Abstract
Objective
The majority of patients with epithelial ovarian cancer achieved a complete clinical remission with normal CA-125 will still relapse and die from their disease. The present study was to determine whether CA-125 levels before, during and after primary treatment provided prognostic information for both Type I and Type II ovarian cancer.
Methods
In this retrospective study, we identified 410 epithelial ovarian cancer patients who had achieved a CCR between 1984 and 2011. A Cox proportional hazards model and log-rank test were used to assess associations between the nadir CA-125, histotype, and prognosis.
Results
The baseline serum CA-125 concentration was higher in patients with type II ovarian cancer than in those with type I (p < 0.001). The nadir CA-125 was an independent predictor of PFS (p < 0.001) and OS (p = 0.035) duration. The PFS and OS durations were 21.7 and 79.4 months in patients with CA-125 ≤ 10 U/ml and 13.6 and 64.6 months in those with 11-35 U/ml (p = 0.01 and 0.002, respectively). Histotype was an independent predictor of PFS (p = 0.041): the PFS and OS durations of type I patients were longer than those in type II (p < 0.001 and < 0.001, respectively).
Conclusions
The nadir CA-125 and the histotype are predictive of PFS and OS duration in ovarian cancers experienced a CCR. PFS and OS durations were shorter in patients with CA-125 levels of 11-35 U/ml and type II disease than in those with ≤ 10 U/ml and type I.
Keywords: ovarian cancer, CA-125, prognosis factors, tumor marker, pathological type
Introduction
Epithelial ovarian cancer leads to more deaths than all other gynecological malignancies in northern and Western Europe and North America. In 2012, an estimated 22,280 new patients will be diagnosed and 15,500 died in the United States[1]. More than 50% of patients enter a complete clinical remission (CCR) after primary treatment, but most ultimately experience tumor relapse and die within 5 years [2]. The high recurrence frequency illustrates the need for prognostic indicators to determine which patients who might benefit from personalized consolidation and maintenance therapy [3].
Over the past three decades, the serum tumor marker CA-125 has been reported to distinguish malignant from benign pelvic masses, monitor therapeutic response and detect recurrent disease [4]. The prognostic significance of CA-125 remains unresolved. Studies have evaluated the prognostic significance of CA-125 levels at various time points, including at diagnosis [5], prior to primary cytoreductive surgery [6], before neo-adjuvant [7] and first-line adjuvant chemotherapy and at relapse, to determine whether they correlate with prognosis, but the role of CA-125 in prognostication remains controversial [8].
Previous studies have reported that the nadir CA-125 is a prognostic factor in ovarian cancer patients who have experienced a CCR [9-15]. Although these studies did not use a unified cut-off for serum CA-125 concentration, most found that a higher CA-125 level within the normal range was associated with a higher hazard ratio for relapse or death. Previous studies have not taken into account the biologic heterogeneity of ovarian cancer.
Ovarian cancer is not a single disease entity, but rather comprises a heterogeneous group of tumors with distinct clinicopathological characteristics [16-17]. Differing CA-125 levels have been found in patients with different ovarian cancer subtypes, such as low levels in mucinous and clear cell cancer [5, 18]. Inconsistent outcomes regarding the prognostic significance of CA-125 may relate to a failure to consider tumor grade and histotype. Recent studies suggest that ovarian cancers can be grouped into two broad categories: type I and type II based upon distinct clinicopathological and molecular genetic features. Type I cancers are of low grade and include well differentiated serous, endometrioid, mucinous, and clear cell carcinomas. Type II tumors include high-grade serous, high-grade endometrioid, undifferentiated carcinomas and malignant mixed mesodermal tumors (carcinosarcomas). Type I ovarian cancers present in early stage, grow slowly and respond less frequently to platinum based therapy. Type II ovarian cancers present at more advanced stages and grow aggressively, constitute approximately 75% of ovarian cancer and are responsible for 90% of ovarian cancer deaths, but respond to platinum based therapy. Type I tumors have few copy number abnormalities, but exhibit activating mutations of Ras, Raf and PTEN. Type II ovarian cancers are driven by multiple areas of amplification and deletion; while virtually all high grade serous cancers have mutations in p53 gene and 40% of these cancer showed mutation or epigenetic alteration in BRCA1 or BRCA2 [16-17, 19-21].
In the present study, we compared the prognostic significance of CA-125 in type I and type II ovarian cancers, evaluating the CA-125 during and at the conclusion of the primary treatment for each type of ovarian cancer in patients at The University of Texas MD Anderson Cancer Center (Houston, TX) who had achieved a CCR without consolidation or maintenance therapy. We then determined whether the CA-125 level, measured at different time points in type I and II tumors, was predictive of PFS and OS duration.
Methods
Study population
Using the tumor registry and pathology databases, we identified patients who had undergone treatment between January 1, 1984, and February 14, 2011 at MD Anderson Cancer Center. Serial serum CA-125 concentrations were obtained retrospectively at different treatment points in time: at baseline, before surgery, before chemotherapy, the narir CA-125, and at relapse. Cases in which a second-look surgery was performed, with detailed surgical notes provided a further group for study.
Clinicopathological data and CA-125 were analyzed from medical records after chart review. The following data were collected: age at diagnosis; ethnicity; height; weight; family cancer history; menopausal status; tumor histological type and grade; International Federation of Gynecology and Obstetrics disease stage; primary treatment, such as cytoreductive surgery, first-line chemotherapy (non-paclitaxel-based [mainly CAP, PVB, or PP) or paclitaxel-based [mainly TC or DC]); clinical treatment response; second-look surgical findings; ascites volume; time and management of recurrence; and date of death.
OS duration was defined as the interval from the time of diagnosis until death or last follow-up for patients who were still alive. The PFS duration was determined by calculating the interval from the time of first evaluation after the end of primary treatment to progression. The pathological disease types for all patients had been previously reviewed by MD Anderson gynecological pathologists (J.L. and J.Z.). The tumors were classified as type I or II according to the criteria proposed by Shih et al [16-17, 19].
Definition of clinical response and CA-125 analysis
Clinical response and progression were assessed according to Response Evaluation Criteria in Solid Tumors (RECIST) [22]. A CCR was defined as (1) no residual tumor on imaging studies; (2) no signs of residual tumor on physical examination; (3) absence of tumor-associated clinical symptoms; and (4) serum CA-125 concentration ≤ 35 U/ml. Pathological complete remission were judged by laparoscopy and/or laparotomy.
The serum CA-125 concentration was determined using a commercially available Roche immunoassay assay system in MD Anderson’s clinical laboratory. In clinical practice, a reference value of 35 U/ml is generally considered to be the upper limit of normal. The measurement time points were defined as follows: baseline, at diagnosis; before surgery, within 2 weeks before the primary surgery; before chemotherapy, within 2 weeks before primary adjuvant chemotherapy; the CA-125 nadir, the lowest CA-125 value from diagnosis to at least 1 month after primary treatment which including surgery and chemotherapy commonly; and at relapse, within 2 weeks of a relapse being confirmed.
Statistical analysis
The Cox proportional hazards model was used to assess the association between absolute serum CA-125 level, pathological type and survival duration. Kaplan-Meier estimates of PFS and OS, stratified by various possible prognostic factor categories including CA-125 level as dichotomous variables around the median value, were calculated and compared using the log-rank test. The CA-125 level was further evaluated survival hazard ratio (HR) using previously reported subgroups: ≤ 10 U/ml, 11–20 U/ml, and 21–35 U/ml. Step-wise regression techniques were used to build multivariate models, using a significance level of 0.15 to remain in the model. Associations were regarded as significant if the two-sided p value was < 0.05. All analyses were conducted using SPSS software (version 18.0; SSPS Inc, Chicago, IL). Baseline serum CA-125 concentrations in the type I and II tumor groups were compared using a Student’s t-test. A multivariate logistic regression analysis model was used to determine the association between CA-125 level and residual tumor and relapse, adjusted by pathological disease type.
This retrospective study was approved by the institutional review board of MD Anderson, and informed patient consent was waived by the M.D. Anderson Institutional Review Board.
Results
Patient characteristics
Nine hundred ninety ovarian cancer patients met the inclusion criteria for this study. Their characteristics are described in Table 1. Among the endometrioid ovarian cancers, we found that 14.1%, 42.3%, and 43.7% of cases were grades 1, 2, and 3, respectively. Most non-endometrioid ovarian carcinomas were high grade (n = 831 [90.4%]) rather than low grade (n = 88 [9.6%]). The most common histological type was high grade serous carcinoma (n= 706 [71.3%]). Five hundred ninety-seven (60.3%) epithelial ovarian cancer patients experienced a radiological remission after primary cytoreduction and first-line chemotherapy, and 410 patients (41.4%) experienced a CCR criterion without undergoing consolidation or maintenance therapy. Seventy-two patients underwent second-look surgery, and 43 (59.7%) of these patients had achieved a pathological complete remission (pCR).
Table 1. Patient characteristics.
| Characteristic | Result |
|---|---|
| Median age, years (range) | 59.7 (20-92.4) |
| Baseline CA-125 level, n (range) (U/ml) | 801 (7-33,439) |
| Ethnic group, n (%) (n = 990 | |
| White | 781 (78.9) |
| Black | 57 (5.8) |
| Hispanic | 122 (12.3) |
| Others* | 30 (3.0) |
| Histology, n (%) (n = 990) | |
| Low-grade serous | 69 (7.0) |
| Low-grade endometrioid | 39 (3.9) |
| Clear cell | 34 (3.4) |
| Mucinous | 20 (2.0) |
| Transitional | 15 (1.5) |
| High-grade serous | 706 (71.3) |
| High-grade endometrioid | 43 (4.3) |
| Undifferentiated | 33 (3.3) |
| MMMT | 31 (3.1) |
| Surgical residual tumor, n (%) (n = | 990) |
| Macroscopic free | 498 (50.3) |
| < 1 cm | 90 (9.1) |
| 1-2 cm | 30 (3.0) |
| > 2 cm | 245 (24.7) |
| Unknown | 127 (12.8) |
| FIGO stage, n (%) (n = 990) | |
| I | 75 (7.6) |
| II | 58 (5.9) |
| III | 653 (66.0) |
| IV | 198 (20.0) |
| Unknown | 6 (0.6) |
| Adjuvant chemotherapy, n (%) (n = 909) | |
| With paclitaxel | 672 (73.9) |
| Without paclitaxel | 237 (26.1) |
Others including 16 Chinese, 8 Middle eastern, 2 Japanese, 2 Korea, and 2 Indian cases.
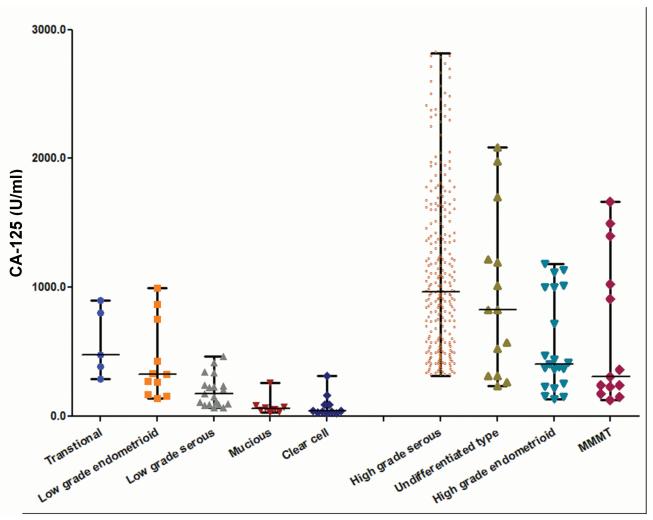
There are 177 type I and 813 type II ovarian cancer cases. The baseline CA-125 level in patients with type II ovarian cancer (median, 937 U/ml; interquartile range, 126-2,820 U/ml) was higher than in those with type I (median, 171 U/ml; interquartile range, 26-996 U/ml, p < 0.001), as shown in Figure 1. Interestingly, in mixed ovarian cancer (two or more components), the median serum CA-125 concentration was higher in those with serous or undifferentiated components than in those without these components (959 U/ml vs. 258.5 U/ml; range, 31-16,852 U/ml vs. 18-9345 U/ml; p < 0.001). In addition, the CA-125 expression level was higher in tissue of type II ovarian cancer than that of type I, as shown in Figure S1. There was no difference of the nadir CA-125 in type I (median 10 U/ml, range 5-34 U/ml) and type II (median 10 U/ml, range 4-35 U/ml) ovarian cancer achieved CCR (p = 0.79).
Figure 1.
The baseline serum CA-125 concentration in type II patients was higher than that in type I patients (median, 937 vs. 171 U/ml; p < 0.001).
The nadir CA-125 is an independent prognostic factor
In all patients with ovarian cancer in CCR, we found that the nadir CA-125 was predictive of PFS (p < 0.001) and OS (p < 0.001) duration in a Cox proportional hazards model univariate analysis. Baseline and preoperative serum concentrations were also related to PFS and OS, as shown in Table 2 and Table S1, but, only the nadir CA-125 was independently associated with PFS (p < 0.001) and OS (p = 0.035) in multivariate analysis (Table 3). The nadir CA-125 was predictive of PFS both in type I and type II ovarian cancer in univariate (p<0.001 and p<0.001) and multivariate (p<0.001 and p<0.001) analysis. The nadir CA-125 was predictive of OS both in type I (p=0.002) and type II (p=0.002) ovarian cancer in univariate analysis and multivariate analysis (p=0.005 and 0.04 respectively).
Table 2. HR of prognosis by CA-125 level in ovarian cancer, as determined by univariate Cox regression analysis.
| CA-125 | HR of PFS | p value | HR of OS | p value |
|---|---|---|---|---|
| Baseline level | 1.000 | < 0.001 | 1.000 | 0.001 |
| Level before surgery | 1.000 | < 0.001 | 1.000 | 0.004 |
| Level after surgery | 1.000 | 0.051 | 1.000 | 0.589 |
| Nadir | 1.046 | < 0.001 | 1.038 | < 0.001 |
| Level at relapse | N/A | N/A | 1.000 | 0.003 |
Table 3. HR of prognosis in ovarian cancer, as determined by multivariate Cox regression analysis.
| Variable | HR of PFS | p value | HR of OS | p value |
|---|---|---|---|---|
| FIGO stage | ||||
| I | 1.000 | Reference | 1.000 | Reference |
| II | 2.361 | 0.138 | 1.848 | 0.296 |
| III | 8.135 | < 0.001 | 4.231 | 0.002 |
| IV | 10.840 | < 0.001 | 5.026 | 0.001 |
| Type | ||||
| I | 1.000 | Reference | 1.000 | Reference |
| II | 1.569 | 0.041 | 1.205 | 0.279 |
| Age | 1.008 | 0.222 | 1.016 | 0.026 |
| The nadir CA-125 Ascites |
1.042 | < 0.001 | 1.022 | 0.035 |
| No | 1.000 | Reference | 1.000 | Reference |
| Yes | 1.147 | 0.206 | 1.552 | 0.002 |
| Surgery residual | ||||
| Macroscopic free | 1.000 | Reference | 1.000 | Reference |
| Tumor residual | 1.575 | 0.008 | 1.272 | 0.226 |
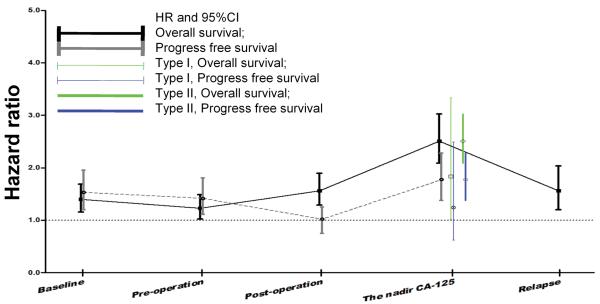
Median CA-125 level as the cut-off point, we compared the relapse and death hazard ratio of patients with higher CA-125 level than those with lower CA-125 at different time points. The PFS and OS HRs of the nadir CA-125 (1.8, 95% CI, 1.43-2.26; and 2.61, 95% CI, 1.18-3.12, respectively) were higher than those at diagnosis and before and after surgery (Figure 2). Stratification analysis revealed PFS and OS HRs of 1.23 (95% CI, 0.92-1.67) and 1.27 (95% CI, 0.98-1.64) for 11-20 U/ml and 2.32 (95% CI, 1.54-3.47) and 2.48 (95% CI, 1.71-3.58) for 21-35 U/ml, respectively.
Figure 2.
The median CA-125 level was used as the cut-off point. The prognosis HR of nadir CA-125 was higher than at other time points.
The nadir CA-125 level is predictive of prognosis but not the presence of residual tumor
Median PFS and OS durations of patients with CCR ovarian cancer were 18.0 months (95% CI, 14.8–21.2) and 72.1 months (95% CI, 63.4–80.8 months), respectively. The median follow-up period of the survivors was 38 months at the end of present study (interquartile range, 20.4–73.2 months).
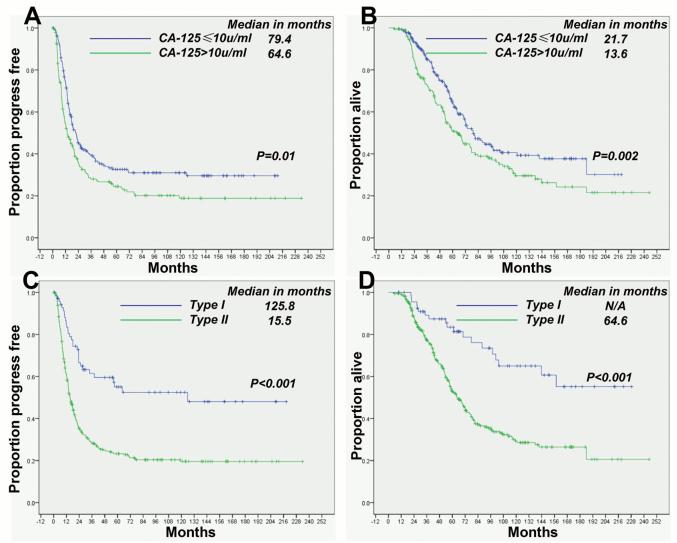
Complete clinical remission ovarian cancer patients with a serum CA-125 concentration of ≤ 10 U/ml (median value) had longer PFS and OS durations than did those with 11-35 U/ml, as shown in Figure 3A and 3B, as well in type I (Figure S4A and S4B) and type II (Figure S4C and S4D) ovarian cancer respectively.
Figure 3.
Patients with CA-125 levels of 11-35 U/ml had shorter PFS durations (A) and OS durations (B) than did those with ≤ 10 U/ml. The PFS (C) and OS (D) durations were shorter in type II patients than in type I patients.
Patients who had experienced a pCR at the time of their second-look surgery had longer PFS and OS durations (median, 20.8 and 76.8 months versus 10.8 and 52.8 months, respectively; p < 0.001 for both) than did those who had not experienced a pCR. No significant correlation was found between CA-125 level and residual tumor found (including microscopic and gross residual tumor) during the second-look surgery (p = 0.094). However, we found a correlation between CA-125 level and tumor relapse (p = 0.003, Table S2, 3, 4). The nadir CA-125 was higher in relapsed cases than in non-relapsed cases. These results suggest that CA-125 level is predictive of relapse but not residual tumor, as shown in Figure S2, 3.
The nadir CA-125 in Type I and type II ovarian cancers predict prognosis
The pathological disease type I and type II was associated with PFS (p < 0.001) and OS (p <0.001) duration in univariate analysis (Table S1), and independently associated with PFS (p =0.041) but not OS (p = 0.279) in multivariate analysis (Table 3). Other than the nadir CA-125, FIGO stage (p<0.001 and p<0.001) were independently associated with PFS and OS in type I ovarian cancers, whereas FIGO stage (p<0.001 and p=0.02), tumor residual (p<0.004 and p=0.09) and ascites (p=0.2 and p=0.008) were significant prognostic factors in type II ovarian cancers. Using the median nadir CA-125 as the cut-off point, we found that the mortality HR (2.51 [95% CI, 2.09-3.02]) and relapse HR (1.77 [95% CI, 1.38-2.28]) were higher in patients with type II tumors than in those with type I tumors (1.84 [95% CI, 1.01-3.34] and 1.25 [95% CI, 0.625-2.49], respectively), as shown in Figure 2. Patients with type II tumors who had experienced a CCR had shorter PFS and OS durations than did those with type I tumors (Figure 3C and 3D). Even among patients with CA-125 10-35 U/ml, the PFS and OS durations were shorter in those with type II tumors than in those with type I, as shown in Figure S5C and S5D.
Discussion
We have determined the prognostic significance of CA-125 in patients with Type I and Type II ovarian cancer who achieved a CCR after primary therapy. The reference value of 35 U/ml for CA-125 is based on levels in healthy women; some studies have suggested using lower cut-off values would be of greater value for monitoring and managing ovarian cancer [23-25]. Our results agree with those of most previous studies that the nadir CA-125 is an appropriate indicator of PFS and OS in patients who have experienced a CCR, although no consensus exists about a fixed cut-off. Some researchers have suggested a reference based on the observed median serum CA-125 concentration [15], whereas others have used an arbitrary cut-off value or an interval to stratify the cohort [9-14, 26-27].
The hazard ratio for prognosis in the present study is similar to that in most related studies, whether the cut-off point is 10 U/ml or three intervals (< 10 U/ml, 10-20 U/ml, and 20-35 U/ml) as shown in table 4. Crawford et al. [13] determined differences in the nadir CA-125 in 106 patients with a CCR and found a statistically significant HR difference in the time to biochemical progression (two values > 60 U/ml) between patients with CA-125 > 11–20 or 21–30 U/ml and those with CA-125 < 10 U/ml. Markman et al. [26] further confirmed that CA-125 > 10 U/ml is predictive of progression. However, Pignata et al. [28] found no difference in PFS between low and intermediate values in an Italian group. Juretzka et al. [15] found a difference in PFS (< 10 U/ml, 3.0 years [median]; 11–20 U/ml, 2.4 years; and 21–30 U/ml, 1.2 years; P = 0.007) but not OS among three intervals. In a study of advanced cancer patients in Spain, Prat et al. [9] found the PFS and OS duration were comparatively longer for CA-125 ≤ 10 U/ml subgroup, respectively. Using the same criteria, Kang et al. [27] found a progression HR of 2.45 (95% CI, 1.55-3.88) in CA-125 > 10 U/ml Korean population. Gard et al. [14] declared a progression HR was higher in patients with CA-125 > 15 U/ml; Kang et al. [10] found a survival hazard ratio increasing for 12-18 U/ml and 18-35U/ml in Korean patients; and Altena et al. [11] claimed an independent progression HR increasing to 1.51 (95% CI, 1.04-2.31 while CA-125 > 5 U/ml in a Netherlands population.
Table 4. Hazard ratio between the nadir CA-125 and survival.
| Author, Year |
N | Country | cutoff (u/ml) | HR,OS | HR,PFS |
|---|---|---|---|---|---|
| Markman,2006 | 384 | USA | <10, | N/A | reference |
| 11-20, | N/A | 1.51(0.002) | |||
| 21-35 | N/A | 2.32(0.001) | |||
| Juretzka,2007 | 241 | USA | 12 | 1/1.41(1.044-1.904) | N/A |
| Prat,2008 | 96 | Spain | 10 | 6.0(0.0002) | 2.15(0.0081) |
| Park,2011 | 267 | Korea | <12 | reference | N/A |
| 12-18 | 1/1.68(1.11-2.56) | N/A | |||
| 18-35 | 1/2.85(1.70-4.76) | N/A | |||
| Altena,2010 | 331 | Netherlands | 5 | 1.62 (95% CI, 1.03-2.54) | 1.75 (95% CI, 1.20-2.57) |
| Kim,2008 | 123 | Korea | <10, | reference | reference |
| 10-21, | 1/2.396(1.270-4.521) | 1/2.508(1.062-5.921) | |||
| >21 | 1/5.384(2.510-11.549) | 1/3.929(1.140-8.607) | |||
| Crawford,2005 | 78 | UK | 10 | 1/D.1336(p<0.001) | N/A |
| Grad,1994 | 223 | Australia | 15 | 1/3.23(2.62-3.99) | N/A |
CA-125’s utility as a prognostic indicator also relies on determining a suitable time point to measure CA-125. We confirmed that the nadir CA-125 is an independent prognostic factor. Other suggested time points, such as at CA-125 at diagnosis, before surgery [12, 29], and after surgery [6, 30], may not be as effective at predicting prognosis alone. The serum CA-125 concentration is believed to be valuable for demonstrating disease persistence. An abnormal CA-125 level indicates > 95% certainty that residual disease is present after standard therapy (e.g., optional cytoreduction and six cycles of cisplatin and paclitaxel) [31]. However, our findings did not support the using CA-125 level to predict a minimal degree of tumor residual in ovarian cancer patients who have experienced a CCR.
Because most of ovarian cancer tumors are high-grade serous carcinomas, ovarian cancer has long been regarded as a single disease. However, the different histological types of ovarian cancer have distinct biology and clinical behavior. Shih et al. [16-17, 19] proposed a dualistic classification to divide the ovarian cancer into two broad categories, type I and type II. Supporting this model, we found that baseline serum CA-125 levels were higher in patients with more aggressive type II ovarian cancers than in those with type I, and the prognosis of patients with type II tumors was poorer. Using the median CA-125 level as a cut-off, we found that the prognostic value of CA-125 level was higher in patients with type II ovarian cancer than in those with type I tumors. On the other hand, we found that CA-125 level is similar between high grade and low grade endometrioid tumors which belong to different subtype, suggesting that CA125 along does not predict whether the tumor belongs to type 1 or type II.
At the present time, primary treatment is generally stopped when patients achieve a CCR. Nadir CA-125 and the pathological disease type are prognostic factors for PFS and OS duration in ovarian cancer patients who have experienced a CCR. Clinical trials of maintenance therapy may be stratified for nadir CA-125 and histotype, and arguably might be limited to patients with a poor prognosis evidenced by CA-125 11-35 U/mL and type II, once our findings have been confirmed in a prospective study.
Supplementary Material
Figure S1. The CA-125 expression level was higher in patients with type II than type I ovarian cancer (p < 0.001).
Figure S2. The CA-125 level was associated with tumor relapse (A) but not residual tumor (B).
Figure S3. An ROC curve shows that the CA-125 level was predictive of tumor relapse (A) and residual tumor (B).
Figure S4. Patients with CA-125 levels of 11-35 U/ml had shorter PFS durations and OS durations than did those with ≤ 10 U/ml in type I ovarian cancer (A, B) and type II ovarian cancer (C, D).
Figure S5. Among patients with a CA-125 level of 11-35 U/ml, patients with type II had shorter PFS (A) and OS (B) durations than did those with type I.
Acknowledgements
We are grateful to Ann Sutton and Elizabeth Gaston’s helpful editing of this manuscript. J.L. was supported by an R01 grant (R01 CA131183-01A2) and an ovarian cancer Specialized Programs of Research Excellence (SPORE) grant (IP50 CA83638) from the National Institutes of Health, the Ovarian Cancer Research Fund and the National Foundation for Cancer Research. JL and RCB are also supported by multi-investigator award from Cancer Prevention and Research Institute of Texas (CPRIT). This work was also supported, in part, by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant (CA016672). Dr. Xiaoxiang Chen is a visiting scientist at MD Anderson, supported by the Jiangsu Health International Exchange Program in 2011 and the Jiangsu Province Institute of Cancer Research Foundation (grant number: ZQ200703).
Abbreviations
- CCR
Complete clinical remission
- PFS
Progression-free survival
- OS
Overall survival
- OR
Odds ratio
- CI
Confidence interval
- HR
Hazard ratio
Footnotes
Conflict of interest: RCB receives royalties from Fujirebio Diagnostics, Inc, for CA-125. The other authors declare no conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Ozols RF. Recurrent ovarian cancer: evidence-based treatment. J Clin Oncol. 2002;20(5):1161–3. doi: 10.1200/JCO.2002.20.5.1161. [DOI] [PubMed] [Google Scholar]
- 3.Foster T, et al. A review of the current evidence for maintenance therapy in ovarian cancer. Gynecol Oncol. 2009;115(2):290–301. doi: 10.1016/j.ygyno.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 4.Meyer T, Rustin GJ. Role of tumour markers in monitoring epithelial ovarian cancer. Br J Cancer. 2000;82(9):1535–8. doi: 10.1054/bjoc.2000.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zorn KK, et al. The prognostic value of pretreatment CA 125 in patients with advanced ovarian carcinoma: a Gynecologic Oncology Group study. Cancer. 2009;115(5):1028–35. doi: 10.1002/cncr.24084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mury D, et al. Prognostic and predictive relevance of CA-125 at primary surgery of ovarian cancer. J Cancer Res Clin Oncol. 2011;137(7):1131–7. doi: 10.1007/s00432-011-0977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang S, et al. Interaction between preoperative CA-125 level and survival benefit of neoadjuvant chemotherapy in advanced epithelial ovarian cancer. Gynecol Oncol. 2011;120(1):18–22. doi: 10.1016/j.ygyno.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 8.Hogdall E. Cancer antigen 125 and prognosis. Curr Opin Obstet Gynecol. 2008;20(1):4–8. doi: 10.1097/GCO.0b013e3282f2b124. [DOI] [PubMed] [Google Scholar]
- 9.Prat A, et al. Nadir CA-125 concentration in the normal range as an independent prognostic factor for optimally treated advanced epithelial ovarian cancer. Ann Oncol. 2008;19(2):327–31. doi: 10.1093/annonc/mdm495. [DOI] [PubMed] [Google Scholar]
- 10.Kang S, et al. Prediction of a high-risk group based on postoperative nadir CA-125 levels in patients with advanced epithelial ovarian cancer. J Gynecol Oncol. 2011;22(4):269–74. doi: 10.3802/jgo.2011.22.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Altena AM, et al. CA125 nadir concentration is an independent predictor of tumor recurrence in patients with ovarian cancer: a population-based study. Gynecol Oncol. 2010;119(2):265–9. doi: 10.1016/j.ygyno.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 12.Kim HS, et al. Significance of preoperative serum CA-125 levels in the prediction of lymph node metastasis in epithelial ovarian cancer. Acta Obstet Gynecol Scand. 2008;87(11):1136–42. doi: 10.1080/00016340802478158. [DOI] [PubMed] [Google Scholar]
- 13.Crawford SM, Peace J. Does the nadir CA125 concentration predict a long-term outcome after chemotherapy for carcinoma of the ovary? Ann Oncol. 2005;16(1):47–50. doi: 10.1093/annonc/mdi012. [DOI] [PubMed] [Google Scholar]
- 14.Gard GB, Houghton CR. An assessment of the value of serum CA 125 measurements in the management of epithelial ovarian carcinoma. Gynecol Oncol. 1994;53(3):283–9. doi: 10.1006/gyno.1994.1135. [DOI] [PubMed] [Google Scholar]
- 15.Juretzka MM, et al. CA125 level as a predictor of progression-free survival and overall survival in ovarian cancer patients with surgically defined disease status prior to the initiation of intraperitoneal consolidation therapy. Gynecol Oncol. 2007;104(1):176–80. doi: 10.1016/j.ygyno.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 16.Shih Ie M, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164(5):1511–8. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurman RJ, Shih Ie M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol. 2011;42(7):918–31. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu D, et al. Comparison of candidate serologic markers for type I and type II ovarian cancer. Gynecol Oncol. 2011;122(3):560–6. doi: 10.1016/j.ygyno.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34(3):433–43. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCluggage WG. My approach to and thoughts on the typing of ovarian carcinomas. J Clin Pathol. 2008;61(2):152–63. doi: 10.1136/jcp.2007.049478. [DOI] [PubMed] [Google Scholar]
- 21.McAlpine JN, et al. BRCA1 and BRCA2 mutations correlate with TP53 abnormalities and presence of immune cell infiltrates in ovarian high-grade serous carcinoma. Mod Pathol. 2012;25(5):740–50. doi: 10.1038/modpathol.2011.211. [DOI] [PubMed] [Google Scholar]
- 22.Therasse P, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Prat A, et al. Risk of recurrence during follow-up for optimally treated advanced epithelial ovarian cancer (EOC) with a low-level increase of serum CA-125 levels. Ann Oncol. 2009;20(2):294–7. doi: 10.1093/annonc/mdn601. [DOI] [PubMed] [Google Scholar]
- 24.Santillan A, et al. Risk of epithelial ovarian cancer recurrence in patients with rising serum CA-125 levels within the normal range. J Clin Oncol. 2005;23(36):9338–43. doi: 10.1200/JCO.2005.02.2582. [DOI] [PubMed] [Google Scholar]
- 25.Wilder JL, et al. Clinical implications of a rising serum CA-125 within the normal range in patients with epithelial ovarian cancer: a preliminary investigation. Gynecol Oncol. 2003;89(2):233–5. doi: 10.1016/s0090-8258(03)00051-9. [DOI] [PubMed] [Google Scholar]
- 26.Markman M, et al. Pretreatment CA-125 and risk of relapse in advanced ovarian cancer. J Clin Oncol. 2006;24(9):1454–8. doi: 10.1200/JCO.2005.04.7373. [DOI] [PubMed] [Google Scholar]
- 27.Kang S, Seo SS, Park SY. Nadir CA-125 level is an independent prognostic factor in advanced epithelial ovarian cancer. J Surg Oncol. 2009;100(3):244–7. doi: 10.1002/jso.21258. [DOI] [PubMed] [Google Scholar]
- 28.Altundag K, et al. CA125 Nadir Values As a Prognostic Factor in Epithelial Ovarian Cancer. Journal of clinical oncology. 2005;23(10):2435–2436. doi: 10.1200/JCO.2005.05.136. [DOI] [PubMed] [Google Scholar]
- 29.Paramasivam S, et al. Prognostic importance of preoperative CA-125 in International Federation of Gynecology and Obstetrics stage I epithelial ovarian cancer: an Australian multicenter study. J Clin Oncol. 2005;23(25):5938–42. doi: 10.1200/JCO.2005.08.151. [DOI] [PubMed] [Google Scholar]
- 30.Markmann S, Gerber B, Briese V. Prognostic value of Ca 125 levels during primary therapy. Anticancer Res. 2007;27(4A):1837–9. [PubMed] [Google Scholar]
- 31.Rubin SC, et al. Serum CA 125 levels and surgical findings in patients undergoing secondary operations for epithelial ovarian cancer. Am J Obstet Gynecol. 1989;160(3):667–71. doi: 10.1016/s0002-9378(89)80054-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The CA-125 expression level was higher in patients with type II than type I ovarian cancer (p < 0.001).
Figure S2. The CA-125 level was associated with tumor relapse (A) but not residual tumor (B).
Figure S3. An ROC curve shows that the CA-125 level was predictive of tumor relapse (A) and residual tumor (B).
Figure S4. Patients with CA-125 levels of 11-35 U/ml had shorter PFS durations and OS durations than did those with ≤ 10 U/ml in type I ovarian cancer (A, B) and type II ovarian cancer (C, D).
Figure S5. Among patients with a CA-125 level of 11-35 U/ml, patients with type II had shorter PFS (A) and OS (B) durations than did those with type I.