI monotherapy in these patients.
The preponderance of data from the three placebo-controlled efficacy studies of T3 augmentation of SSRI response in enhancement and acceleration design studies is that there is no added benefit to co-initiate treatment with the thyroid hormone (Appelhof et al., 2004; Cooper-Kazaz et al., 2007; Papkostas et al., 2009). All three of these trials employed very similar designs and patient populations in terms of disease severity and were adequately powered, so are comparable. In total these three investigations include 362 patients treated with SSRI and T3 in the enhancement/acceleration design. All three trials clearly demonstrate that combining both agents at treatment initiation does not accelerate onset of antidepressant response. A smaller investigation (50 patients) that included a range of antidepressants, reported a very modest signal for acceleration over the first three weeks of treatment, but no statistical difference for response or remission at end point (Posternak et al., 2007).
Two of the three trials, this report and that of Appelhof et al. utilizing paroxetine, demonstrate no enhancement of antidepressant response. The one trial that did find a benefit to the combination of T3 and sertraline at 8 weeks of treatment used a maximal daily dose of sertraline of 100 mg (mean dose 87 mg/day), raising the possibility of SSRI under dosing in these patients (Cooper-Kazaz et al., 2007). In contrast, our protocol used flexible dose titration of sertraline up to 200 mg/day, with the mean daily dose approximately 150 mg/day. One potential interpretation is that in the former study the patients were relatively undertreated with antidepressant, which allowed detection of the antidepressant action of the T3, compared to the present study in which the potential benefit of augmentation was masked by the robust response to sertraline at the higher dose. The titration schedule of the sertraline in this study may be more aggressive than typical in routine clinical practice, which may have also contributed to masking a potential benefit from the T3. Interestingly a secondary analysis of the Cooper-Kazaz et al. subjects suggests that a polymorphism in the type 1 deiodinase gene was associated with the antidepressant response to T3 augmentation (Cooper-Kazaz et al., 2009). This appears to be a low activity allele of the gene, resulting in lower T4 to T3 conversion and it is hypothesized that subjects constitutionally with lower T3 levels may be more likely to respond to augmentation. A pooled analysis of all of the three aforementioned trials came to the conclusion of no added benefit to the combination of T3 with an antidepressant in non treatment resistant patients (Papkostas et al., 2009).
This investigation has several strengths. This is the largest patient sample to be studied utilizing a T3 enhancement design, co-initiating with an SSRI. Moreover our sample contained 18.3% African American subjects, reflecting diversity absent in previous efficacy trials of this combination. More than two thirds of the patients completed the protocol. A high mean sertraline dose was achieved, reducing the potential confound of inadequate antidepressant exposure. The vast majority (96%) of T3 treated subjects were able to tolerate the target dose (50 μg) from the second post randomization visit to study end point, again reducing any potential confounds based on inadequate or variable medication exposure.
There are certain limitations to this study. The first is that this was a single site protocol so all subjects were identified, evaluated and treated by the same study staff and may not represent a comprehensive cross section of all patients seeking treatment for depression. That being said this is the largest such study utilizing this design and the vast majority of patients did complete the entire protocol. Patients were recruited from a variety of sources including those responding to advertisements and those spontaneously seeking care and there may be differences in motivations and investment in treatment by these different groups. Another potential limitation is that the included patients were not treatment resistant or chronically ill, which may have made them more likely to be SSRI responsive and therefore not a population in which the potential additional benefit of T3 augmentation could be observed. There was no placebo only group in this study, so we cannot assess the rate of sertraline versus placebo response and therefore cannot conclude the improvement we observed was due to the actions of the antidepressant. That being said, the overall response rate of 63.4% we observed is very consistent with response rates reported in placebo controlled trials of sertraline in major depressive disorder compared to placebo response (Fabre et al., 1995; Lydiard et al., 1997).
The results of this study and of the pooled analysis of all three T3 coinitiation trials do not support the routine use of this combination at the beginning of treatment, in the setting of patients who are not resistant. The use of T3 augmentation in treatment resistant patients may have some therapeutic value, as suggested by the STAR-D trial (Nierenberg et al., 2006). The lack of inclusion of a placebo arm in STAR-D confounds the interpretation of those results as separating the added therapeutic benefit of continued time in treatment from that due to T3 remains obscure. Future investigations of the use of T3 augmentation of antidepressant action should focus on treatment resistant and chronically ill patients for whom there are few effective, evidence-based interventions.
Acknowledgments
Disclosures: This research funded by K23 RR15531-01 (SJG), RO1 MH56946 (PTN), K23 MH 086690 (BWD) and UL1 RR025008 (Emory University). The National Institutes of Health funded this research and they did not participate in the design or conduct of the study, collection and analysis of data, or in the presentation of the results. Pfizer Pharmaceutical Inc donated the sertraline and did so with no stipulation or limitation. Pfizer was not involved in the design or conduct of this research, the collection of analysis of data or the presentation of results. Jones Medical Industries donated the triiodothyronine for this study and did so with no stipulation or limitation. Jones Industries was not involved in the design or conduct of this research, the collection of analysis of data, or in the presentation of results.
Dr. Garlow receives research support from Dana Foundation and has been on scientific advisory board and speaker bureau for Eli Lilly Inc. He was previously on speaker bureau for GSK and Pfizer. He also has received research support from Janssen Pharmaceuticals.
Dr. Dunlop has received research support from Evotec, Forest, GSK, NIMH, Novartis, Ono, Pfizer. He has performed consulting with Imedex LLC and Medavante.
Dr Ninan is currently an employee of Pfizer Pharmaceutical Inc.
Dr. Nemeroff has served on the scientific advisory boards of American Foundation for Suicide Prevention (AFSP); AstraZeneca; Forest Laboratories; NARSAD; Quintiles; Janssen/Ortho-McNeil, PharmaNeuroboost, and Mt. Cook Pharma, Inc. He holds stock/equity in Corcept; Revaax; NovaDel Pharma; CeNeRx, and PharmaNeuroboost. He is on the board of directors of the AFSP; George West Mental Health Foundation; NovaDel Pharma, and Mt. Cook Pharma, Inc. Dr. Nemeroff holds a patent on the method and devices for transdermal delivery of lithium (US 6,375,990 B1) and the method to estimate serotonin and norepinephrine transporter occupancy after drug treatment using patient or animal serum (provisional filing April, 2001). Grants/research: Abbott Laboratories; AFSP; AstraZeneca; Bristol-Myers-Squibb; Eli Lilly; Forest Laboratories; GlaxoSmithKline; Janssen Pharmaceutica; Merck; NARSAD; NIMH; Pfizer Pharmaceuticals; Stanley Foundation/NAMI; Wyeth-Ayerst. Consultant: Abbott Laboratories; Acadia Pharmaceuticals; AstraZeneca; Bristol-Myers-Squibb; Corcept; Cypress Biosciences; Cyberonics; Eli Lilly; Forest Laboratories; GlaxoSmithKline; Janssen Pharmaceutica; Neurocrine Biosciences; Novartis; NPS Pharmaceuticals; Organon; Otsuka; Sanofi; Scirex; Somerset; Wyeth-Ayerst. Speakers bureau: Abbott Laboratories; AstraZeneca; Bristol-Myers-Squibb; Eli Lilly; Forest Laboratories; GlaxoSmithKline; Janssen Pharmaceutica; Organon; Otsuka; Pfizer Pharmaceuticals; Wyeth-Ayerst. Stockholder: Corcept; Neurocrine Biosciences.Board of directors:American Foundation for Suicide PrBievention (AFSP); Cypress Biosciences; George West Mental Health Foundation; Novadel Pharma; Heinz C. Prechter Fund for Manic Depression.
Footnotes
A preliminary version of this analysis was presented as a poster at the ACNP annual meeting in 2007.
Contributors: Steven J. Garlow MD, PhD, conducted much of the hands on clinical care and assessments, was a significant source of funding in the completion of the study, managed the database and conducted the analysis and wrote the manuscript.
Boadie W. Dunlop MD, conducted much of the hands on clinical care and assessments and participated in writing the manuscript.
Philip T. Ninan MD, was Director of the Mood Disorders Research clinic while study was conducted and was the PI on the grant that supported much of the project and participated in writing the manuscript.
Charles B. Nemeroff MD, PhD, was the department chairman at the time the research was conducted, had participated in writing the original grant, and participated in writing the manuscript.
References
- Abraham G, Milev R, Lawson JS. T3 augmentation of SSRI resistant depression. Journal of Affective Disorders. 2006;91:211–5. doi: 10.1016/j.jad.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Altshuler L, Bauer M, Frye MA, Gitlin MJ, Mintz J, Szuba MP, et al. Does thyroid supplementation accelerate tricyclic antidepressant response? A review and meta-analysis of the literature. American Journal of Psychiatry. 2001;158(10):1617–22. doi: 10.1176/appi.ajp.158.10.1617. [DOI] [PubMed] [Google Scholar]
- Appelhof BC, Brouwerr JP, van Dyck R, Fliers E, Hoogendijk WJG, Huyser J, et al. Triiodothyronine addition to paroxetine in the treatment of major depressive disorder. Journal of Clinical Endocrinology & Metabolism. 2004;89:6271–6. doi: 10.1210/jc.2004-1147. [DOI] [PubMed] [Google Scholar]
- Aronson R, Offman H, Joffe R, Naylor D. Triiodothyronine augmentation in the treatment of refractory depression: a meta-analysis. Archives of General Psychiatry. 1996;53:842–8. doi: 10.1001/archpsyc.1996.01830090090013. [DOI] [PubMed] [Google Scholar]
- Bauer M, Forsthoff A, Baethge C, Adli M, Berghofer A, Dopfmer S, et al. Lithium augmentation therapy in refractory depression – update 2002. European Archives of Psychiatry and Clinical Neuroscience. 2003;253(3):132–9. doi: 10.1007/s00406-003-0430-9. [DOI] [PubMed] [Google Scholar]
- Blier P, Ward HE, Tremblay P, Laberge L, Hebert C, Bergeron R. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. American Journal of Psychiatry. 2010;167:281–8. doi: 10.1176/appi.ajp.2009.09020186. [DOI] [PubMed] [Google Scholar]
- Cooper-Kazaz R, Apter JT, Cohen R, Karagichev L, Muhammed-Moussa S, Grupper D, et al. Combined treatment with sertraline and liothyronine in major depression. Archives of General Psychiatry. 2007;64:679–88. doi: 10.1001/archpsyc.64.6.679. [DOI] [PubMed] [Google Scholar]
- Cooper-Kazaz R, van der Deure WM, Medici M, Visser TJ, Alkelai A, Glaser B, et al. Preliminary evidence that a functional polymorphism in type 1 deiodinase is associated with enhanced potentiation of the antidepressant effect of sertraline by triiodothyronine. Journal of Affective Disorders. 2009;116:113–6. doi: 10.1016/j.jad.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Fabre LF, Abuzzahab FS, Amin M, Claghorn JL, Mendels J, Petrie WM, et al. Setraline safety and efficacy in major depression: a double-blind, fixed-dose comparison with placebo. Biological Psychiatry. 1995;38:592–602. doi: 10.1016/0006-3223(95)00178-8. [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ. Current status of augmentation and combination treatments for major depressive disorder: a literature review and proposal for a novel approach to improve practice. Psychotherapy and Psychosomatics. 2006;75(3):139–53. doi: 10.1159/000091771. [DOI] [PubMed] [Google Scholar]
- Feldmesser-Reiss EE. The application of triiodothyronine in the treatment of mental disorders. Journal of Nervous and Mental Disease. 1958;127:540–6. doi: 10.1097/00005053-195812000-00007. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, et al. Structured clinical interview for DSM-IV axis I disorders (SCID-I/P, v 2.0, 8/98 revision) New York: Biometrics Research Department; 1998. [Google Scholar]
- Flach FF, Celian CI, Rawson WW, et al. Treatment of psychiatric disorders with triiodothyronine. American Journal of Psychiatry. 1958;114:841–2. doi: 10.1176/ajp.114.9.841. [DOI] [PubMed] [Google Scholar]
- Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Archives of General Psychiatry. 1991;48:851–5. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- Guy W, editor. ECDEU assessment manual for psychopharmacology. Rockville, U.S: Department of Health, Education, and Welfare; 1976. Clinical Global Impression (CGI) [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosifescu DV, Nierenberg AA, Mischoulon D, Perlis RH, Papkostas GI, Ryan JL, et al. An open study of triiodothyronine augmentation of selective serotonin reuptake inhibitors in treatment-resistant major depressive disorder. Journal of Clinical Psychiatry. 2005;66:1038–42. doi: 10.4088/jcp.v66n0812. [DOI] [PubMed] [Google Scholar]
- Keitner GI, Garlow SJ, Ryan CE, Ninan PT, Solomon DA, Nemeroff CB, et al. A randomized, placebo-controlled trial of risperidone augmentation for patients with difficult-to-treat unipolar, non-psychotic major depression. Journal of Psychiatric Research. 2009;43(3):205–14. doi: 10.1016/j.jpsychires.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydiard RB, Stahl SM, Hertzman M, Harrison WM, et al. A double-blind, placebocontrolled study comparing the effects of sertraline versus amitriptyline in the treatment of major depression. Journal of Clinical Psychiatry. 1997;58(11):484–91. doi: 10.4088/jcp.v58n1104. [DOI] [PubMed] [Google Scholar]
- Maes M, Libbrecht I, van Hunsel F, Campens D, Meltzer HY, et al. Pindolol and mianserin augment the antidepressant activity of fluoxetine in hospitalized major depressed patients, including these with treatment resistance. Journal of Clinical Psychopharmacology. 1999;19(2):177–82. doi: 10.1097/00004714-199904000-00014. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Fava M, Trivedi M, Wisniewski SR, Thase ME, McGrath PJ, et al. Comparison of lithium and T3 augmentation following two failed medication treatments for depression: a STAR*D report. American Journal of Psychiatry. 2006;163:1519–30. doi: 10.1176/ajp.2006.163.9.1519. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Wright EC. Evolution of remission as the new standard in the treatment of depression. Journal of Clinical Psychiatry. 1999;60(Suppl. l2):7–11. [PubMed] [Google Scholar]
- Papkostas GI, Cooper-Kazaz R, Appelhof BC, Posternak MA, Johnson DP, Klibanski A, et al. Simultaneous initiation (coinitiation) of pharmacotherapy with triiodothyronine and a selective serotonin reuptake inhibitor for major depressive disorder: a quantitative synthesis of double-blind studies. International Clinical Psychopharmacology. 2009;24:19–25. doi: 10.1097/YIC.0b013e328314dfaf. [DOI] [PubMed] [Google Scholar]
- Papkostas GI, Shelton RC. Use of atypical antipsychotics for treatment-resistant major depressive disorder. Current Psychiatry Reports. 2008;10(6):481–6. doi: 10.1007/s11920-008-0077-3. [DOI] [PubMed] [Google Scholar]
- Posternak MA, Novak S, Stern R, Hennessey J, Joffe R, Prange A, et al. A pilot effectiveness study: placebo-controlled trial of adjunctive L-triiodothyronine (T3) used to accelerate and potentiate the antidepressant response. International Journal of Neuropsychopharmacology. 2007;11:15–25. doi: 10.1017/S1461145707007663. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Kraemer HC, Sackeim HA, Fava M, Trivedi M, Frank E, et al. Report by the ACNP task force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006a;31:1841–53. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi M, Carmody TJ, Biggs MM, Shores-Wilson K, Ibrahim H, et al. One-year clinical outcomes of depressed public sector outpatients: a benchmark for subsequent studies. Biological Psychiatry. 2004;56:46–53. doi: 10.1016/j.biopsych.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi M, Stewart JW, Nierenberg AA, Fava M, Kurian BT, et al. Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. American Journal of Psychiatry. 2011;168:689–701. doi: 10.1176/appi.ajp.2011.10111645. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi M, S R, Stewart JW, Nierenberg AA, Thase ME, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. New England Journal of Medicine. 2006b;354(12):1231–42. doi: 10.1056/NEJMoa052963. [DOI] [PubMed] [Google Scholar]
- Seemuller F, Riedel M, Obermeier M, Bauer M, Adli M, Kronmuller K, et al. Outcomes of 1014 naturalistically treated inpatients with major depressive episode. European Neuropsychopharmacology. 2010;20(5):346–55. doi: 10.1016/j.euroneuro.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Stewart JW, McGrath PJ, Deliyannides DA, Quitkin FM. Does dual antidepressant therapy at initial treatment hasten and increase remission from depression. Journal of Psychiatric Practice. 2009;15(5):337–45. doi: 10.1097/01.pra.0000361276.88339.44. [DOI] [PubMed] [Google Scholar]
- Thase ME, Entsuah AR, Rudolph RL. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. British Journal of Psychiatry. 2001;178:234–41. doi: 10.1192/bjp.178.3.234. [DOI] [PubMed] [Google Scholar]
- Trivedi M, Fava M, Wisniewski SR, Thase ME, Quitkin FM, Warden D, et al. Medication augmentation after the failure of SSRIs for depression. New England Journal of Medicine. 2006a;354(12):1243–52. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- Trivedi M, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. American Journal of Psychiatry. 2006b;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Valenstein M, McCarthy JF, Austin KL, Greden JF, Young EA, Blow FC. What happened to lithium? Antidepressant in clinical settings American Journal of Psychiatry. 2006;163:1219–25. doi: 10.1176/ajp.2006.163.7.1219. [DOI] [PubMed] [Google Scholar]