Abstract
Background
Mechano-receptive C-fiber (MR-CF) stimulation via slow stroking of C-fiber rich skin areas can be used to probe the relationship between reward and interoception. Individuals with substance use disorders show impaired reward processing, and dysfunctional interoceptive processing of MR-CF may contribute to this dysfunction. This study predicted that methamphetamine dependent (MD) individuals would exhibit altered responses to MR-CF stimulation in brain regions important for interoception.
Methods
Recently abstinent MD (n=25) and comparison (CTL, n=17) subjects received a pleasant interoceptive stimulus (“Soft Touch” consisting of a slow brush stroke) to the palm or forearm during functional magnetic resonance imaging. Subjects were provided with cues signaling stimulation to examine anticipatory and stimulus-related processing. Subjective responses were measured using visual analog scales (VAS).
Results
Groups were similar on behavioral performance and ratings of the interoceptive stimuli, yet MD exhibited lower anterior insula, dorsal striatum, and thalamus activation than CTL, across anticipation and soft touch conditions. The lower the anterior insula activation, the faster the reaction time across conditions in MD, whereas the opposite pattern was evident in CTL. Striatal activation in MD was greater than CTL during anticipation, but lower during soft touch. Greater striatal attenuation was associated with higher VAS pleasantness ratings of soft touch.
Conclusions
MD expend fewer brain processing resources during soft touch, a form of positively-valenced interoceptive stimuli, in brain areas that are important for both interoception and reward. Future studies will ascertain if sustained abstinence from methamphetamine use can normalize aberrant neural interoceptive processing.
Keywords: methamphetamine, interoception, reward, fMRI
1. INTRODUCTION
Over 35 million individuals have abused methamphetamine worldwide (UNODC, 2010). In addition to cognitive deficits, greater unemployment, violent crime, and family dysfunction (Darke et al., 2010; Henry et al., 2010; Pennar et al., 2012; Weber et al., 2012), methamphetamine users suffer adverse physical health consequences such as cardiovascular complications, seizures, weight loss, tooth decay, and skin lesions (Darke et al., 2008; Sommers et al., 2006; Padilla and Ritter, 2008; MacKenzie and Heischober, 1997). Methamphetamine addiction causes detrimental effects to the brain, body, and society, constituting a major public health concern. However, the neural basis for physical and mental health dysfunction in methamphetamine use disorders is not well understood.
One recent approach to understanding dysfunction in methamphetamine dependence (MD) is based on the idea that this condition is linked to attenuated experience of body-relevant signals, and as a result, individuals with MD may not adjust behavior based on visceral sensations associated with consequences of their choices (Verdejo-Garcia and Bechara, 2008). Interoception involves representation of feelings originating from inside the body, our internal state, and motivated actions to maintain homeostasis (Craig, 2002; Naqvi and Bechara, 2009; Paulus et al., 2009). It has been proposed that the difference between the expected and observed body state provides a learning signal, a body prediction error, enabling one to adjust behavior (Paulus et al., 2009). The thalamus and insula are involved in interoception as a relay system between other brain regions, initiating motivated action to achieve homeostasis and minimize body prediction error (Craig, 2003; Paulus et al., 2009). The thalamus delivers sensory information first to posterior insula then to anterior insula, resulting in interoceptive feeling states. Individuals with substance use disorders may have inadequate function in this relay system, resulting in unstable adjustment of the body prediction error (Naqvi and Bechara, 2009; Paulus et al., 2009; Verdejo-Garcia et al. 2012). The interaction between compromised interoception and reward systems may result in suboptimal decision-making that could maintain and exacerbate substance use. Substance dependent individuals exhibit decreased striatal response to natural rewards, in turn seeking out alternative stimuli (drugs) in order to maintain bodily equilibrium (Garavan et al., 2000; Kelley and Berridge, 2002; Koob, 2001; Lubman et al., 2009; Paulus et al., 2005, 2009; Volkow et al., 2009, 2010). Therefore, brain regions subserving interoceptive processing may be altered in MD, resulting in diminished response to naturally rewarding interoceptive stimuli.
The insula has been implicated in a range of psychological processes characterized as involving interoceptive awareness and decision making such as drug craving, error monitoring, empathy, and emotion detection. Although prior work has shown that MD individuals exhibit attenuated insula activation during error processing (London et al., 2005; Nestor et al., 2011) and emotion matching (Kim et al., 2011), no studies have examined insula function within the context of a task probing responses to interoceptive sensations in MD, despite assertions that interoceptive dysfunction via the insula is implicated in addiction and interacts with decision making (e.g., Garavan, 2010; Naqvi and Bechara, 2009, 2010; Naqvi et al., 2007; Paulus et al., 2009; Verdejo-Garcia and Bechara, 2009; Verdejo-Garcia et al., 2012). With respect to thalamic function, although stimulant dependent individuals exhibit attenuated activation during decision making in the context of a working memory task (Moeller et al., 2010), the role of the thalamus in interoception of MD has not yet been examined. Moreover, although a large literature implicates reduced reward responsivity in substance dependence as reflected in attenuated striatal function (e.g., Ahmed and Koob, 2005; Berridge et al., 2011; Koob and Volkow, 2009), few studies have examined neural attenuation of pleasant or rewarding stimuli in MD individuals (Hoffman et al., 2007; Monterosso et al., 2007). Therefore, further research is warranted to determine whether the nature of brain dysfunction in MD extends to the processing of pleasant interoceptive stimuli.
To examine brain markers of interoception and reward processing in MD, the present study employed a Soft Touch paradigm wherein subjects completed a simple attentional task while anticipating and receiving soft brush strokes to the forearm and palm during functional magnetic resonance imaging (fMRI). Research has demonstrated that gentle stroking along the skin is experienced as pleasant compared to constant pressure to the same region (Björnsdotter et al., 2009, 2010; McGlone et al., 2012; Morrison et al., 2011). Forearm skin contains unmyelinated mechanoreceptive C-tactile fibers (MR-CF) that respond to gentle tactile stimulation and project to anterior insula via the thalamus for emotional processing and interoceptive awareness (Loken et al., 2009; Olausson et al., 2002). In contrast to forearm skin, palm skin contains less MR-CF and touch to this region results in lower pleasantness ratings than forearm skin (Loken et al., 2009; Morrison et al., 2011, Olausson et al., 2002). Specifically, Loken et al. (2009) showed pleasantness to be significantly lower for the slow stroke to the palm than for the forearm, with subjective pleasantness ratings peaking at a velocity of 2.8 cm/s. In addition, previous research demonstrated increased posterior insula activation during MR-CF stimulation at a velocity of 1–10 cm/ (Löken et al., 2009; Morrison et al., 2011). These findings suggest that MR-CF are involved in positively valenced interoceptive processing. The goal of the present investigation was to determine whether MD is linked to attenuated brain activation in regions involved in reward and interoception only during pleasant touch of MR-CF located in the forearm, or across both types of pleasant touch. Moreover, we aimed to determine whether brain regions involved in interoception and reward responsivity were reduced not only during the sensation of pleasant touch, but also in anticipation of pleasant touch.
This investigation compared brain activation between MD and healthy comparison subjects (CTL) during a Soft Touch continuous performance task (CPT) involving pleasant interoceptive stimulation. Three primary hypotheses were tested. First, given that substance dependent individuals have been shown to exhibit decreased brain response to natural rewards, we predicted that MD would display lower striatal activation than CTL in response to both forearm and palm pleasant touch. Second, due to recent assertions that substance users may possess a dysfunctional interoceptive system (Verdejo-Garcia et al., 2012), we predicted that MD would exhibit lower left anterior insula activation than CTL during pleasant touch. Third, given reduced reward and interoceptive sensitivity we predict in MD, we also hypothesized that MD will subjectively report soft touch as less pleasant than CTL. Moreover, a secondary hypothesis was tested; we predicted that group differences in brain activation and subjective ratings of pleasantness would be stronger for forearm soft touch due to differences in fiber type between palm and forearm. Finally, brain activation was correlated with subjective ratings and behavioral performance within group to assist with interpretations of group differences.
2. METHODS
2.1 Subjects
Twenty five MD meeting criteria for DSM-IV methamphetamine dependence (19M, 6F) and 17 healthy CTL (10M, 7F) with no history of any substance use disorder completed clinical assessments and an fMRI session. The University of California San Diego Human Research Protections Program and the Veterans Affairs San Diego Healthcare System Research and Development Office approved the study protocol and written informed consent was obtained from all participants prior to study enrollment.
Participants underwent a clinical interview using the Semi-Structured Assessment for Drug Dependence and Alcoholism (SSADDA; Koob, 2001) to determine lifetime substance use and patterns of use including days and amounts used. Similar to the Structured Clinical Interview for DSM disorders (SCID; First, 1997), the SSADDA assesses Axis I mood, anxiety, schizophrenia, and substance use disorders as well as Axis II antisocial personality disorder (ASPD) and includes greater in-depth questioning of substance taking behaviors. Individuals were ruled out if they met the following exclusionary criteria: (1) current Axis I diagnoses (other than substance dependence for MD subjects); (2) lifetime bipolar disorder, schizophrenia and obsessive compulsive disorder; (3) ASPD; (4) positive urine toxicology test; (5) pregnancy; (6) left handedness; (7) head injuries or loss of consciousness > 5 minutes; (8) irremovable metal in body and (9) claustrophobia. Clinical diagnoses were presented at a consensus meeting and confirmed by a psychiatrist trained in substance use disorders (MPP).
During the interview session, participants completed personality measures including the Barratt Impulsiveness Scale (BIS; Patton et al., 1995) and the Sensation seeking Scale form V (SSS-V; Zuckerman, 1996) as measures of impulsivity and sensation seeking. At the fMRI session, the Beck Depression Inventory II (BDI-II; Beck et al., 1993) was completed to assess depression and urine samples were obtained to test for toxicology. A positive result for any substance other than cannabis excluded individuals from the study. MD were all receiving treatment at the time of participation and were required to be between 15 and 120 days sober (M = 41.13, SD =19.51). No subjects reported symptoms of withdrawal during study participation.
2.2 Soft Touch Task
The interoceptive stimulus consisted of MR-CF stimulation (“Soft Touch”) administered by trained research assistants using a soft boar bristle brush (OXO International Ltd., NY). MR-CF stimulation was administered on pre-measured and marked 4cm long regions of skin on the ventral surface of the left forearm, thought to be dense in MR-CF, and on the palm, thought to be devoid of these fibers (Loken et al., 2009; Olausson et al., 2000; Vallbo et al., 1993). Each soft touch brush stroke was performed at a velocity of 2cm/sec in a proximal to distal direction with a force equal to the weight of the brush.
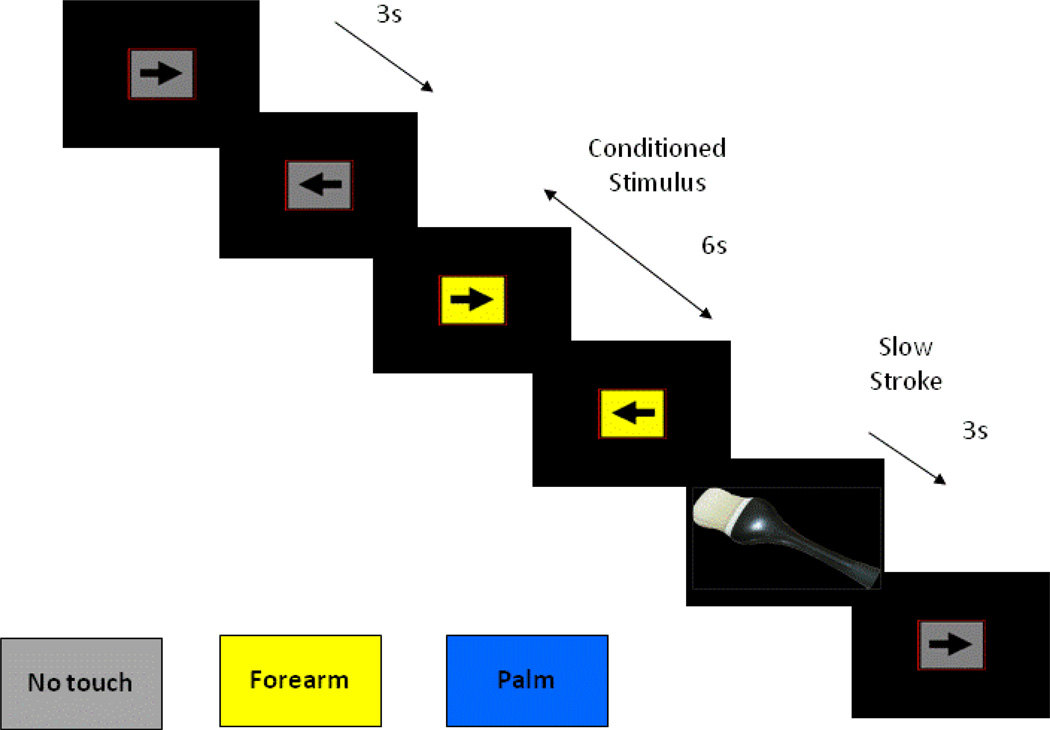
During the fMRI session, participants performed a CPT with cued stimulus presentation, which was designed to focus their attention on visual stimuli while maintaining a stable cognitive load. This task was intentionally chosen to keep participants engaged while not being too complex that it would distract from external stimuli. A screen presented a left or right pointing black arrow surrounded by a colored rectangle in successive three second intervals (see Figure 1). Subjects were instructed to respond to the orientation of the arrow by pressing a left or right button on a button box. The arrow remained on the screen for the entire 3 seconds during which the subject could press a button at any time. The colored rectangle background was used to signify one of three conditions for cued MR-CF stimulus presentation: (1) a baseline condition (gray background) during which no tactile stimulus was expected or administered, averaging nine seconds (three consecutive arrows trials) duration; (2) an anticipation condition lasting six seconds (two consecutive arrows trials) duration, wherein the background color of the presentation indicated an impending soft touch on the left palm (blue background) or left forearm (yellow background); (3) a Soft Touch condition of three second (one arrows trial) duration, during which a soft touch to the skin was administered to either the palm or the forearm for the first two seconds of the trial. Anticipation and soft touch conditions were presented a total of twenty times throughout the task (120 seconds for anticipation, 60 seconds for soft touch) for each stimulus type (palm, forearm). Total task duration was 840 seconds.
Figure 1.
Illustration of continuous performance task
Response accuracy and RT were obtained during all conditions. Participants received instruction on task structure and background color meanings prior to fMRI acquisition and completed pre- and post-fMRI visual analog scale (VAS) questionnaires. VAS instructions indicated that participants should provide a rating from ‘0 - not at all’ to ‘10 - extremely’ about their experience of the Soft Touch task for seven dimensions: pleasant, unpleasant, intensity, tickle, warm, cold, and soft.
2.3 fMRI Data Acquisition
The Soft Touch task was conducted during one fMRI scan sensitive to blood oxygenation level-dependent (BOLD) contrast using a Signa EXCITE (GE Healthcare, USA) 3.0 Tesla scanner (T2*-weighted echo planar imaging (EPI) scans, TR=2000 ms, TE=30 ms, FOV=24 cm (squared), 64×64x40 matrix, forty 3.0 mm axial slices with an in-plane resolution of 3.75×3.75×3mm, flip angle=90 degrees, 420 whole-brain acquisitions). For anatomical reference, a high-resolution T1-weighted image (spoiled gradient recalled [SPGR], TR=8 ms, TE=3 ms, slices=172, FOV=25 cm approximately 1mm (cubed) voxels) was obtained. The CPT was presented in an event related design.
2.4 fMRI Data Analysis
2.4.1 Single subject analysis
fMRI data were preprocessed with the Analysis of Functional Neuroimages (AFNI) software package (Cox, 1996). GE slices were first reconstructed into AFNI BRIK format. The largest temporal region containing the fewest voxel-wise outliers was used as a base for 3d registration. All other time points in dx, dy, dz, and roll, pitch, yaw directions were adjusted to align data to the base image. The functional echoplanar image underwent automatic coregistration to the high resolution anatomical image and each dataset was inspected to confirm successful alignment. New outliers were generated for the volume-registered dataset based on whether a given time point greatly exceeded the mean number of voxel outliers for the time series. Deconvolution was performed to determine Soft Touch decision phase activations. Three movement regressors (roll, pitch, yaw), a baseline and linear drift regressor, and four decision-making regressors (trials for anticipation palm, anticipation forearm, soft touch palm, soft touch forearm), were convolved with a modified hemodynamic response function. The baseline condition, wherein participants were neither anticipating nor receiving the soft touch stimulus, served as the baseline for this analysis. A Gaussian Spatial Filter (4mm FWHM) was used to spatially blur data to account for anatomical differences. Voxels were resampled into 4 × 4 × 4 mm space. Automated Talairach transformations were applied to anatomical images and echoplanar images were subsequently transformed into Talairach space. Percent signal change was determined by dividing the signal for each regressor of interest by the baseline regressor.
2.4.2 Group analysis
A linear mixed effects (LME) analysis (r-project.org) examined group differences in brain activation. Subjects were treated as random effects while group (MD, CTL), condition (anticipation, soft touch), and stimulus type (palm, forearm) were treated as fixed effects. Percent signal change was the dependent variable. The main effect of group was examined to determine whether MD and CTL differed across all conditions. The group by condition interaction was the primary effect of interest in order to test hypotheses involving anticipation and receipt of pleasant touch in MD versus CTL. The secondary effect of interest was the group by condition by stimulus type interaction in order to determine whether group differences in soft touch were stronger for the forearm than the palm. Results for the remaining LME effects are presented in Supplemental Material (Table S1–S4)1. To guard against identifying false positive areas of activation, a threshold adjustment method based on Monte-Carlo simulations (via AFNI Alpha Sim program) was applied. For whole brain analysis, AlphaSim identified a minimum cluster volume of 512 µL (8 contiguous voxels) corresponding to a cluster significance of p<.01 (one-sided) to result in a voxel-wise probability of p<.01 (one-sided) corrected for multiple comparisons. In addition, to further examine the role of brain regions involved in apriori hypotheses, limbic masks for the insula and striatum were applied to LME results. For these masks, AlphaSim identified a minimum cluster volume of 256 µL (4 contiguous voxels) for bilateral insula and a minimum cluster of 192 µL (3 contiguous voxels) for bilateral (dorsal and ventral) striatum. Limbic mask minimum cluster volumes were also corrected for multiple comparisons at p<.01.
2.4.3 Follow-up analysis: Robust regressions
Voxel-based robust regression analyses (Huber, 1973) were used to provide a conservative estimate of correlations between brain activation and VAS ratings by minimizing the effect of outliers. Regressions were performed within MD and CTL separately to examine whether brain activation was associated with subjective VAS ratings of two predictors: ‘pleasant’ (an index of positive valence) and ‘intensity’ (a measure of arousal). Four regressions were performed for each group, one for each of the following dependent variables: percent signal change for (1) anticipation palm, (2) anticipation forearm, (3) soft touch palm, and (4) soft touch forearm. Whole brain and limbic masks were again utilized to obtain significant clusters of activation. Regression results are reported for apriori regions of interest (insula, striatum). Predictors were natural log transformed, due to non-normal distributions, and z-scored prior to regression entry.
2.5 Behavioral Data Analysis
2.5.1 VAS Scales
Two VAS predictors (‘pleasant’ and ‘intensity’) were dependent measures in independent-samples t-tests to examine group differences in subjective reports.
2.5.2 RT and Accuracy
Participants responded to the orientation of an arrow on the screen using the first two buttons of a four-button response box for left and right respectively. Accuracy and RT of each button press was recorded from onset of arrow presentation. A repeated measures ANOVA was performed to investigate RT differences, wherein group (MD, CTL) was the between subject variable and stimulus type (palm, forearm) was the within-subjects variable.
2.6 Follow-up analysis
2.6.1 Brain-behavior relationships
Due to the role of the insula in interoception, analyses were conducted to investigate the relationship between RT and anterior insula activation. Within each group, average RT across all conditions was correlated with group differences in anterior insula activation that emerged from the LME.
2.6.2 Brain-Clinical relationships
Analyses were also conducted to investigate the role of abstinence. Number of days since last methamphetamine use was correlated with RT, VAS pleasantness and intensity ratings, and activation in the insula, striatum and thalamus. Likewise, age of first use was correlated with these variables.
3. RESULTS
3.1 Subject Characteristics
Groups did not differ significantly for age (t(40)=.09, p=.93) or ethnicity ( χ²(4)=2.46, p=.65). CTL, however, had significantly more years of education than MD (t(39)=−4.23, p<.001). MD also reported greater cocaine, cannabis, alcohol and nicotine use (see Table 1).
Table 1.
Group Characteristics
| Gender | MD (19M, 6F) |
CTL (10M, 7F) |
|---|---|---|
| M (SD) | M (SD) | |
| Demographics | ||
| Age (in years) | 38.84(9.16) | 38.77(9.40) |
| Education (in years) | 13.56(1.12) | 15.88(2.18) |
| Drug Use | ||
| Lifetime Sessions of Methamphetamine Use | 14912.78(18686.76) | -- |
| Age of First Methamphetamine Use | 22.26(7.29) | -- |
| # of Participants with Past Cannabis Use | 23* | 10** |
| Lifetime Sessions of Cannabis Use | 4413.61(6212.24) | 70.63(174.01) |
| Age of First Cannabis Use | 13.78(5.46) | 18.17(5.19)□ |
| % Lifetime Cannabis Abuse | 33.33%≠ | 5.88% |
| % Lifetime Cannabis Dependence | 19.05%≠ | -- |
| # of Participants with Past Cocaine Use | 21* | 2** |
| Lifetime Sessions of Cocaine Use | 2918.96(5320.57) | 0.25(0.68) |
| Age of First Cocaine Use | 19.95(3.72) | 19.00(1.41) |
| % Lifetime Cocaine Abuse | 38.09% | -- |
| % Lifetime Cocaine Dependence | 38.09% | -- |
| % Regular Tobacco Use | 100% | 29.41% |
| % Lifetime Tobacco Dependence | 57.14%≠ | -- |
| % Lifetime Alcohol Abuse | 80.95%≠ | 5.88% |
| % Lifetime Alcohol Dependence | 66.67%≠ | -- |
| Questionnaires | ||
| Barratt Impulsivity Scale Total | 74.31(7.88) n=16 | 51.19(7.76) n=16 |
| Sensation Seeking Scale Total | 21.78(5.20) n=18 | 16.81(6.22) n=16 |
Note: MD = methamphetamine dependent subjects. CTL = control subjects.
Use data based on self-report from n=23
Use data based on self-report from n=16
Use data based on self-report from n=6
Use data based on self-report from n=21
3.2 VAS scales
Groups did not differ in VAS pleasantness of soft touch to their palm (MD: M=5.55, SE=.44; CTL: M=5.31, SE=.57) or forearm (MD: M=5.21, SE=.41; CTL: M=5.05, SE=.52) (p=.76). VAS ratings of soft touch intensity to their palm (MD: M=1.71, SE=.38; CTL: M=1.13, SE=.49) and forearm (MD: M=1.47, SE=.27; CTL: M=.85, SE=.35) (p=.21) also did not differ between groups.
3.3 RT and accuracy
A condition by stimulus type interaction (F(1, 40)=7.16, p=.011) indicated that RT during the anticipation condition was slower for forearm than palm, and RT during the soft touch condition was slower for palm than forearm. Groups performed similarly with no significant differences in accuracy for palm anticipation (MD: M=.98, SE=.02; CTL: M=.96, SE=.03), forearm anticipation (MD: M=.97, SE=.02; CTL: M=1.00, SE=.02), palm soft touch (MD: M=.97, SE=.02; CTL: M=.98, SE=.03) and forearm soft touch (MD: M=.97 SE=.02; CTL: M=.97, SE=.02) (p=.09).
3.4 fMRI Data
3.4.1 Group main effect
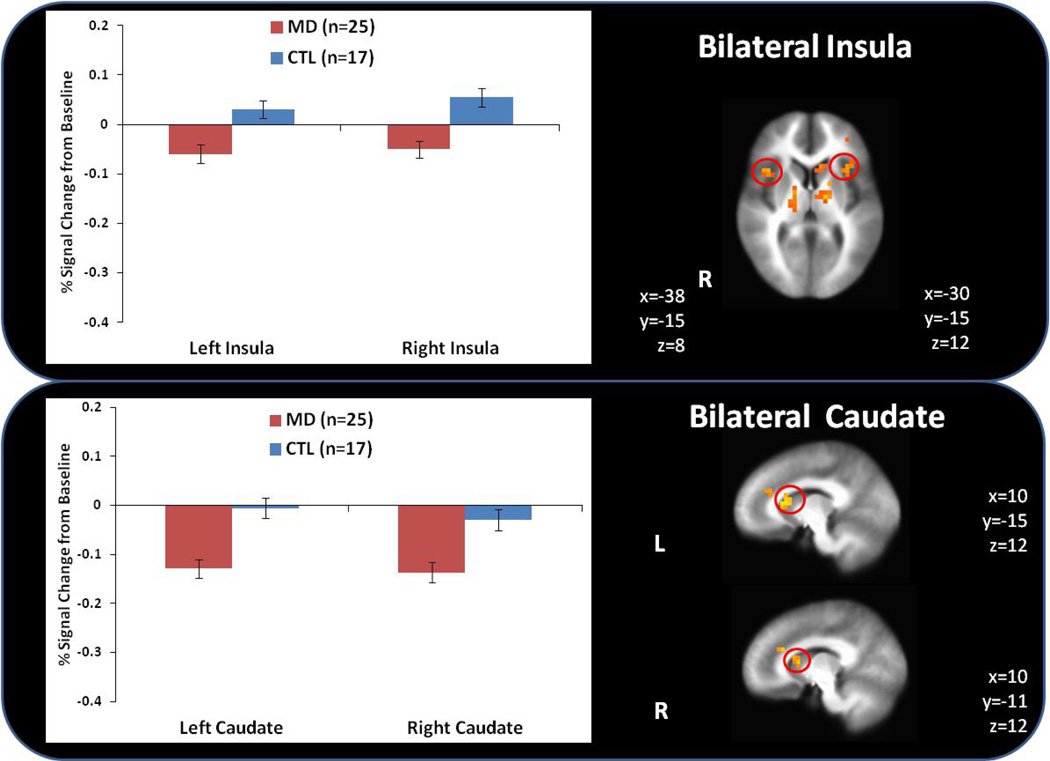
MD exhibited lower activation than CTL during anticipation and soft touch to the palm and forearm within bilateral anterior insula (see Figure 2), bilateral dorsal striatum (caudate), left middle frontal gyrus and bilateral thalamus (see Table 2).
Figure 2.
Methamphetamine dependent (MD) subjects exhibited lower bilateral anterior insula and caudate activation than healthy comparison subjects (CTL) across all conditions.
Table 2.
fMRI Results for Group Main Effect
| Mask | Volume (µL) | x | y | z | F | p | L/R | Area |
|---|---|---|---|---|---|---|---|---|
| CTL>MD | ||||||||
| whole brain | 11008 | 47 | −37 | 40 | 9.04 | .0078 | R | Inferior Parietal Lobule |
| whole brain | 9728 | 8 | 23 | 34 | 7.91 | .0097 | R | Cingulate Gyrus |
| whole brain | 5696 | −31 | 40 | 27 | 7.72 | .0099 | L | Middle Frontal Gyrus |
| whole brain | 2944 | −15 | 10 | 16 | 8.26 | .0080 | L | Caudate |
| whole brain | 2432 | 19 | −20 | 12 | 8.62 | .0081 | R | Thalamus |
| whole brain | 2176 | 12 | −30 | 46 | 8.39 | .0088 | R | Paracentral Lobule |
| whole brain | 2048 | −16 | −9 | 6 | 7.99 | .0098 | L | Thalamus |
| limbic | 1664 | −11 | 13 | 15 | 8.14 | .0083 | L | Caudate |
| whole brain | 1344 | 11 | 6 | 53 | 7.05 | .0127 | R | Medial Frontal Gyrus |
| whole brain | 1216 | −54 | −35 | 26 | 7.92 | .0094 | L | Inferior Parietal Lobule |
| whole brain | 1152 | −39 | −44 | 40 | 7.43 | .0101 | L | Inferior Parietal Lobule |
| whole brain | 1088 | −34 | −12 | −4 | 8.00 | .0104 | L | Claustrum |
| whole brain | 1088 | 39 | 13 | 12 | 8.21 | .0092 | R | Anterior Insula |
| whole brain | 1088 | −40 | 2 | 17 | 7.78 | .0101 | L | Anterior Insula |
| whole brain | 1088 | −2 | 28 | 48 | 8.01 | .0098 | L | Superior Frontal Gyrus |
| whole brain | 960 | −37 | 17 | 20 | 9.26 | .0079 | L | Middle Frontal Gyrus |
| limbic | 896 | 38 | 13 | 11 | 8.05 | .0098 | R | Anterior Insula |
| whole brain | 832 | −33 | 17 | 10 | 8.27 | .0087 | L | Anterior Insula |
| limbic | 704 | −34 | 17 | 10 | 8.31 | .0086 | L | Anterior Insula |
| limbic | 640 | −40 | 4 | 14 | 7.70 | .0099 | L | Anterior Insula |
| whole brain | 576 | 11 | 0 | 63 | 8.69 | .0071 | R | Superior Frontal Gyrus |
| whole brain | 512 | −43 | 12 | 0 | 8.39 | .0084 | L | Anterior Insula |
| limbic | 448 | −43 | 11 | 1 | 8.06 | .0093 | L | Anterior Insula |
| limbic | 448 | 5 | 31 | 24 | 7.31 | .0115 | R | Anterior Cingulate |
| limbic | 320 | −10 | 33 | 23 | 6.65 | .0143 | L | Anterior Cingulate |
| limbic | 320 | 10 | 13 | 13 | 7.07 | .0128 | R | Caudate |
| limbic | 256 | −38 | −13 | −6 | 7.32 | .0111 | L | Anterior Insula |
Note: MD = methamphetamine dependent subjects. CTL = control subjects. L = left hemisphere. R = right hemisphere.
3.4.2 Group by condition interaction
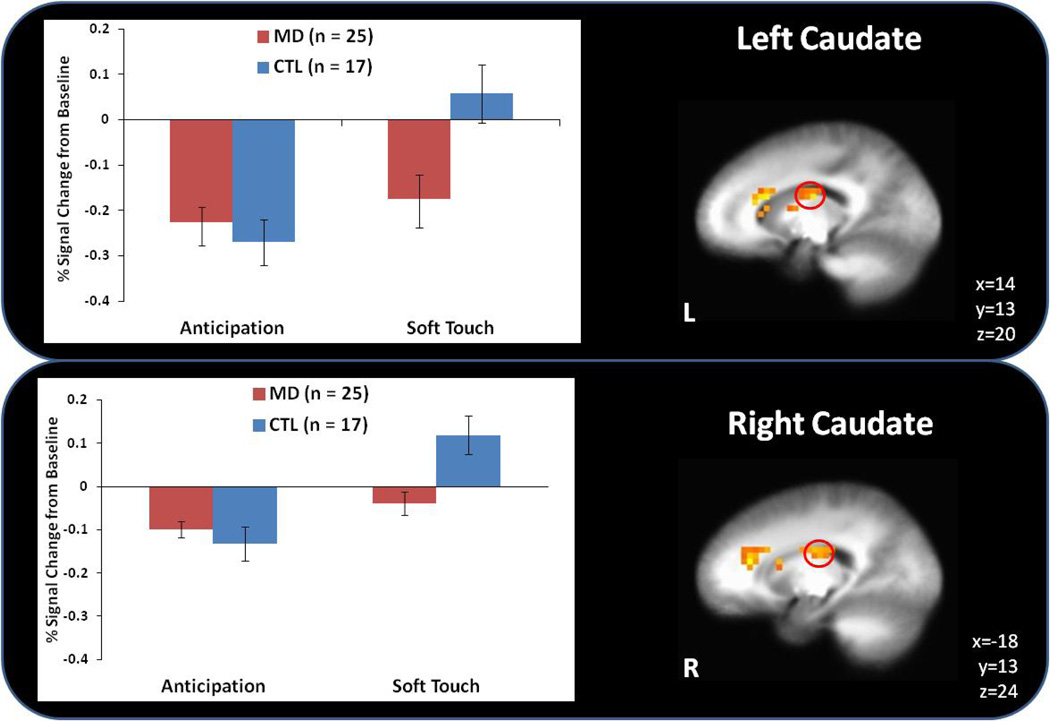
During the anticipation condition, MD displayed lower left thalamus activation than CTL but higher bilateral dorsal striatum (caudate) activation than CTL (see Figure 3). In contrast, during the soft touch condition, MD demonstrated lower activation than CTL in bilateral dorsal striatum (caudate) and the left thalamus (see Table 3).
Figure 3.
Although methamphetamine dependent (MD) subjects showed greater caudate activation during anticipation of soft touch than healthy comparison subjects (CTL), this pattern reversed during the experience of soft touch.
Table 3.
fMRI Results for Group by Condition Interaction
| Mask | Volume (µL) | x | y | z | F | p | L/R | Area | Anticipation | Soft Touch |
|---|---|---|---|---|---|---|---|---|---|---|
| whole brain | 41984 | −20 | −16 | 27 | 9.85 | .0058 | L | Cingulate Gyrus | CTL>MD | CTL>MD |
| whole brain | 29440 | 20 | −2 | 30 | 9.14 | .0064 | R | Cingulate Gyrus | CTL>MD | CTL>MD |
| Limbic | 3520 | −11 | 18 | 17 | 9.24 | .0060 | L | Caudate | MD>CTL | MD>CTL |
| Limbic | 2688 | 15 | 26 | 21 | 8.47 | .0072 | R | Anterior Cingulate | MD>CTL | CTL>MD |
| whole brain | 1152 | −23 | −78 | 14 | 7.45 | .0090 | L | Middle Occipital Gyrus | CTL>MD | CTL>MD |
| Limbic | 832 | −13 | −10 | 21 | 8.46 | .0082 | L | Caudate | MD>CTL | CTL>MD |
| whole brain | 640 | −10 | −32 | 11 | 8.70 | .0073 | L | Thalamus | CTL>MD | CTL>MD |
| whole brain | 640 | −33 | −75 | 38 | 8.94 | .0051 | L | Precuneus | CTL>MD | CTL>MD |
| whole brain | 640 | −14 | 4 | 54 | 9.08 | .0056 | L | Medial Frontal Gyrus | CTL>MD | MD>CTL |
| whole brain | 576 | 28 | 49 | 27 | 7.15 | .0100 | R | Superior Frontal Gyrus | CTL>MD | MD>CTL |
| Limbic | 576 | 18 | −16 | 23 | 9.69 | .0029 | R | Caudate | MD>CTL | CTL>MD |
| whole brain | 512 | −44 | −66 | −5 | 7.67 | .0078 | L | Middle Occipital Gyrus | CTL>MD | MD>CTL |
| Limbic | 512 | 14 | 13 | 13 | 7.55 | .0089 | R | Caudate | MD>CTL | CTL>MD |
| Limbic | 320 | −21 | −30 | 17 | 7.19 | .0010 | L | Caudate | MD>CTL | CTL>MD |
| Limbic | 192 | 25 | −35 | 12 | 7.77 | .0078 | R | Caudate | MD>CTL | CTL>MD |
Note: MD = methamphetamine dependent subjects. CTL = control subjects. L = left hemisphere. R = right hemisphere.
3.4.3 Group by condition by stimulus type interaction
No significant results emerged.
3.4.4 Robust regressions
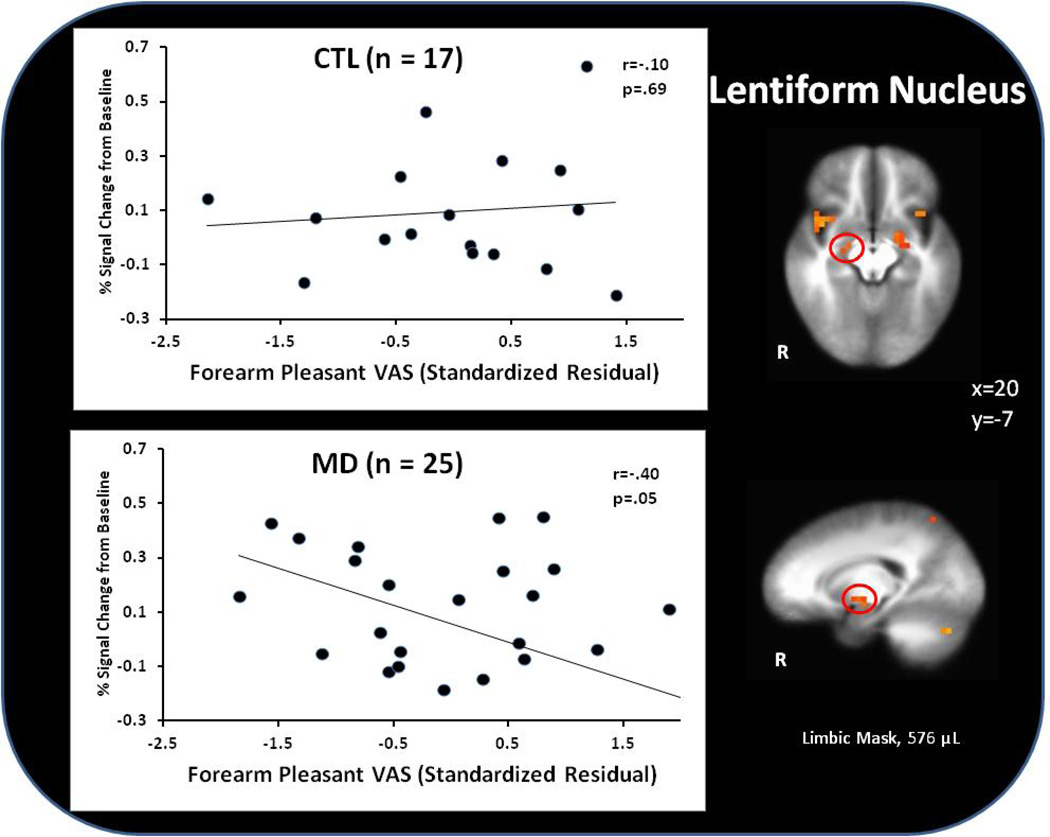
Within MD, higher VAS pleasantness soft touch ratings were associated with lower lentiform nucleus activation during forearm soft touch (r=−0.40, p=.05). No significant VAS results emerged within CTL in this condition (r=0.10, p=.69) (see Figure 4).
Figure 4.
Within methamphetamine dependent (MD) subjects, unique variance associated with visual analog scale (VAS) pleasantness ratings (with shared variance between pleasantness and intensity ratings removed) predicted attenuated lentiform nucleus activation during the experience of soft touch.
3.4.5 Brain-behavior relationships
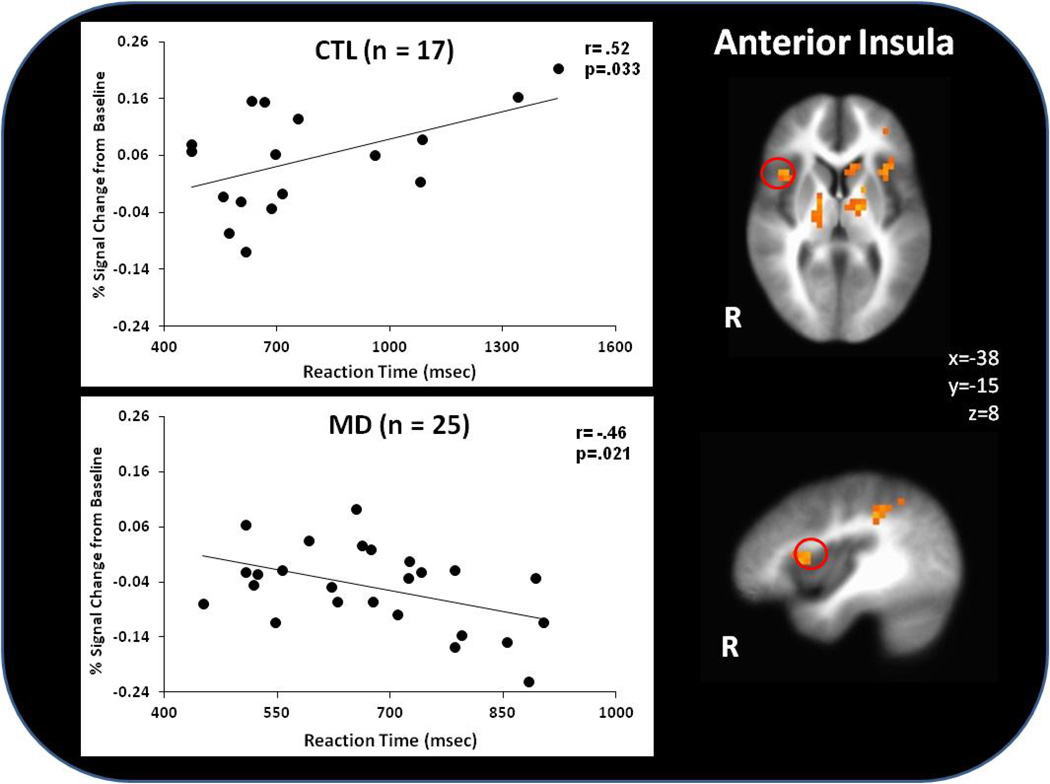
Figure 5 illustrates that within MD, subjects with greater right anterior insula activation responded faster irrespective of condition (r=−.46, p=.02). In comparison, CTL with higher anterior insula activation displayed slower RT across conditions (r=.52, p=.03).
Figure 5.
Within methamphetamine dependent (MD) subjects, lower insula activation was associated with faster RT across conditions, whereas healthy comparison (CTL) subjects displayed the opposite pattern.
3.4.6 Brain-Clinical relationships
No significant correlations were found between days of abstinence or age of first methamphetamine use with RT, VAS ratings of pleasantness and intensity, or activation in the insula, caudate and thalamus.
4. DISCUSSION
This study examined three hypotheses regarding interoceptive processing differences between MD and CTL. Consistent with our first and second predictions, MD exhibited less caudate and left anterior insula activation than CTL to forearm and palm pleasant touch. Third, regarding behavioral differences, we hypothesized that MD would subjectively report soft touch as less pleasant than CTL. Although results did not support this prediction, we did find that higher soft touch pleasantness ratings were linked to lower striatal activation to forearm soft touch within the MD group, suggesting a disconnect between neural responses to pleasant interoceptive stimuli and subjective ratings of this experience. Taken together, relative to CTL, MD deploy fewer brain processing resources to pleasant interoceptive stimuli and may show a paradoxical behavioral response to such stimuli, which provides further evidence of altered interoceptive processing in drug abusing individuals. This lack of self-reported differences suggest that although there may not be an overall subjective attenuation of positive feeling states, there does appear to be an altered interoceptive system in MD compared to CTL. Groups showed no significant differences in accuracy as none were hypothesized, given the simplicity of the task.
Drugs of abuse alter the body state of the individual and can directly affect the centrally generated internal states via direct neurotransmitter-modulating properties (Everitt et al., 2001). Drug use can be seen as a repeated perturbation of the current body state, which becomes associated with conditioned stimuli that may ultimately contribute to a change of the body prediction error. In drug addicted individuals the internally generated states are a result of long-term adjustments due to allostatic dysregulation (Koob and Le Moal, 1997), rendering the individual in an unstable and presumably aversive body state. We have previously proposed that addicted individuals exhibit two types of abnormalities: (1) an increase in body prediction error and insula response to anticipating and experiencing aversive events potentially due to allostatic dysregulation; and (2) an increased decay of the body prediction error over time and insula activity following the experience of an aversive event, resulting in a decreased flexibility to adjust behavior when anticipating or experiencing an aversive event. This may be linked to the transition from goal-directed to habitual behavior (Robbins and Everitt, 1999). Thus, there is an inability of the predicted aversive body states to appropriately influence cognitive control mechanisms (Garavan and Hester, 2007) which may result in response patterns that are primarily driven by striatal responses and associated with the habit system (Everitt and Robbins, 2005). It has been argued that inadequate insula function in drug-dependent individuals may result in an insufficient, instable, or non-adaptive adjustment of the body prediction error (Paulus et al., 2009). Although results from this study support the idea that MD is associated with attenuated processing of afferent body signals, additional research is needed to determine whether these attenuated signals result in inadequate bodily prediction error.
There are several limitations to this study. First, all MD participants had a history of regular nicotine use, whereas this was only true for 29% of the CTL group, leaving the possibility that results could be due, at least in part, to tobacco use. Second, data was collected during only forty repetitions of soft touch total which may not provide enough power for statistical analyses. Future studies should consider using more repetitions and a longer duration of the soft touch to collect more complete information. Third, groups did not differ in their subjective ratings of the soft touch experience. This could be because VAS ratings were provided after the fMRI session. To differentiate between groups, future studies might employ a rating scale during the task to more accurately capture subjective ratings in the moment. Fourth, lack of group differences in reaction time during the soft touch condition is another limitation of the study. A more difficult paradigm requiring a higher cognitive load during complex decision-making could be useful in determining whether interoceptive manipulations result in behavioral performance differences between MD and CTL. Despite null group differences in behavior, however, differential brain-behavior relationships emerged as a function of group status that assists in the interpretation of our results. More specifically, higher anterior insula activation in CTL was associated with slower performance, suggesting that CTL with higher interoceptive awareness become more distracted. The opposite pattern was evident in MD, suggesting an altered mapping of attention to interoceptive functioning within this group. Fifth, changes in caudate activation are difficult to interpret as there was a decrease from baseline for both groups. Lastly, the current sample is also heavily weighted with male participants and as a result, may be less characteristic of female MD.
Despite these limitations, these results provide evidence that MD have neural deficits in brain regions involved in the processing of reward and interoception. The present study is the first to examine positive interoceptive stimulation in a sample of primarily MD individuals. Findings are strengthened by the large sample size as well as the specificity of the current dependence for only one stimulant drug. This study also consists of individuals that are on average at least 2 weeks sober, suggesting that findings are not due to methamphetamine withdrawal. The variety of data from behavioral, neural and self-report measures also strengthens the results of this study. Further investigation is needed to determine whether abstinence from methamphetamine use can normalize interoceptive processing in MD.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
REFERENCES
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II (BDI-II). Manual for Beck Depression Inventory-II. San Antonio: Psychological Corporation; 1993. [Google Scholar]
- Bjornsdotter M, Loken L, Olausson H, Vallbo A, Wessberg J. Somatotopic organziation of gentle touch processing in the posterior insulare cortex. J. Neurosci. 2009;29:9314–9320. doi: 10.1523/JNEUROSCI.0400-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsdotter M, Morrison I, Olausson H. Feeling good: on the role of C fiber mediated touch in interoception. Exp. Brain Res. 2010;207:149–155. doi: 10.1007/s00221-010-2408-y. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the snese of the physiological condition of the body. Curr. Opin. Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Darke S, Kaye S, McKetin R, Duflou J. Major physical and psychological harms of methamphetamine use. Drug Alcohol Rev. 2008;27:253–262. doi: 10.1080/09595230801923702. [DOI] [PubMed] [Google Scholar]
- Darke S, Torok M, Kaye S, Ross J, McKetin R. Comparative rates of violent crime among regular methamphetamine and opioid users: offending and victimization. Addiction. 2010;105:916–919. doi: 10.1111/j.1360-0443.2009.02872.x. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res.B rain Res. Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Clinical Version (SCID-I/CV) Washington, DC: American Psychiatric Association; 1997. [Google Scholar]
- Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol. Rev. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am. J. Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addict. Behav. 2010;35:593–598. doi: 10.1016/j.addbeh.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman WF, Schwartz DL, Huckans MS, McFarland BH, Meiri G, Stevens AA, Mitchell SH. Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology (Berl.) 2008;201:183–193. doi: 10.1007/s00213-008-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber PJ. Robust Regression: Asymptomatics, conjectures, and Monte Carlo. Ann. Stats. 1973;1:799–821. [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J. Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YT, Song HJ, Seo JH, Lee JJ, Lee J, Kwon DH, Yoo DS, Lee HJ, Suh KJ, Chang Y. The differences in neural network activity between methamphetamine abusers and healthy subjects performing an emotion-matching task: functional MRI study. NMR Biomed. 2011;24:1392–1400. doi: 10.1002/nbm.1702. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward and allostasis. Neuropsychopharmacology. 2001;24:97–128. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Loken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat. Neurosci. 2009;12:547–548. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- London ED, Berman SM, Voytek B, Simon SL, Mandelkern MA, Monterosso J, Thompson PM, Brody AL, Geaga JA, Hong MS, Hayashi KM, Rawson RA, Ling W. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biol. Psychiatry. 2005;58:770–778. doi: 10.1016/j.biopsych.2005.04.039. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Kettle JW, Scaffidi A, Mackenzie T, Simmons JG, Allen NB. Responsiveness to drug cues and natural rewards in opiate addiction: associations with later heroin use. Arch. Gen. Psychiatry. 2009;66:205–212. doi: 10.1001/archgenpsychiatry.2008.522. [DOI] [PubMed] [Google Scholar]
- McGlone F, Olausson H, Boyle JA, Jones-Gotman M, Dancer C, Guest S, Essick G. Touching and feeling: differences in pleasant touch processing between glabrous and hairy skin in humans. Eur. J. Neurosci. 2012;35:1782–1788. doi: 10.1111/j.1460-9568.2012.08092.x. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Schmitz JM, Ma L, Liu S, Kjome KL, Rathnayaka N, Kramer LA, Narayana PA. Working memory fMRI activation in cocaine-dependent subjects: association with treatment response. Psychiatry Res. 2010;181:174–182. doi: 10.1016/j.pscychresns.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison I, Loken LS, Minde J, Wessberg J, Perini I, Nennesmo I, Olausson H. Reduced C-afferent fibre density affects perceived pleasantness and empathy for touch. Brain. 2011;134:1116–1126. doi: 10.1093/brain/awr011. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor LJ, Ghahremani DG, Monterosso J, London ED. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res. 2011;194:287–295. doi: 10.1016/j.pscychresns.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, Ekholm S, Strigo I, Worsley K, Vallbo AB, Bushnell MC. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat. Neurosci. 2002;5:900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Olausson H, Wessberg J, Kakuda N. Tactile directional sensibility: peripheral neural mechanisms in man. Brain Res. 2000;866:178–187. doi: 10.1016/s0006-8993(00)02278-2. [DOI] [PubMed] [Google Scholar]
- Padilla R, Ritter AV. Meth mouth: Methamphetamine and oral health. Talking with patients. J. Esthet. Restor. Dent. 2008;20:148–149. doi: 10.1111/j.1708-8240.2008.00167.x. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch. Gen. Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schulteis G. The role of interoception and alliesthesia in addiction. Pharmacol. Biochem. Behav. 2009;94:1–7. doi: 10.1016/j.pbb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennar AL, Shapiro AF, Krysik J. Endangered children: examining children removed from methamphetamine laboratories. Child. Youth Serv. Rev. 2012;34:1777–1785. [Google Scholar]
- Robbins TW, Everitt BJ. Drug addiction: bad habits add up [news] Nature. 1999;398:567–570. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- Sommers I, Baskin D, Baskin-Sommers A. Methamphetamine use among young adults: health and social consequences. Addict. Behav. 2006;31:1469–1476. doi: 10.1016/j.addbeh.2005.10.004. [DOI] [PubMed] [Google Scholar]
- UNODC. World Drug Report 2010. Vienna: United Nations Publication; 2010. [Google Scholar]
- Vallbo A, Olausson H, Wessberg J, Norrsell U. A system of unmyelinated afferents for innocuous mechanoreception in the human skin. Brain Res. 1993;628:301–304. doi: 10.1016/0006-8993(93)90968-s. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A. A somatic marker theory of addiction. Neuropharmacology. 2008;56:48–62. doi: 10.1016/j.neuropharm.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Clark L, Barnaby DD. The role of interoception in addiction: a critical review. Neurosci. Biobehav. Rev. 2012;36:1857–1869. doi: 10.1016/j.neubiorev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;1(56 Suppl):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays. 2010;32:748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E, Blackstone K, Iudicello JE, Morgan EE, Grant I, Moore DJ, Woods SP. Neurocognitive deficits are associated with unemployment in chronic methamphetamine users. Drug Alcohol Depend. 2012;125:146–153. doi: 10.1016/j.drugalcdep.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Item revisions in the sensation seeking scale form V (SSS-V) Person. Individ. Diff. 1996;20:515–515. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.