Abstract
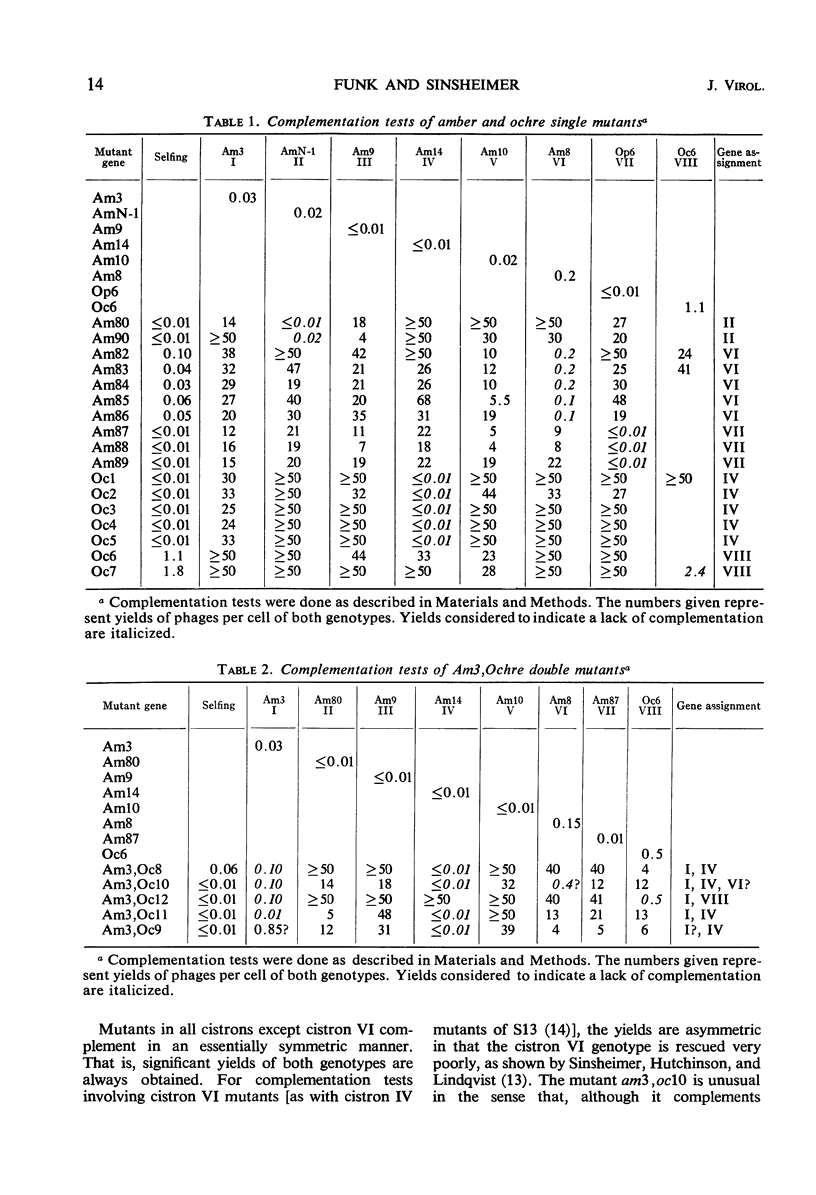
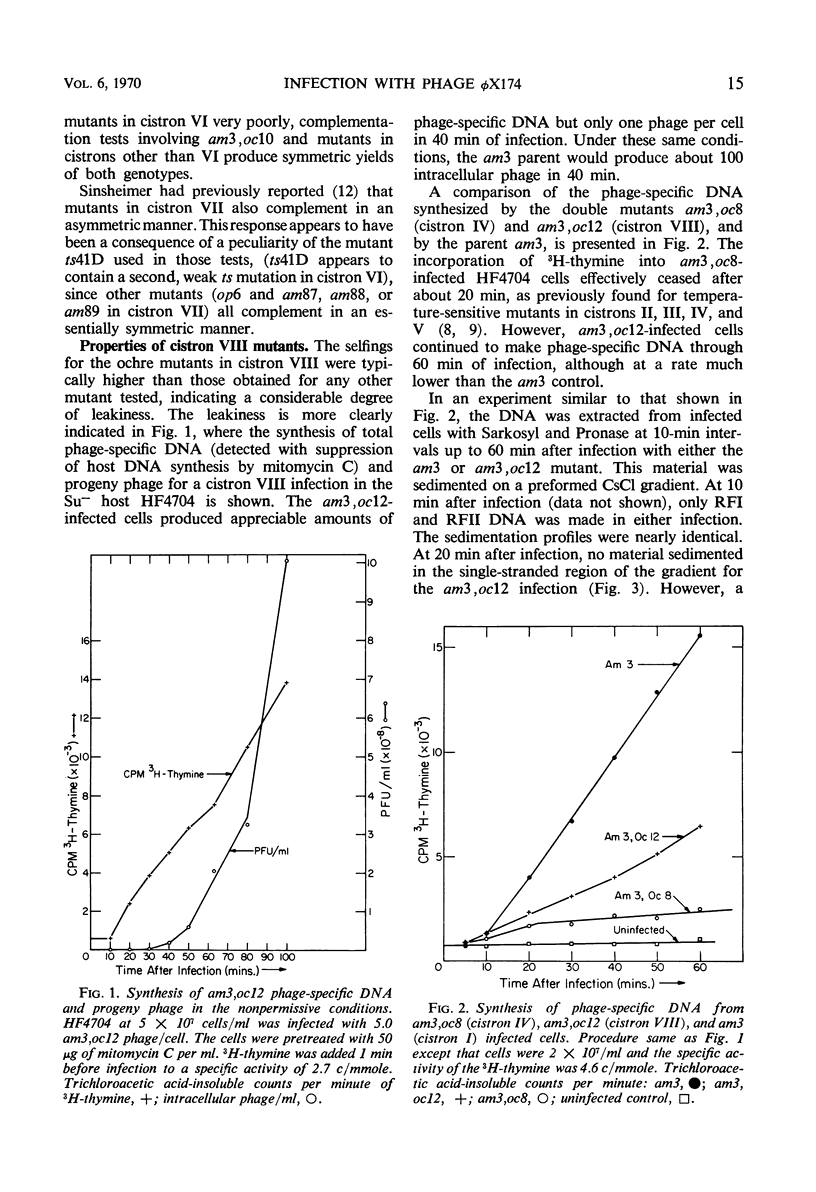
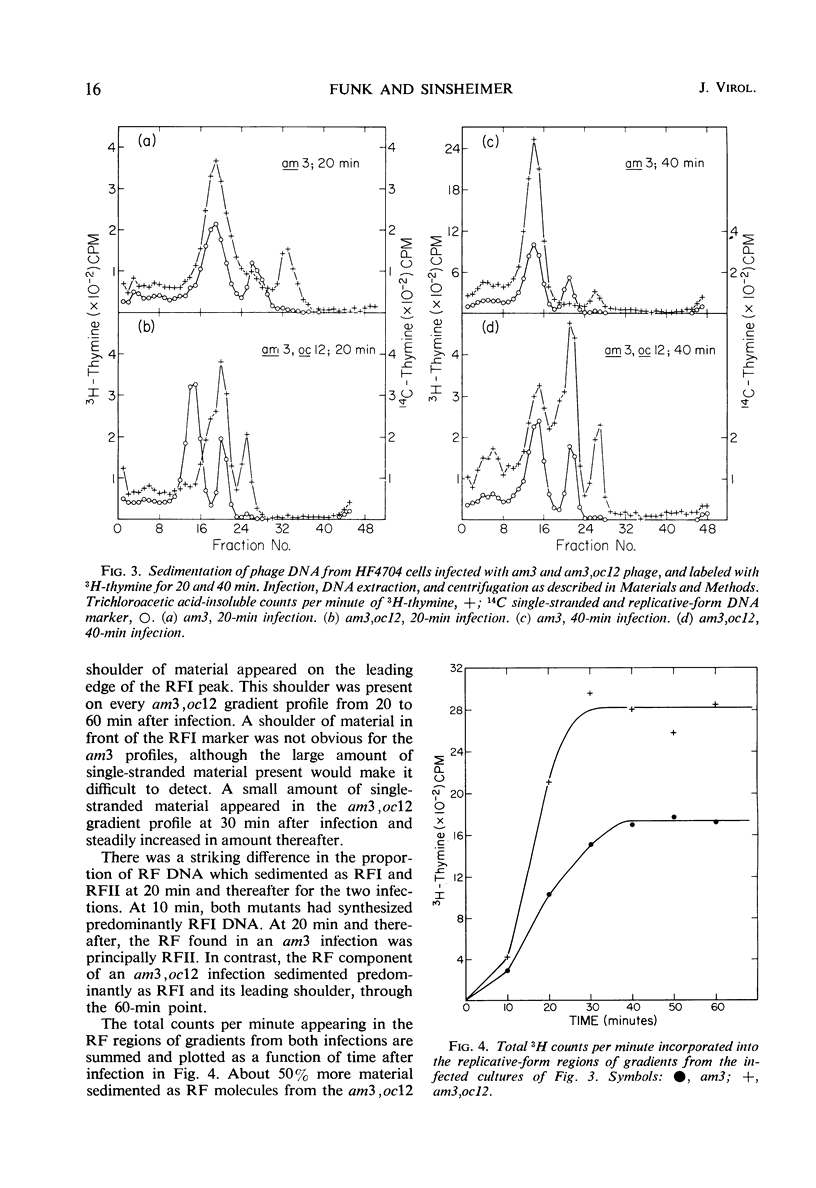
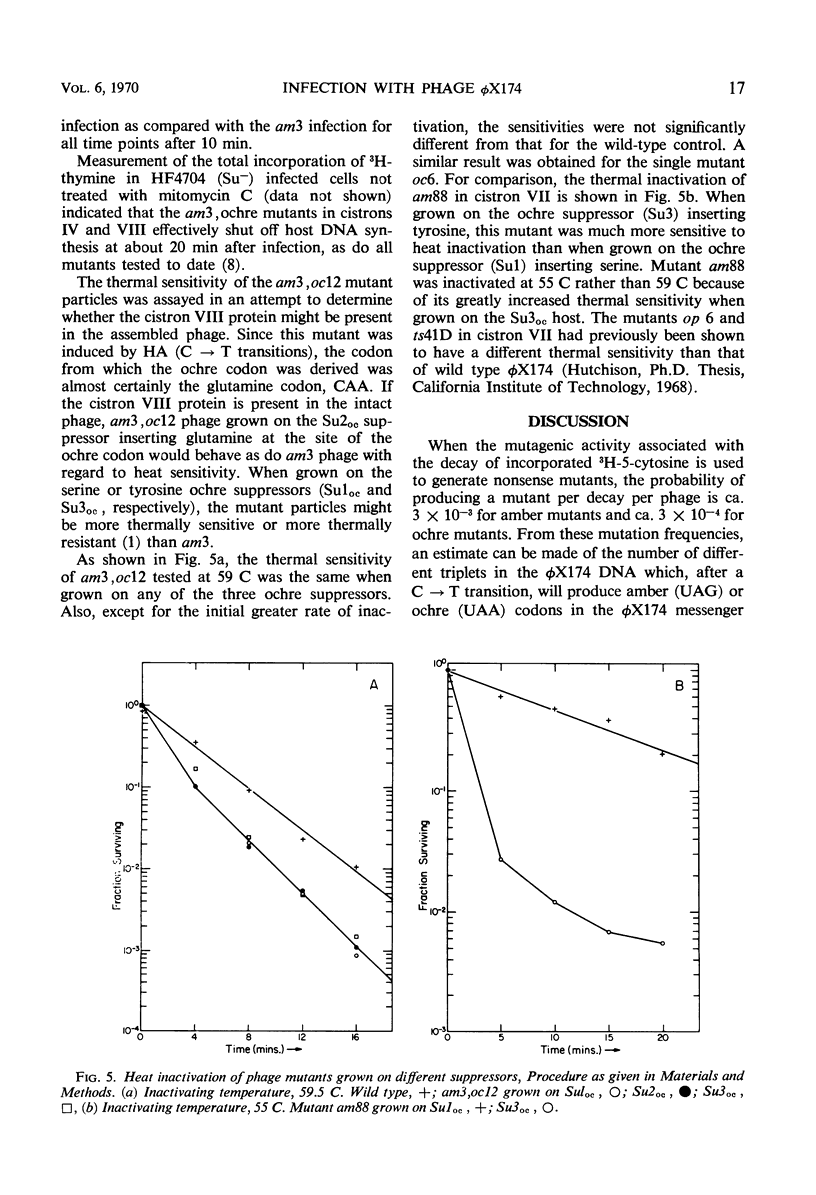
Twenty-two new amber and ochre mutants of φX174 were isolated and classified into complementation groups. Three ochre mutants gave positive complementation tests with reference mutants in the seven previously defined groups and thus represent an eighth cistron. Studies of the physiology of infection in the nonpermissive condition for mutants in cistron VIII yielded the following information. (i) Replicative-form synthesis proceeds at a normal rate, and is turned off at the usual time. (ii) Synthesis of single-stranded deoxyribonucleic acid (DNA) is delayed until nearly 40 min after infection (in the absence of lysis), at which time a slow synthesis of infectious phage particles commences. The synthesis of infectious particles at late times is interpreted as a consequence of “leakage,” and indicates that the cistron VIII product is required in very small quantities. (iii) During the normal period of single-strand synthesis, most of the replicative-form DNA is found in a form with properties similar to those of the transient intermediates of single-strand DNA synthesized during normal infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker R., Tessman I. Heat stability of mutants in genes II, 3a, and VI of phage S13. Virology. 1968 May;35(1):179–181. doi: 10.1016/0042-6822(68)90321-8. [DOI] [PubMed] [Google Scholar]
- Burgess A. B., Denhardt D. T. Studies on phiX174 proteins. I. Phage-specific proteins synthesized after infection of Escherichia coli. J Mol Biol. 1969 Sep 28;44(3):377–386. doi: 10.1016/0022-2836(69)90367-2. [DOI] [PubMed] [Google Scholar]
- Dowell C. E., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. IX. Studies on the physiology of three phi-X174 temperature-sensitive mutants. J Mol Biol. 1966 Apr;16(2):374–386. doi: 10.1016/s0022-2836(66)80180-8. [DOI] [PubMed] [Google Scholar]
- Funk F., Person S. Cytosine to thymine transitions from decay of cytosine-5-3H in bacteriophage S13. Science. 1969 Dec 26;166(3913):1629–1631. doi: 10.1126/science.166.3913.1629. [DOI] [PubMed] [Google Scholar]
- Funk F., Sinsheimer R. L. Process of infection with bacteriophage phiX174. 33. Templates for the synthesis of single-stranded deoxyribonucleic acid. J Virol. 1970 Mar;5(3):282–288. doi: 10.1128/jvi.5.3.282-288.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Sinsheimer R. L. The process of infection with bacteriophage phi-X174. X. Mutations in a phi-X Lysis gene. J Mol Biol. 1966 Jul;18(3):429–447. doi: 10.1016/s0022-2836(66)80035-9. [DOI] [PubMed] [Google Scholar]
- Knippers R., Razin A., Davis R., Sinsheimer R. L. The process of infection with Bacteriophage phi-X174. XXIX. In vivo studies on the synthesis of the single-stranded DNA of progeny phi-X174 bacteriophage. J Mol Biol. 1969 Oct 28;45(2):237–263. doi: 10.1016/0022-2836(69)90103-x. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H., Sinsheimer R. L. Process of infection with bacteriophage phi-X174. XIV. Studies on macromolecular synthesis during infection with a lysis-defective mutant. J Mol Biol. 1967 Aug 28;28(1):87–94. doi: 10.1016/s0022-2836(67)80079-2. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. XV. Bacteriophage DNA synthesis in abortive infections with a set of conditional lethal mutants. J Mol Biol. 1967 Nov 28;30(1):69–80. doi: 10.1016/0022-2836(67)90244-6. [DOI] [PubMed] [Google Scholar]
- Newbold J. E., Sinsheimer R. L. Process of infection with bacteriophage phi-X174. XXXIV. Kinetic of the attachment and eclipse steps of the infection. J Virol. 1970 Apr;5(4):427–431. doi: 10.1128/jvi.5.4.427-431.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person S., Osborn M. The conversion of amber suppressors to ochre suppressors. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1030–1037. doi: 10.1073/pnas.60.3.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinsheimer R. L. Bacteriophage phi-X174 and related viruses. Prog Nucleic Acid Res Mol Biol. 1968;8:115–169. [PubMed] [Google Scholar]
- TESSMAN I., PODDAR R. K., KUMAR S. IDENTIFICATION OF THE ALTERED BASES IN MUTATED SINGLE-STRANDED DNA. I. IN VITRO MUTAGENESIS BY HYDROXYLAMINE, ETHYL METHANESULFONATE AND NITROUS ACID. J Mol Biol. 1964 Aug;9:352–363. doi: 10.1016/s0022-2836(64)80212-6. [DOI] [PubMed] [Google Scholar]
- Tessman E. S. Mutants of bacteriophage S13 blocked in infectious DNA synthesis. J Mol Biol. 1966 May;17(1):218–236. doi: 10.1016/s0022-2836(66)80104-3. [DOI] [PubMed] [Google Scholar]
- Tessman I., Ishiwa H., Kumar S., Baker R. Bacteriophage S13: a 7th gene. Science. 1967 May 12;156(3776):824–825. doi: 10.1126/science.156.3776.824. [DOI] [PubMed] [Google Scholar]



