Abstract
Background
Reducing inappropriate use of imaging to stage incident prostate cancer is a challenging problem highlighted recently as a Physician Quality Reporting System quality measure and by the American Society of Clinical Oncology and the American Urological Association in the Choosing Wisely campaign. Since 2000, the National Prostate Cancer Register (NPCR) of Sweden has led an effort to decrease national rates of inappropriate prostate cancer imaging by disseminating utilization data along with the latest imaging guidelines to urologists in Sweden. We sought to determine the temporal and regional effects of this effort on prostate cancer imaging rates.
Methods
We performed a retrospective cohort study among men diagnosed with prostate cancer from the NPCR from 1998 to 2009 (n = 99 879). We analyzed imaging use over time stratified by clinical risk category (low, intermediate, high) and geographic region. Generalized linear models with a logit link were used to test for time trend.
Results
Thirty-six percent of men underwent imaging within 6 months of prostate cancer diagnosis. Overall, imaging use decreased over time, particularly in the low-risk category, among whom the imaging rate decreased from 45% to 3% (P < .001), but also in the high-risk category, among whom the rate decreased from 63% to 47% (P < .001). Despite substantial regional variation, all regions experienced clinically and statistically (P < .001) significant decreases in prostate cancer imaging.
Conclusions
A Swedish effort to provide data on prostate cancer imaging use and imaging guidelines to clinicians was associated with a reduction in inappropriate imaging over a 10-year period, as well as slightly decreased appropriate imaging in high-risk patients. These results may inform current efforts to promote guideline-concordant imaging in the United States and internationally.
Widespread use of prostate-specific antigen (PSA) screening has caused a stage migration in prostate cancer, rendering the imaging evaluation of low-risk disease, defined as clinical stage T1 to T2, Gleason score 6 or less, and PSA less than 10ng/mL, largely unnecessary (1,2). Through the development of quality measures and clinical guidelines, policy organizations such as the Physicians Quality Reporting System and professional societies have tried to limit the use of whole body radionuclide bone scan and axial imaging of the abdomen and pelvis among patients with low-risk prostate cancer because the rare detection of metastases is outweighed by the harms of false-positive results (3–9). The success of these efforts on nationwide imaging rates has been mixed, as there continue to be overuse of “inappropriate” imaging among low-risk prostate cancer patients and, paradoxically, simultaneous underuse of appropriate imaging among high-risk prostate cancer patients (10–16). Recently, the American Society of Clinical Oncology and the American Urological Association again highlighted the need to reduce inappropriate imaging for low-risk prostate cancer in the Choosing Wisely campaign, a multidisciplinary effort to reduce unnecessary medical testing, decrease overuse of health-care resources, and improve quality of care (5,17–19). Given the Choosing Wisely campaign’s particular emphasis on the reduction of unnecessary diagnostic imaging (20) and the freedom it affords participants to affect its goals, the description and evaluation of policies to reduce inappropriate prostate cancer imaging are especially important.
Since 2000, the National Prostate Cancer Register (NPCR) of Sweden has engaged in an effort to reduce inappropriate diagnostic imaging among men with low-risk prostate cancer (21). The NPCR collects data on diagnostic and therapeutic procedures performed within 6 months of prostate cancer diagnosis in over 97% of incident cases in Sweden (22,23). These data were used to generate local, hospital-level reports of the frequency of imaging use for patients with low-risk prostate cancer. Along with the most recent versions of imaging guidelines from professional societies in Europe, the United States, and Sweden, as well as important contemporary literature in the field, these utilization data were presented annually to Swedish urologists attending regional and national urology meetings along with the message that reducing inappropriate imaging was important (8,21,23,24). The results of this effort have not been previously reported but are extremely important to determine whether such a strategy effectively reduces inappropriate imaging. These results may also demonstrate unintended consequences, as studies have suggested that policy efforts to reduce inappropriate imaging may also reduce appropriate imaging (10,25).
The aim of this study was to assess the NPCR effort to reduce inappropriate prostate cancer imaging in Sweden by examining imaging trends across the country. We hypothesized that although rates of inappropriate imaging would decrease, rates of appropriate imaging would also decrease. If this hypothesis were true, it would be an important lesson that policy efforts to curb inappropriate prostate cancer imaging, such as Choosing Wisely, might need to be augmented, perhaps with further efforts to encourage appropriate use. If rates of inappropriate imaging did not change or increased during the study period, then the Swedish intervention would be unlikely to be successful in other settings. However, if inappropriate imaging declined during the study period while appropriate imaging improved or remained unchanged, the Swedish intervention might be used as a model to encourage stewardship of health-care resources in other health-care systems and countries.
Methods
Study Design and Patients
We performed a retrospective cohort study to analyze temporal and geographic patterns of prostate cancer imaging in Sweden. The study population consisted of men from the NPCR, which is a national prostate cancer quality registry. Information is provided to patients about the NPCR in all urology clinic waiting rooms as well as online; no written consent is collected, but patients may opt out of the registry at any time. Data from the NPCR, when cross-referenced with the Cancer Register of Sweden (to which reporting is legally mandated), includes information on more than 97% of incident prostate cancer cases (22,26). All patients included in the sample have data on age, date of diagnosis, and name and location of the diagnosing hospital (22). We identified 100 832 men in NPCR diagnosed from 1998 to 2009. We excluded 953 patients with incomplete imaging data, leaving a final cohort of 99 879 men. This study was approved by the Research Ethics Board of Umeå University Hospital.
Definition of Variables
Our primary dependent variable of interest was receipt of imaging such as radionuclide bone scan, computed tomography (CT), or magnetic resonance imaging (MRI) to assess for skeletal metastases. The NPCR records whether this imaging was performed within 6 months of the date of cancer diagnosis. Because NPCR does not specify which imaging modality was used, we examined a randomly selected subgroup of 500 men diagnosed with prostate cancer in 2009 to determine the relative frequencies of each imaging modality.
The independent variables of interest included year of diagnosis and clinical risk category, defined using a modified National Comprehensive Cancer Network (NCCN) risk stratification (7): 1) low-risk (clinical stage T1–2, Gleason score ≤6, andPSA < 10ng/mL); 2) intermediate-risk (clinical stage T1–2, Gleason score 7, and/or PSA 10–20ng/mL); 3) high-risk (clinical stage T3–4 or Gleason score 8–10 or PSA > 20ng/mL). Categorizing a patient as high-risk required only one high-risk feature, even if other data were missing; for low- and intermediate-risk classification, all three features were required. We selected this classification because of its frequent contemporary use and because its definition of low-risk prostate cancer ensures imaging would have been inappropriate regardless of the imaging guideline employed or the calendar year of diagnosis (8,27). Because of missing data, 627 men who received imaging and, 819 men who did not receive imaging could not be classified into a risk category; they were included in descriptions of “all risk categories” but were excluded from analyses stratified by risk category. Covariates included patient age at diagnosis (years); serum PSA (nanograms per milliliter, collected before treatment and within 6 months of cancer diagnosis), clinical tumor stage (modified International Union Against Cancer staging recommendations), and Gleason score (22).
Statistical Analysis
We analyzed yearly imaging patterns and stratified them by demographic and geographic factors. First, we performed a bivariate analysis to determine the association between receipt of imaging and the described independent variables. We reported P values based on χ2 tests for categorical variables and Mann–Whitney tests for continuous, nonparametric variables. We determined whether the trend for the differences in yearly imaging rates for each risk category was statistically significantly different from their baseline imaging rate using generalized linear models with a logit link.
We next examined patterns of use between six different regions in Sweden (North, South, Southeast, Stockholm/Gotland, Uppsala/Orebro, and West). We categorized the time periods of diagnosis as 1998 to 1999 (a baseline period before the intervention), 2000 to 2005, and 2006 to 2009. The trend for the difference in imaging rate between time periods was assessed for each region and stratified by risk group using generalized linear models with a logit link, adjusting for patient age and comorbidity.
Statistical analysis was performed using R version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria). All P values were two-sided with statistical significance at α = .05.
Results
Among 99 879 men diagnosed with prostate cancer between 1998 and 2009, 36 414 (36%) underwent imaging within 6 months of diagnosis (Table 1). Men undergoing imaging were younger (P < .001) than those not undergoing imaging. Seventy percent of men undergoing imaging were high-risk vs only 36% of those not undergoing imaging (P < .001). Men undergoing imaging also had higher-risk features (clinical stage, PSA, and Gleason score) at presentation compared with those not undergoing imaging (all P < .001). Use of prostate cancer imaging demonstrated wide regional variation, with men in the north and southeast regions undergoing imaging 42% of the time, compared with 30% in the western region (P < .001). Overall use of prostate cancer imaging trended down from a high of 58% among men diagnosed in 1998 to a low of 23% among men diagnosed in 2008 (P < .001).
Table 1.
Characteristics of men diagnosed with prostate cancer from the National Prostate Cancer Register of Sweden from 1998 to 2009 (N = 99 879)*
| Characteristic | Men receiving imaging (n = 36,414) | Men not receiving imaging (n = 63,465) | P† | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, y | <.001 | ||||
| <55 | 1056 | 2.9 | 1925 | 3.0 | |
| 55–59 | 2722 | 7.5 | 4897 | 7.7 | |
| 60–64 | 5531 | 15.2 | 9398 | 14.8 | |
| 65–69 | 7436 | 20.4 | 10 950 | 17.3 | |
| 70–74 | 7543 | 20.7 | 10 782 | 17.0 | |
| 75–79 | 6251 | 17.2 | 11 062 | 17.4 | |
| ≥80 | 5875 | 16.1 | 14 451 | 22.8 | |
| Charlson Comorbidity Index | <.001 | ||||
| 0 | 24 273 | 66.7 | 40 755 | 64.2 | |
| 1–2 | 9768 | 26.8 | 18 043 | 28.4 | |
| ≥3 | 2373 | 6.5 | 4667 | 7.4 | |
| Risk category | <.001 | ||||
| Low | 3264 | 9.0 | 21 199 | 33.4 | |
| Intermediate | 7112 | 19.5 | 16 520 | 26.0 | |
| High | 25 411 | 69.8 | 22 927 | 36.1 | |
| Missing | 627 | 1.7 | 2819 | 4.4 | |
| PSA level, ng/mL | <.001 | ||||
| <10 | 7811 | 21.5 | 31 936 | 50.3 | |
| 10–20 | 8120 | 22.3 | 13 127 | 20.7 | |
| >20 | 20 056 | 55.1 | 16 430 | 25.9 | |
| Missing | 427 | 1.2 | 1972 | 3.1 | |
| Median PSA (IQR) | <.001 | ||||
| 23.2 | (11.0–75.0) | 9.4 | (5.8–21.0) | ||
| Clinical local stage | <.001 | ||||
| T1 | 8902 | 24.4 | 30 901 | 48.7 | |
| T2 | 12 213 | 33.5 | 19 188 | 30.2 | |
| T3+ | 14 465 | 39.7 | 12 072 | 19.0 | |
| Other/missing | 834 | 2.0 | 1304 | 2.1 | |
| Gleason score | <.001 | ||||
| 2–6 | 10 567 | 29.0 | 34 197 | 53.9 | |
| 7 | 14 064 | 38.6 | 18 783 | 29.6 | |
| 8–10 | 11 012 | 30.2 | 9034 | 14.2 | |
| Missing | 771 | 2.1 | 1451 | 2.3 | |
| Region | <.001 | ||||
| North | 4336 | 11.9 | 5890 | 9.3 | |
| South | 6906 | 19.0 | 12 101 | 19.1 | |
| Stockholm/Gotland | 5961 | 16.4 | 11 723 | 18.5 | |
| Southeast | 4862 | 13.4 | 6623 | 10.4 | |
| Uppsala/Örebro | 8379 | 23.0 | 12 878 | 20.3 | |
| West | 5970 | 16.4 | 14 250 | 22.5 | |
| Year of diagnosis | <.001 | ||||
| 1998 | 3505 | 9.6 | 2544 | 4.0 | |
| 1999 | 3790 | 10.4 | 3245 | 5.1 | |
| 2000 | 3501 | 9.6 | 3630 | 5.7 | |
| 2001 | 3304 | 9.1 | 4087 | 6.4 | |
| 2002 | 3151 | 8.7 | 4398 | 6.9 | |
| 2003 | 3493 | 9.6 | 5222 | 8.2 | |
| 2004 | 3337 | 9.2 | 6296 | 9.9 | |
| 2005 | 3009 | 8.3 | 6580 | 10.4 | |
| 2006 | 2657 | 7.3 | 6322 | 10.0 | |
| 2007 | 2231 | 6.1 | 6571 | 10.4 | |
| 2008 | 2008 | 5.5 | 6770 | 10.7 | |
| 2009 | 2428 | 6.7 | 7800 | 12.3 | |
* IQR = interquartile range; PSA = prostate-specific antigen.
† All P values are from the χ2 test, except for median PSA, which is from the Mann–Whitney test. All tests were two-sided.
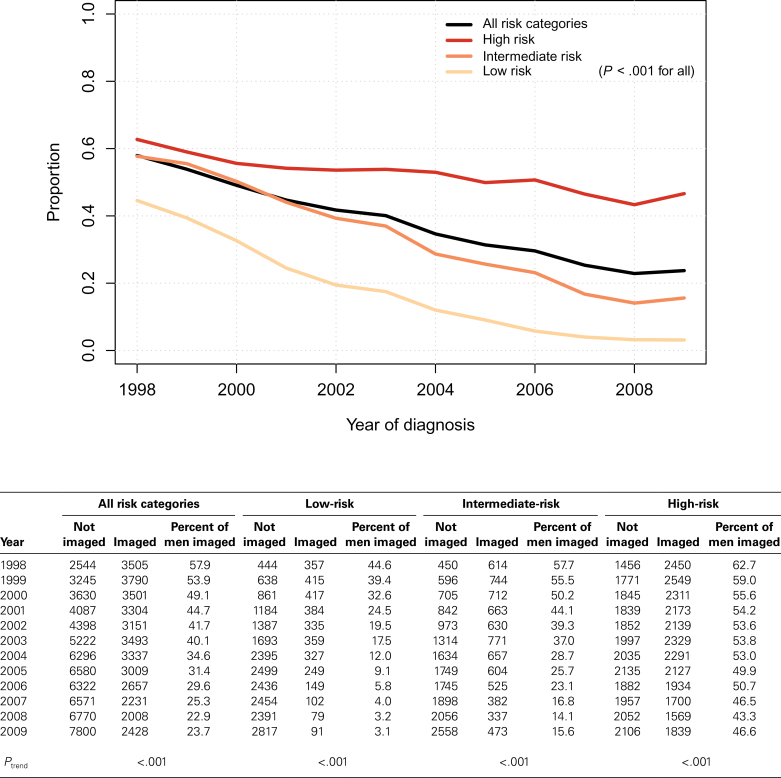
Among a randomly sampled subset of 500 patients with detailed imaging information, bone scan was the most common imaging modality. Overall, 88% of men underwent one or more bone scans; bone scan was the sole imaging modality in 75% of patients and was performed in conjunction with other imaging in 13% (Table 2). An additional 6% and 2% of patients underwent only MRI or CT scan, respectively. Prostate cancer imaging rates decreased during the study period (Figure 1). At each time point, imaging use was greater among men with high-risk prostate cancer than among those with intermediate-risk disease. Similarly, imaging use at each time point was greater among men with intermediate-risk prostate cancer than among those with low-risk disease. The decline over time in prostate cancer imaging use was statistically significant among the overall population and within each clinical risk category individually (P < .001 for all three [high-, intermediate-, and low-risk] categories). Whereas in 1998 45% of men with low-risk prostate cancer underwent imaging, the rate declined to a nadir of 3% in 2008 and 2009 (P < .001). Similarly, 63% of men with high-risk prostate cancer underwent imaging in 1998, which also declined over time (43% in 2008 and 47% in 2009) (P < .001).
Table 2.
Diagnostic modality used to stage men diagnosed in 2009 with prostate cancer from the National Prostate Cancer Register of Sweden*
| Hospital | Bone scan | Bone scan + CT scan | Scint + MRI | Bone scan + plain film | CT scan | CT + plain film | MRI | Plain film | No information available | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Malmö | 28 | 80.0% | 4 | 11.4% | 1 | 2.9% | 1 | 2.9% | 1 | 2.9% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 35 |
| Helsingborg | 21 | 91.3% | 1 | 4.3% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 1 | 4.3% | 0 | 0.0% | 0 | 0.0% | 23 |
| Ystad | 9 | 100.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 9 |
| Uppsala | 16 | 72.7% | 1 | 4.5% | 1 | 4.5% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 2 | 9.1% | 0 | 0.0% | 2 | 9.1% | 22 |
| Västerås | 27 | 62.8% | 7 | 16.3% | 4 | 9.3% | 0 | 0.0% | 3 | 7.0% | 1 | 2.3% | 1 | 2.3% | 0 | 0.0% | 0 | 0.0% | 43 |
| Umeå | 21 | 77.8% | 1 | 3.7% | 1 | 3.7% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 2 | 7.4% | 2 | 7.4% | 27 |
| Skellefteå | 5 | 45.5% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 6 | 54.5% | 0 | 0.0% | 0 | 0.0% | 11 |
| Total | 127 | 74.7% | 14 | 8.2% | 7 | 4.1% | 1 | 0.6% | 4 | 2.4% | 1 | 0.6% | 10 | 5.9% | 2 | 1.2% | 4 | 2.4% | 170 |
* CT = computed tomography; MRI = magnetic resonance imaging.
Figure 1.
Time trends in imaging use among men with newly diagnosed prostate cancer by clinical risk categories. Low risk includes patients with tumors designated as clinical stage T1 to T2, Gleason score of 6 or less, and prostate-specific antigen (PSA) of less than 10ng/mL. Intermediate risk includes patients with tumors designated as clinical stage T1 to T2, Gleason score of 7, and/or PSA of 10 to 20ng/mL. High risk includes patients with tumors designated as clinical stage T3 and/or Gleason score of 8 to 10 and/or PSA greater than 20ng/mL. P values from generalized linear models with logit link. All statistical tests were two-sided.
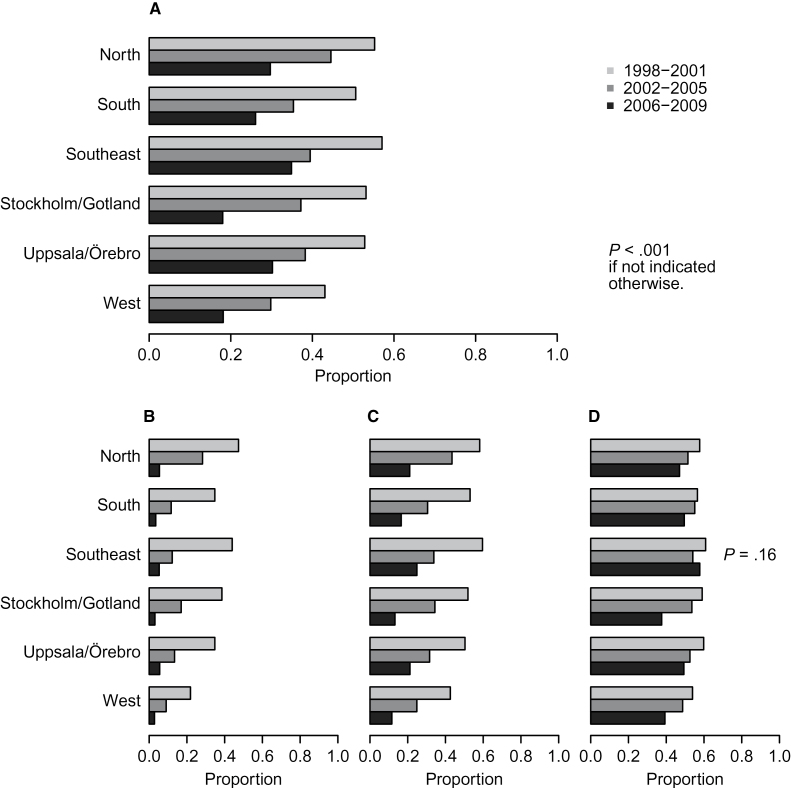
There was considerable regional variation in prostate cancer imaging (Figure 2A). Nevertheless, across all six regions, prostate cancer imaging declined over time (all P < .001). In virtually all regional and clinical risk subgroups, there was a statistically significant decline in imaging use over time (P < .001 for all, except high-risk patients in the southeast) (Figure 2, B–D). The decline was most pronounced among low-risk patients, where relative imaging rates decreased almost 10-fold. The same monotonic decrease in all time periods across all regions was observed among intermediate-risk patients, as well as high-risk patients in all regions except the southeast. Adjusting for age and comorbidity did not statistically significantly affect the magnitude or the significance of the association between imaging rates and time.
Figure 2.
Temporal trends in imaging use for newly diagnosed prostate cancer by region. P values from generalized linear models with logit link. All statistical tests were two-sided. A) Temporal trends in imaging use for newly diagnosed prostate cancer by region in the overall population. Temporal trends in imaging use are given for newly diagnosed prostate cancer by region within low-risk (B), intermediate-risk (C), and high-risk (D) categories.
Discussion
This study is the first to report the outcomes of a Swedish effort to reduce national rates of inappropriate prostate cancer imaging. As in the United States, Swedish prostate cancer imaging rates demonstrate wide regional variation. The effort to reduce inappropriate prostate cancer imaging, however, seemed to be effective in nearly all Swedish regions, decreasing imaging among low-risk men from 45% to 3% in a decade. These results compare favorably with previous local efforts to reduce inappropriate imaging (28) and build on those results substantially by demonstrating the ability to improve inappropriate prostate cancer imaging at a national scale. While inappropriate imaging decreased dramatically among low-risk men, this was accompanied by a small, yet statistically significant decrease in appropriate imaging among high-risk men from a peak of 63% to a recent nadir of 47%.
In 1998, the baseline low-risk prostate cancer imaging rate in Sweden was 45%. Per the NCCN guidelines (7), none of these men should have received bone imaging unless they presented with symptoms suggestive of bone pain (8,24). In the United States, the imaging rate among men with low-risk prostate cancer has been reported to be 19% to 74% in a community cohort and 10% to 48% in a Surveillance Epidemiology and End Results (SEER)–Medicare cohort (10–13,16). It is challenging to compare these rates directly across the two countries because the NPCR aggregates all staging imaging into one variable. However, our sampling revealed that 88% of those undergoing imaging had at least a bone scan, whereas only 11% had any CTs and 10% had any MRI. This suggests that baseline rates of bone scan among low-risk men in Sweden were similar to those among their low-risk counterparts in the United States, whereas rates of axial imaging were likely much lower. During the study period, rates of prostate cancer imaging among low-risk men in Sweden decreased to 3%, substantially lower than those reported in the United States at any time. Because guidelines suggest imaging for prostate cancer patients with bone pain (7) and 5.6% to 28.7% of men aged 50 to 80 years have back pain (29), the optimal rate of imaging should not be zero, even among men with low-risk features. This 3% rate undoubtedly encompasses some patients presenting with prostate cancer and unrelated back pain, in whom a bone scan is indicated but whose rates of metastatic prostate cancer are vanishingly small (2,30).
Given our retrospective study design, we can only infer an association between the decline in inappropriate prostate cancer imaging in Sweden and the NPCR’s efforts to promote guideline-concordant imaging use. Similar to previous work by Miller et al., it is not possible to determine causality with this type of study design (28). The associations described in the analysis could be affected by unmeasured confounding or could result from secular trends unrelated to any specific policy effort, as occurred with imaging rates in the United States (11). Another potential explanation for the decline in inappropriate imaging is the Hawthorne effect, where the behavior of study subjects is modified as the result of the awareness that they are being observed, an explanation supported perhaps by the decline in imaging rates in 1998 and 1999, a time period before the initiation of the NPCR’s effort (31). In spite of these alternate explanations, it remains plausible that interventions such as those in this report and those described by Miller et al. (28) could have had an effect on prostate cancer imaging rates. Miller et al. describe a decline in imaging associated with a small-scale intervention administered in three urology practices located in the United States participating in a quality-improvement consortium. Our study’s contribution is to demonstrate that a similar strategy can be applied effectively at a national scale with an associated decline in inappropriate imaging rates, a finding of great interest for policy makers in the United States seeking to improve health-care quality.
In 1998, the baseline high-risk prostate cancer imaging rates in Sweden were 63%, and decreased by 43% in 2008 (rising slightly to 47% in 2009). Based on our risk category definitions and the guidelines advocated in Sweden, all of these men should have undergone an imaging evaluation (8,24). Swedish rates of prostate cancer imaging among men with high-risk disease are considerably lower than those reported from the SEER–Medicare cohort, where 70% to 75% underwent bone scan and 57% to 58% underwent CT (13,16). These already low rates of imaging among men with high-risk prostate cancer only decreased further during the NPCR’s effort to promote guideline-concordant imaging. Clearly in both countries, imaging for high-risk prostate cancer remains underused despite the general overuse of imaging and numerous guidelines encouraging its appropriate use (3–9).
The results of several studies suggest a mechanism for the observed decline in appropriate imaging among men with high-risk prostate cancer during the NPCR’s effort to reduce inappropriate prostate cancer imaging. Ko et al. analyzed cardiac catheterization rates among Medicare patients with acute myocardial infarction and found patients living in regions with higher catheterization rates were more likely to undergo catheterization, regardless of whether they needed the procedure (32). Abraham and colleagues found that, after implementation of the initial American Urological Association prostate cancer imaging guidelines, bone scan rates declined substantially among SEER–Medicare patients in whom bone scan was not indicated but also decreased slightly among men in whom bone scan was indicated (10). A follow-up study found a regional-level association between appropriate imaging among high-risk men and inappropriate imaging among low-risk men, a finding termed the thermostat model of health-care resource allocation (25). According to this model, men with high-risk prostate cancer are more likely to undergo appropriate imaging if they reside in a region where inappropriate imaging is more common. A corollary to this model is that policies aimed at lowering rates of inappropriate imaging among men with low-risk prostate cancer may have the unintended consequence of lowering appropriate imaging rates among men with high-risk prostate cancer (25). This is borne out in our analysis, which demonstrates a modest, but statistically significant, decline in imaging among high-risk prostate cancer patients in Sweden during an effective national effort to decrease inappropriate imaging among low-risk patients.
Our analysis has several key strengths, most notably, the high quality of data from the NPCR of Sweden. This population-based registry represents virtually all men diagnosed with prostate cancer across Sweden and eliminates selection bias in the study sample. Similarly, as reporting to the register is mandatory, the health-care resource use records are extremely accurate, eliminating a source of verification bias. Additionally, the registry is well established, allowing us to observe the long-term effects of policy changes.
This analysis also has several limitations. Because this is an initial exploration of imaging use patterns from Sweden, we focused on descriptive statistics rather than complex, multivariable modeling. Although superficially a limitation, it is actually a testament to the clear trends in imaging use that took place during the study period. The body parts imaged by CT and MRI are not recorded, although all studies were obtained to evaluate patients for bone metastasis, making it highly likely that these were images of the spine. The high rate of bone scan use relative to other modalities in 2009 the year from which we reviewed 500 incident prostate cancer cases to determine the types and frequencies of diagnostic tests used, is likely to have been a year in which use of MRI would have peaked (8,27). This suggests bone scan was at least similarly common throughout the entire study period though it is not possible to confirm whether these patters were constant in other years where such data could not be reviewed. It is challenging to compare directly the various imaging rates across countries and across studies. Some of the reported US imaging rates are based on the same criteria for low-risk disease as this study, whereas other used a broader definition (incorporating patients with more aggressive features). This phenomenon could help explain the higher imaging rates observed in other studies (13). Finally, differences across health-care systems might reduce the generalizability of the NPCR effort to a country not having a similar uniform national health-care system where decisions regarding capacity for diagnostic imaging are made by regional authorities (33,34). Such centralization facilities the dissemination of information, implementation of policy, and accurate record-keeping, which are critical for continuous quality improvement efforts. A final limitation is the lack of consensus over the interpretation of data supporting imaging in high-risk patients (35–38). Some of the underuse of imaging among high-risk patients may be the result of physicians’ reluctance to use imaging in an era when metastatic disease is so uncommon.
A national effort to reduce inappropriate prostate cancer imaging by disseminating hospital-level utilization data and contemporary imaging guidelines to urologists in Sweden was associated with a reduction in inappropriate imaging from 45% to 3%. Although appropriate imaging suffered to a small extent, these national-level results are truly remarkable because many previous guidelines and policy efforts have failed to reduce inappropriate prostate cancer imaging in the United States. The Swedish experience could inform future US health policy efforts, such as the Choosing Wisely campaign, in several ways. Policymakers should be encouraged that they have selected a solvable problem. However, to avoid unintended consequences, our analysis suggests that efforts to curb inappropriate prostate cancer imaging might best be coupled with efforts to encourage appropriate use. Without some sort of further modification, policies to reduce inappropriate prostate cancer imaging may improve care for patients with low-risk disease at the expense of those with high-risk features.
Funding
This work was supported by the Swedish Research Council (825-2012-5047) and The Swedish Cancer Foundation (11 0471). SL and DVM (VA HSR&D CDA & CDP 11–257) are supported by the United States Department of Veterans Affairs, Health Services Research and Development Service and the Louis Feil Charitable Lead Trust.
P. Stattin and L. Drevin had full access to the data and can take responsibility for the integrity of the data and the accuracy of the data analysis.
The study sponsor(s) had no role in the design of the study; no role in the collection, analysis, or interpretation of the data; no role in the writing of the manuscript; and no role in the decision to submit the manuscript for publication.
D.V. Makarov is a VA HSR&D Career Development awardee (VA HSR&D CDA & CDP 11–257) at the Manhattan VA. The views expressed in this article are those of the author(s) and do not necessarily represent the views of the Department of Veterans Affairs.
The authors would like to acknowledge David F. Penson, MD, MPH, for his thoughtful review of the manuscript. This project was made possible by the continuous work of the National Prostate Cancer Register (NPCR) of Sweden steering group: Pär Stattin chairman, Anders Widmark, Lars Egevad, Magnus Törnblom, Stefan Carlsson, Jan Adolfsson, Anna Bill-Axelson, Jan-Erik Johanssson, Ove Andrén, Mats Lambe, Erik Holmberg, David Robinson, Bill Pettersson, Jonas Hugosson, Jan-Erik Damber, Ola Bratt, and Göran Ahlgren, Karin Hellström, and Maria Nyberg.
References
- 1. Han M, Partin AW, Pound CR, et al. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28(3):555–565 [DOI] [PubMed] [Google Scholar]
- 2. Cooperberg MR, Lubeck DP, Meng MV, et al. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004;22(11):2141–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thompson I, Clauser S, Albertsen P, et al. Prostate cancer: percentage of patients, regardless of age, with a diagnosis of prostate cancer, at low risk of recurrence, receiving interstitial prostate brachytherapy, OR external beam radiotherapy to the prostate, OR radical prostatectomy, OR cryotherapy who did not have a bone scan performed at any time since diagnosis of prostate cancer. http://www.qualitymeasures.ahrq.gov/summary/summary.aspx?ss=1&doc_id=11481 Accessed June 11, 2013
- 4. Miller DC, Murtagh DS, Suh RS, et al. Establishment of a urological surgery quality collaborative. J Urol. 2010;184(6):2485–2490 [DOI] [PubMed] [Google Scholar]
- 5. Schnipper LE, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: the top five list for oncology. J Clin Oncol. 2012;10;30(14):1715-–1724 [DOI] [PubMed] [Google Scholar]
- 6. Roach M, Tempany C, Choyke P, et al. Expert Panel on Radiation Oncology—Prostate Work Group (ROP) and Urologic Imaging. Pretreatment Staging Prostate Cancer. Reston, VA: American College of Radiology; 1995: 11 [Google Scholar]
- 7. Prostate cancer In: NCCN Clinical Practice Guidelines in Oncology (NCCN Guideline). 2.2013 ed: National Comprehensive Cancer Network; 2013:66 Fort Washington, PA. [Google Scholar]
- 8. Aus G, Abbou CC, Pacik D, et al. EAU guidelines on prostate cancer. Eur Urol. 2001;40(2):97–101 [DOI] [PubMed] [Google Scholar]
- 9. Middleton R, Thompson I, Austenfeld M. Report on the management of clinically localized prostate cancer. In: AUA Clinical Practice Guidelines. Baltimore: American Urological Association; 1995. [PubMed] [Google Scholar]
- 10. Abraham N, Wan F, Montagnet C, et al. Decrease in racial disparities in the staging evaluation for prostate cancer after publication of staging guidelines. J Urol. 2007;178(1):82–87; discussion 87. [DOI] [PubMed] [Google Scholar]
- 11. Cooperberg MR, Lubeck DP, Grossfeld GD, et al. Contemporary trends in imaging test utilization for prostate cancer staging: data from the cancer of the prostate strategic urologic research endeavor. J Urol. 2002;168(2):491–495 [PubMed] [Google Scholar]
- 12. Kindrick AV, Grossfeld GD, Stier DM, et al. Use of imaging tests for staging newly diagnosed prostate cancer: trends from the CaPSURE database. J Urol. 1998;160(6 Pt 1):2102–2106 [DOI] [PubMed] [Google Scholar]
- 13. Choi WW, Williams SB, Gu X, et al. Overuse of imaging for staging low risk prostate cancer. J Urol. 2011;185(5):1645–1649 [DOI] [PubMed] [Google Scholar]
- 14. Lavery HJ, Brajtbord JS, Levinson AW, et al. Unnecessary imaging for the staging of low-risk prostate cancer is common. Urology. 2011;77(2):274–278 [DOI] [PubMed] [Google Scholar]
- 15. Saigal CS, Pashos CL, Henning JM, et al. Variations in use of imaging in a national sample of men with early-stage prostate cancer. Urology. 2002;59(3):400–404 [DOI] [PubMed] [Google Scholar]
- 16. Makarov DV, Desai RA, Yu JB, et al. The population level prevalence and correlates of appropriate and inappropriate imaging to stage incident prostate cancer in the medicare population. J Urol. 2012;187(1):97–102 [DOI] [PubMed] [Google Scholar]
- 17. American Board of Internal Medicine Foundation The Choosing Wisely Campaign: Five Things Physicians and Patients Should Question. http:// choosingwisely.org/wp-content/uploads/2011/12/about_choosingwisely.pdf Accessed June 11, 2013 [Google Scholar]
- 18. Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801–1802 [DOI] [PubMed] [Google Scholar]
- 19. Isett WW. As Part of Choosing Wisely Campaign American Urological Assocation Identifies List of Commonly Used Tests and Treatments to Question. http://www.auanet.org/advnews/press_releases/article.cfm?articleNo=285 Accessed June 11, 2013 [Google Scholar]
- 20. Rao VM, Levin DC. The overuse of diagnostic imaging and the Choosing Wisely initiative. Ann Intern Med. 2012;157(8):574–576 [DOI] [PubMed] [Google Scholar]
- 21. Nationella prostatacancerregistret (NPCR) http://www.cancercentrum.se/INCA/kvalitetsregister/Prostatacancer332/ Accessed June 11, 2013
- 22. Van Hemelrijck M, Wigertz A, Sandin F, et al. Cohort profile: the National Prostate Cancer Register of Sweden and Prostate Cancer Data Base Sweden 2.0. Int J Epidemiol. 2012. first published online May 4, 2012 10.1093/ije/dys068. [DOI] [PubMed] [Google Scholar]
- 23. Annual Report NPCR http://www.cancercentrum.se/uppsalaorebro/ vardprocesser/Prostatacancer/vardprogram/ Accessed June 11, 2013
- 24. Vårdprogram för Prostatacancer http://www.urolog.se/professionen/Vardprg_2004_2.PDF Accessed June 11, 2013
- 25. Makarov DV, Desai RA, Yu JB, et al. Appropriate and inappropriate imaging rates for prostate cancer go hand in hand by region, as if set by thermostat. Health Aff (Millwood). 2012;31(4):730–740 [DOI] [PubMed] [Google Scholar]
- 26. PcBaSe Sweden http://snd.gu.se/en/catalogue/study/612 Accessed June 11, 2013
- 27. Heidenreich A, Bolla M, Joniau S, et al. Guidelines on Prostate Cancer. Arnhem, The Netherlands: European Association of Urology; 2009. [Google Scholar]
- 28. Miller DC, Murtagh DS, Suh RS, et al. Regional collaboration to improve radiographic staging practices among men with early stage prostate cancer. J Urol. 2011;186(3):844–849 [DOI] [PubMed] [Google Scholar]
- 29. Loney PL, Stratford PW. The prevalence of low back pain in adults: a methodological review of the literature. Phys Ther. 1999;79(4):384–396 [PubMed] [Google Scholar]
- 30. Paquette EL, Sun L, Paquette LR, et al. Improved prostate cancer-specific survival and other disease parameters: impact of prostate-specific antigen testing. Urology. 2002;60(5):756–759 [DOI] [PubMed] [Google Scholar]
- 31. Wickstrom G, Bendix T. The “Hawthorne effect”—what did the original Hawthorne studies actually show? Scand J Work Environ Health. 2000;26(4):363–367 [PubMed] [Google Scholar]
- 32. Ko DT, Wang Y, Alter DA, et al. Regional variation in cardiac catheterization appropriateness and baseline risk after acute myocardial infarction. J Am Coll Cardiol. 2008;51(7):716–723 [DOI] [PubMed] [Google Scholar]
- 33. Pettersson H. Reorganization of diagnostic imaging in south Sweden: realization and cost-effectiveness. Academ Radiol. 1998;5(Suppl 2):S315–S316 [DOI] [PubMed] [Google Scholar]
- 34. Thomson S, Osborn R, Squires D, et al. International Profiles of Health Care Systems: Australia, Canada, Denmark, England, France, Germany, Iceland, Italy, Japan, the Netherlands, New Zealand, Norway, Sweden, Switzerland, and the United States. New York: The Commonwealth Fund; 2012. [Google Scholar]
- 35. Oesterling JE. Prostate specific antigen: a critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. J Urol. 1991;145(5):907–923 [DOI] [PubMed] [Google Scholar]
- 36. Levran Z, Gonzalez JA, Diokno AC, et al. Are pelvic computed tomography, bone scan and pelvic lymphadenectomy necessary in the staging of prostatic cancer? Br J Urol. 1995;75(6):778–781 [DOI] [PubMed] [Google Scholar]
- 37. Chybowski FM, Keller JJ, Bergstralh EJ, et al. Predicting radionuclide bone scan findings in patients with newly diagnosed, untreated prostate cancer: prostate specific antigen is superior to all other clinical parameters. J Urol. 1991;145(2):313–318 [DOI] [PubMed] [Google Scholar]
- 38. O’Dowd GJ, Veltri RW, Orozco R, et al. Update on the appropriate staging evaluation for newly diagnosed prostate cancer. J Urol. 1997;158(3 Pt 1): 687–698 [DOI] [PubMed] [Google Scholar]