Abstract
Objective
Preterm infants are exposed to multiple painful procedures in the neonatal intensive care unit (NICU) during a period of rapid brain development. Our aim was to examine relationships between procedural pain in the NICU and early brain development in very preterm infants.
Methods
Infants born very preterm (n=86, 24–32 weeks gestational age) were followed prospectively from birth, and studied with MRI, 3D MR spectroscopic imaging (MRSI) and diffusion tensor imaging (DTI): scan 1 early in life (median 32.1 weeks) and scan 2 at term-equivalent age (median 40 weeks). We calculated N-acetylaspartate to choline ratios (NAA/choline), lactate to choline ratios, average diffusivity (DAV) and white matter fractional anisotropy (FA) from up to seven white and four subcortical grey matter regions of interest. Procedural pain was quantified as the number of skin-breaking events from birth to term or scan 2. Data were analysed using generalized estimating equation modelling adjusting for clinical confounders such as illness severity, morphine exposure, brain-injury and surgery.
Results
After comprehensively adjusting for multiple clinical factors, greater neonatal procedural pain was associated with reduced white matter FA (β= −0.0002, p=0.028) and reduced subcortical grey matter NAA/choline (β= −0.0006, p=0.004). Reduced FA was predicted by early pain (before scan 1), whereas lower NAA/choline was predicted by pain exposure throughout the neonatal course, suggesting a primary and early effect on subcortical structures with secondary white matter changes.
Interpretation
Early procedural pain in very preterm infants may contribute to impaired brain development.
Introduction
Physiologically immature infants born at very low gestational age are exposed to multiple stressful and painful procedures in the neonatal intensive care unit (NICU). This newborn period is a time of rapid brain development and potential vulnerability.1 Thus there has been long-standing concern regarding potential effects of neonatal pain on the immature brain in preterm neonates.2, 3 Nociceptive stimuli reach the cortex, induce pain-specific activation,4, 5 and alter peripheral6, 7 and central4, 8–11 pain processing in neonates. Moreover, repetitive neonatal pain in rats accentuated neuronal excitation and increased cell death in several cortical and subcortical areas,12 suggesting that pain may have a wide-spread effect on the developing brain. Essential painful clinical interventions may thus contribute to activity-dependent modelling of neuronal connectivity during this vulnerable newborn period in very preterm infants.13 In line with this, previous work from our group showed that higher numbers of neonatal skin-breaking procedures (skin breaks) were associated with poorer cognitive and motor function in very preterm infants, after adjusting for specific medical confounders.14
Children born very preterm display poorer cognition, more behavioural problems, poorer executive functions and lower academic achievement compared to their full-term peers.15 Cognitive deficits have been associated with smaller volumes of certain white and grey matter areas in children and adolescents born preterm.16, 17 The specific role that early pain-related stress during neonatal brain development may play in mediating altered structure and function remains unknown.
New advanced in vivo imaging techniques such as Diffusion Tensor imaging (DTI) and MR spectroscopic imaging (MRSI) now allow us to quantify early measures of microstructural and metabolic brain development.13, 18, 19 This is the first prospective longitudinal study, to our knowledge, to address the hypothesis that higher procedural pain-related stress (quantified as the number of skin breaks) will be associated with abnormal brain maturation, after adjusting for confounding clinical factors such as illness severity, infection, hypotension, brain injuries and morphine exposure, that may mediate apparent effects of pain. We applied these non-invasive brain imaging methods in the present study to directly address the question of the consequences of neonatal pain exposure on early brain development in very preterm infants in the NICU.
Materials and Methods
Study design and patients
Participants in this prospective cohort study comprised infants born very preterm (24 to 32 weeks gestation) and admitted from April 2006 to January 2009 to the level-III NICU at Children’s & Women’s Health Centre of British Columbia, the provincial tertiary-level neonatal referral centre, as part of an on-going longitudinal research program of pain-related stress and brain development in very preterm infants.14, 20 Exclusion criteria were: 1) major congenital malformation or syndrome (none), 2) maternal illicit drug use during pregnancy (n=4) and 3) clinically unstable for transport to the MRI scanner resulting in only one scan (n=20). Ten infants were excluded due to missing chart data, which did not allow us to calculate the daily number of skin breaks. The study sample comprised N=86 infants (Figure 1). This study was approved by the University of British Columbia Children’s and Women’s Research Ethics Board and written informed consent was obtained from parents.
Figure 1. Study profile.
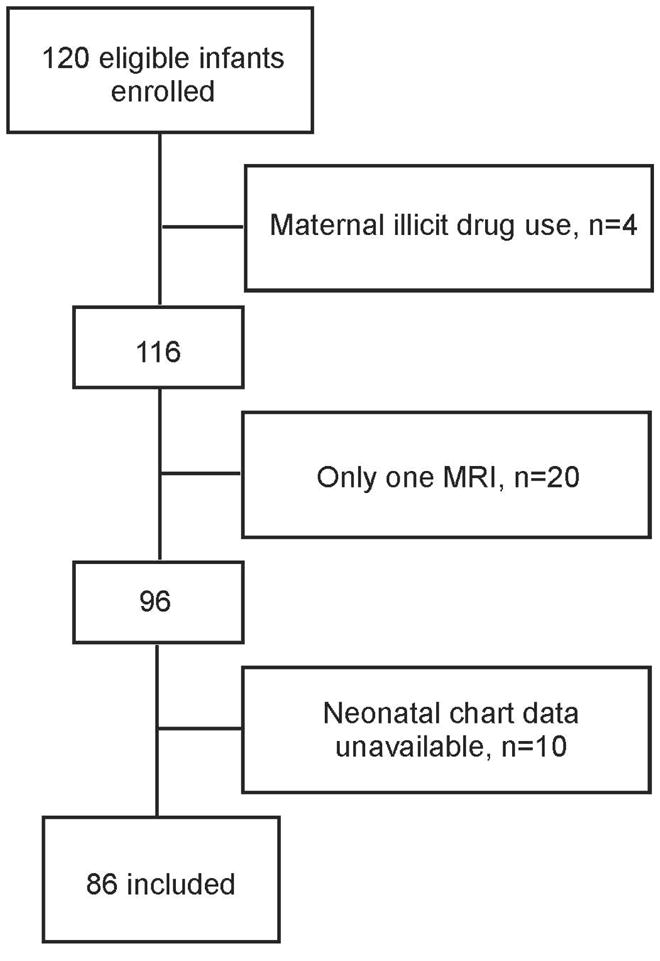
MRI= magnetic resonance imaging
Procedures
MAGNETIC RESONANCE IMAGING
MR-compatible isolette (Lammers Medical Technology, Luebeck, Germany) and specialized neonatal head coil (Advanced Imaging Research, Cleveland, OH) were used for all scans. Newborns were scanned as soon as they were clinically stable (scan 1) and again at term-equivalent age (scan 2), without pharmacological sedation as described previously.20 MRI studies were carried out on a Siemens (Berlin, Germany) 1.5T Avanto using VB 13A software and included the following sequences: 3D coronal volumetric T1-weighted images (repetition time [TR], 36; echo time [TE], 9.2; field of view [FOV], 200mm; slice thickness, 1mm; no gap) and axial fast spin echo T2-weighted images (TR, 4610; TE, 107; FOV, 160mm; slice thickness, 4mm; gap, 0.2mm). An experienced neuroradiologist (K.J.P.) reviewed the images blinded to the newborn’s medical history for severity of white matter injury (WMI), intraventricular haemorrhage (IVH) ventriculomegaly and cerebellar haemorrhage as previously described.20 Moderate-severe brain injury was defined as moderate or severe WMI, IVH grades 3 or 4, or ventriculomegaly.
DIFFUSION TENSOR IMAGING (DTI)
DTI was acquired with a multi repetition, single-shot echo planar sequence with 12 gradient directions (TR, 4,900; TE, 104; FOV, 160mm; slice thickness, 3 mm; no gap) and three averages of two diffusion weightings of 600 and 700s/mm2 (b value) and an image without diffusion weighting, resulting in an in-plane resolution of 1.3mm. DTI measures the rate of diffusion of water molecules, which differs depending on surrounding restricting structures, such as white matter tracts. The diffusion tensor describes an ellipsoid in space, with size, shape and orientation given by three eigenvalues and their corresponding eigenvectors (λ1, λ 2 and λ3), with the principal eigenvector (λ1) reflecting axial diffusion, such as that parallel to organized white-matter tracts and λ2 and λ3 reflecting radial diffusion, perpendicular to white-matter tracts.21 Mean diffusivity [DAV] reflects the mean of these eigenvalues, expressed as 10−3mm2/s, whereas fractional anisotropy [FA] reflects their variance and thus overall directionality. DAV and FA were calculated from seven white matter regions bilaterally and DAV was also obtained from four subcortical grey matter regions bilaterally (Figure 2). With increasing maturity, DAV decreases,22, 23 presumably from decreased water content and developing neuronal and glial cell membranes.19 FA increases with white matter maturation, particularly with the maturation of the oligodendrocyte lineage and early events of myelination.23, 24
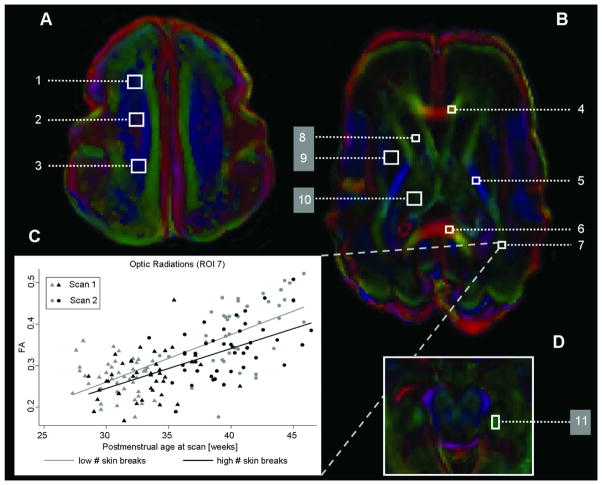
Figure 2. Diffusion tensor imaging and regions of interest.
The figure shows the axial diffusion tensor imaging encoded anisotropy colour map and the seven white (1–7; FA and DAV analysed) and four grey matter (8–11; only DAV analysed) regions of interest (ROIs) that were analysed in a premature newborn with normal magnetic resonance imaging and born at 26+6/7 weeks gestation and scanned at 29+3/7 weeks postmenstrual age, at (A) the high centrum semiovale, (B) the basal ganglia and (D) the hippocampal area. The values of each region were averaged bilaterally: high white matter ([1] anterior, [2] central, [3] posterior), (4) genu of the corpus callosum, (5) posterior limb of the internal capsule, (6) splenium of the corpus callosum, (7) optic radiations, (8) caudate, (9) lentiform nuclei, (10) thalamus and (11) hippocampus. The colour convention used to display the predominant diffusion direction has red representing right-left, green representing anterior-posterior and blue representing superior-inferior anatomical directions. (C) represents mean fractional anisotropy (FA) for the optic radiations (ROI 7) for infants with low (below median: grey colour) or high (above median: black colour) numbers of skin-breaking procedures at scan 1 (triangles) and scan 2 (circles).
MAGNETIC RESONANCE SPECTROSCOPIC IMAGING (MRSI)
MRSI was acquired using multivoxel chemical shift imaging (TR, 1,500; TE, 144; averaging 4) as previously described.20 MRSI measures the concentration of biochemical compounds such as N-acetylaspartate (NAA), choline and lactate which reflect regional brain metabolism and maturation.25 NAA, an acetylated amino acid found in high concentrations in neurons, increases with advancing cerebral maturity.26 Though lactate is elevated with disturbances in cerebral oxidative metabolism, elevated lactate is observed in premature newborns in the absence of overt brain injury.25 All spectra were analysed offline by a single observer with voxels (6 × 6 × 10mm) centred bilaterally on four predefined white matter (anterior, central, posterior high white matter and optical radiations) and three subcortical anatomical regions (Figure 3). The mean NAA/choline and lactate/choline ratios were calculated bilaterally for each region of interest and log-transformed for analysis.
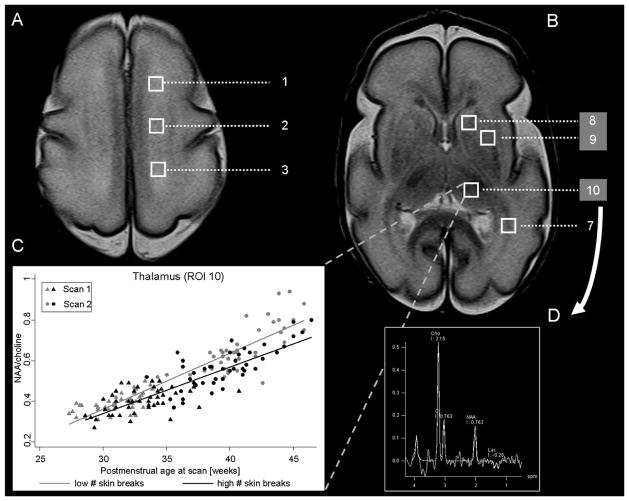
Figure 3. Proton magnetic resonance imaging and regions of interest.
The figure shows the magnetic resonance (MR) spectroscopic imaging and the four white (1–3, 7) and three grey (8–10) regions of interest that were analysed at the level of (A) the high centrum semiovale and (B) the basal ganglia. The values of each region were averaged bilaterally: high white matter ([1] anterior, [2] central and [3] posterior), (7) optic radiations, (8) caudate, (9) lentiform nuclei and (10) thalamus as in Figure 2. (C) represents the mean NAA/choline ratio for the thalamus (ROI 10) for infants with low (below median: grey colour) or high (above median: black colour) numbers of skin-breaking procedures at scan 1 (triangles) and scan 2 (circles). The spectrum of the right thalamus is shown in D. Cho = choline; Cr = creatine; NAA = N-acetylaspartate; Lac = lactate.
CLINICAL DATA COLLECTION
Medical and nursing chart review was carried out by highly trained neonatal research nurses, including but not limited to, birth weight, gestational age, illness severity on day 1 (SNAP-II27), days of mechanical ventilation, daily doses of morphine and other medications, number of surgeries, presence of hypotension, infections and necrotizing enterocolitis (NEC). Cumulative exposure to morphine, midazolam, fentanyl and dexamethasone was calculated for each period as the average daily dose (i.e. intravenous dose plus converted oral dose) adjusted for daily body weight, multiplied by the number of days the drug was given, as we have used previously.14 To be conservative in our evaluation of the role of procedural pain, both clinically suspicious and culture-positive postnatal infections were included in analyses, as well as NEC (stage 1–3); using specific criteria described previously.20 The number of days on mechanical ventilation was stratified into five categories (none, 1–20, 21–40, 41–60, 61 days or more) for analysis. Newborns were considered to have hypotension if they were treated with saline boluses or vasopressors (including dopamine) for low blood pressure. To operationalize procedural pain-related stress, which was an a priori goal of this research program, the number of skin-breaking procedures (e.g. heel lance, intravenous or central line insertion, intramuscular injection, chest tube insertion, gastrostomy tube insertion, tape removal), as well as nasogastric tube insertion, as used previously,14, 28–30 were recorded for each time window. To simplify the terminology we refer to all included procedures as “skin breaks” throughout the manuscript. Each attempt at a procedure was included, thus the sum reflected all skin breaks. While it is recognized that procedures differ in pain intensity, we count every skin break as a “marker” of acute pain-related stress exposure in the NICU, since due to sensitization, the impact of a procedure is not a direct function of the degree of tissue damage.31 All nursing staff in our NICU have been trained to carry out precise recordings of every skin-breaking procedure, including each attempt; therefore we have highly consistent chart information on every infant. Since ongoing chart review would require 24 hour presence of a research nurse, we conducted chart review when the infant was discharged. Data entry was independently checked by a second research nurse. A soother with facilitated tucking, or breast milk, is typically used for routine procedures (e.g. heel lance) in our NICU. Measures from chart review were calculated from birth to scan 1 and from scan 1 to scan 2 or term (i.e. 39+6 postmenstrual age), whichever occurred first, for each infant.
Statistical Analysis
Statistical analysis was performed using Stata 9.2 software (Stata Corporation, College Station, TX). Generalized estimating equation (GEE) modelling with Huber–White sandwich estimator for robust standard errors was used to evaluate the association of procedural pain and clinical variables with white and subcortical grey matter diffusivity (λ1, λ2 and λ3, DAV, FA) and brain metabolites (NAA/choline and lactate/choline). GEE accounts for the repeated measures within each subject over time (scan 1 and scan 2) by allowing for the correlation between the observations. All models include terms for multiple regions of interest (ROIs: four to seven white matter areas or three to four subcortical) and are adjusted for clustering by individuals. Independent variables entered in the initial model based on previously reported impact on brain development or association with pain were: days of life at MRI scan, gestational age at birth, moderate-severe brain injury, sex, SNAP-II, morphine and the number of skin breaks).14, 20 If skin breaks were significant predictors of the outcome variable in this basic model, we extended the model to include additional clinical confounders (i.e. hypotension, infection, NEC, dexamethasone, midazolam, mechanical ventilation). Surgery (none, one, or two and more) and Fentanyl were only included in the final model if they were significant in a separate surgery model (see Tables 2–4).
Table 2.
Results of GEE models for mean FA in seven white matter regions of interest
| Basic Model | Surgery Model | Extended / Final Model | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Effect size | p value | Effect size | p value | Effect size | p value | |
| Gestational age at birth | 0.0056 | 0.016* | 0.0050 | 0.024* | 0.006 | 0.006* |
| Days of life at MRI | 0.0013 | <0.0001* | 0.0013 | <0.0001* | 0.0013 | <0.0001* |
| Brain Injury | −0.0224 | <0.0001* | −0.0234 | <0.0001* | −0.020 | 0.002* |
| Sex | −0.0002 | 0.97 | −0.0011 | 0.85 | −0.001 | 0.79 |
| SNAP II | −0.0001 | 0.65 | −0.00002 | 0.95 | −0.00004 | 0.87 |
| Morphine | 0.0002 | 0.62 | 0.0005 | 0.36 | 0.0006 | 0.19 |
| Mechanical ventilation | −0.005 | 0.24 | ||||
| Infection | −0.0031 | 0.86 | ||||
| NEC | −0.008 | 0.33 | ||||
| Hypotension | −0.014 | 0.061 | ||||
| Dexamethasone | −0.011 | 0.02* | ||||
| Midazolam | −0.0004 | 0.33 | 0.00009 | 0.78 | ||
| Fentanyl | 0.0006 | 0.27 | ||||
| One surgery | −0.0179 | 0.051 | ||||
| Two or more surgery | −0.011 | 0.31 | ||||
| Skin breaks | −0.00027 | 0.002* | −0.00022 | 0.012* | −0.00024 | 0.028* |
| Skin breaks to scan 1a | −0.00026 | 0.029* | ||||
| Skin breaks after scan 1a | −0.00018 | 0.22 | ||||
The effect size describes the expected change per unit in the outcome variable (FA) with a change in the predictor variable. For example, each additional skin break decreased FA by 0.00024 in the final model.
When added to final model instead of cumulative skin breaks,
p<0.05
Table 4.
Results of GEE models for mean NAA/choline in three grey matter regions of interest
| Basic Model | Surgery Model | Extended / Final Model | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Effect size | p value | Effect size | p value | Effect size | p value | |
| Gestational age at birth | 0.0472 | <0.0001* | 0.0453 | <0.0001* | 0.0432 | <0.0001* |
| Days of life at MRI | 0.0073 | <0.0001* | 0.0074 | <0.0001* | 0.0073 | <0.0001* |
| Brain Injury | −0.0267 | 0.11 | −0.0336 | 0.031* | −0.0238 | 0.16 |
| Sex | 0.0058 | 0.75 | −0.0039 | 0.80 | −0.0018 | 0.91 |
| SNAP II | 0.00068 | 0.36 | 0.0014 | 0.012* | 0.0019 | 0.001* |
| Morphine | −0.0017 | 0.19 | −0.0013 | 0.43 | −0.00056 | 0.77 |
| Mechanical ventilation | 0.0044 | 0.75 | ||||
| Infection | −0.0183 | 0.40 | ||||
| NEC | 0.022 | 0.29 | ||||
| Hypotension | −0.0531 | 0.003* | ||||
| Dexamethasone | −0.0177 | 0.36 | ||||
| Midazolam | −0.0012 | 0.23 | −0.00055 | 0.63 | ||
| Fentanyl | −0.0038 | 0.25 | −0.0033 | 0.20 | ||
| One surgery | −0.0764 | 0.001* | −0.0776 | 0.001* | ||
| Two or more surgery | 0.0310 | 0.17 | 0.0329 | 0.20 | ||
| Skin breaks | −0.00069 | 0.008* | −0.00064 | 0.001* | −0.00061 | 0.004* |
| Skin breaks to scan 1a | −0.00056 | 0.013* | ||||
| Skin breaks after scan 1a | −0.00063 | 0.018* | ||||
The effect size describes the expected change per unit in the outcome variable (NAA/choline) with a change in the predictor variable. For example, each additional skin break decreased NAA/choline by 0.00061 in the final model.
When added to final model instead of cumulative skin breaks,
p<0.05
Results
Demographic and clinical characteristics of the cohort are presented in Table 1.
Table 1.
Infant characteristics for the full cohort and separately for high and low pain exposure (median split on number of skin breaks) (n=86)
| Median (IQR) or N (%) | Infants with low number of skin breaks (n=42) | Infants with high number of skin breaks (n=44) | p value | |
|---|---|---|---|---|
| Neonatal characteristics | ||||
| Gestational age at birth, weeks | 27.4 (25.9–29.6) | 29.4 (27.6–31.1) | 25.9 (25.2–27.3) | <0.001* |
| Weight at birth, g | 956 (790–1285) | 1230 (1055–1380) | 814 (705–944) | <0.001* |
| Male, n (%) | 42 (48) | 17 (40) | 25 (57) | 0.14 |
| SNAP II | 14 (9–24) | 9 (0–14) | 19 (9–32) | <0.001* |
| Apgar score 5 min | 8 (6.5–9) | 8 (7–9) | 7 (6–8) | 0.009* |
| Multiple gestations, n (%) | 40 (47) | 19 (45) | 21 (48) | 0.83 |
| Small for gestation age, n (%) | 17 (20) | 7 (17) | 10 (23) | 0.59 |
| NICU experience/ Clinical factors | ||||
| Days of mechanical ventilation | 6 (1–31) | 1 (0–3) | 30 (13–51.5) | <0.001* |
| Postnatal infection, n (%) | 44 (52) | 8 (19) | 36 (81) | <0.001* |
| Necrotizing enterocolitis (NEC), n (%) | 16 (19) | 4 (10) | 12 (27) | 0.051 |
| NEC stage I, n (% of all NEC) | 13 (81) | 4(100) | 9(75) | 0.23 |
| Hypotension, n (%) | 40 (47) | 10 (24) | 30 (68) | <0.001* |
| Surgeries, n with at least 1 (%) | 30 (35) | 3(7) | 27 (61) | <0.001* |
| Surgeries, n with more than 1 (%) | 14 (16) | 0 | 14 (32) | <0.001* |
| Number of skin breaks | 109 (69–179) | 69 (49–90) | 178.5 (129–229.5) | <0.001* |
| Number of skin breaks before scan 1 | 61 (35–131) | 34 (22–46) | 130 (88–178) | <0.001* |
| Number of skin breaks after scan 1a | 31 (20–55) | 26 (17–48) | 38 (29–62) | 0.007* |
| Medication (doseb (IQR), n exposed) | ||||
| Dexamethasone | 0.9 (0.6–1.6), 17 | 0 (0–0), 0 | 0 (0–0.71), 17 | 0.003* |
| Morphine | 2.5 (0.2–5.1), 57 | 0 (0–0.1), 16 | 3.2 (0.5–10.6), 41 | 0.001* |
| Midazolam | 18.1 (2.5–41.5), 21 | 0 (0–0), 1 | 0 (0–12.53), 20 | 0.004* |
| Fentanyl | 0.01 (0.008–0.032), 15 | 0 (0–0), 2 | 0 (0–0.006), 13 | 0.98 |
| Magnetic resonance imaging | ||||
| Moderate-severe brain injury, n (%) | 31 (36) | 11 (26) | 20 (45) | 0.075 |
| Days of life at scan 1, days | 21.5 (11–47) | 12 (7–17) | 46.5 (30.5–59.5) | <0.001* |
| Days of life at scan 2, days | 80.5 (70–102) | 80 (62–94) | 97 (80.5–105.5) | 0.009* |
| Post-conceptional age at scan 1 | 32.1 (30.4–33.6) | 31.0 (29.6–32.4) | 32.8 (31–34.4) | 0.001* |
| Post-conceptional age at scan 2 | 40.0 (38.4–42) | 40.6 (39.3–43) | 39.4 (37.1–41.2) | 0.0499* |
P-values provide results using t-test for continuous measures and Fisher exact tests for categorical measures
‘After scan 1’ indicates the time between scan 1 and scan 2 or term (whichever was first)
cumulative dose [mg] adjusted for daily body weight
Relationship of skin breaks with early white matter diffusivity (FA and DAV)
Entering the pre-defined factors (days of life at MRI scan, gestational age at birth, moderate-tosevere brain injury, sex) and factors associated with early pain (SNAP-II, morphine) into the initial model revealed a significant association of the total number of skin breaks (to scan 2 or term) with mean FA (β= −0.00027, 95% CI: −0.00044 – −0.000094, p=0.002). We then extended the model and added mechanical ventilation, hypotension, infection, NEC, dexamethasone and midazolam. The number of skin breaks remained significant (β= −0.0002, 95% CI: −0.00045 – −0.00003, p=0.028) after adjusting for all these factors.
We analysed the number of surgeries in a separate model including days of life, gestational age, sex, SNAP-II, morphine, midazolam, fentanyl and skin breaks. There was only a trend (p=0.051) for an association of a surgery with white matter FA, while skin breaks remained significant even after controlling for surgeries (p=0.012). Examination of the data by scan for the skin breaks in the extended model revealed that skin breaks before scan 1 were the driving factor of the overall effect of pain (before scan 1: β= −0.00026, 95%:−0.00049 – −0.000026, p=0.029, after scan 1: p=0.22, Table 2).
The three eigenvectors (λ1, λ2 and λ3) representing each axis of the diffusion tensor ellipsoid, were investigated using the extended model. Though skin breaks were not significantly associated with either λ1 (p=0.20) or λ2 and λ3 (p=0.54), the observed difference in FA reflected a stronger negative impact of skin breaks on axial diffusion (λ1) relative to radial diffusion (λ2 and λ3) (−4 × 10−4 vs. 1.9 × 10−4 mm2/s).
DAV in white matter was analysed using the same approach, but skin breaks were not significantly associated with this measure (p=0.16) in the initial model.
Relationship of skin breaks with brain metabolites (NAA/choline and lactate/choline) in white matter
In the initial model the number of skin breaks was significantly associated with NAA/choline in white matter (β= −0.00099, 95% CI: −0.0017 – −0.00025, p=0.009). In the extended model the number of skin breaks were no longer significantly associated with white matter NAA/choline (p=0.12) and there was no independent effect of surgeries (Table 3).
Table 3.
Results of GEE models for mean NAA/choline in four white matter regions of interest
| Basic Model | Surgery Model | Extended / Final Model | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Effect size | p value | Effect size | p value | Effect size | p value | |
| Gestational age at birth | 0.0489 | <0.0001* | 0.0483 | <0.0001* | 0.0472 | <0.0001* |
| Days of life at MRI | 0.0081 | <0.0001* | 0.0082 | <0.0001* | 0.0091 | <0.0001* |
| Brain Injury | −0.0244 | 0.32 | −0.0330 | 0.18 | −0.0257 | 0.31 |
| Sex | 0.0260 | 0.28 | 0.0193 | 0.40 | 0.0271 | 0.26 |
| SNAP II | 0.00037 | 0.75 | 0.0013 | 0.20 | 0.0014 | 0.20 |
| Morphine | 0.000026 | 0.99 | 0.00007 | 0.98 | 0.0026 | 0.42 |
| Mechanical ventilation | 0.00055 | 0.98 | ||||
| Infection | −0.0464 | 0.16 | ||||
| NEC | −0.0170 | 0.55 | ||||
| Hypotension | −0.0561 | 0.05 | ||||
| Dexamethasone | 0.0069 | 0.80 | ||||
| Midazolam | −0.0014 | 0.34 | −0.0022 | 0.15 | ||
| Fentanyl | −0.0069 | 0.18 | ||||
| One surgery | −0.0442 | 0.24 | ||||
| Two or more surgery | 0.0284 | 0.58 | ||||
| Skin breaks | −0.00099 | 0.009* | −0.00092 | 0.009* | −0.00065 | 0.12 |
| Skin breaks to scan 1a | −0.00072 | 0.15 | ||||
| Skin breaks after scan 1a | −0.00045 | 0.38 | ||||
The effect size describes the expected change per unit in the outcome variable (NAA/choline) with a change in the predictor variable. For example, each additional skin break decreased NAA/choline by 0.00065 in the final model.
When added to final model instead of cumulative skin breaks,
p<0.05
Lactate/choline in white matter was analysed using the same initial model, but skin breaks were not significantly associated with this measure (p=0.27).
Relationship of skin breaks with early subcortical grey matter diffusivity (DAV)
Skin breaks were not significantly associated with subcortical DAV values in the initial model (p=0.89).
Relationship of skin breaks with brain metabolites (NAA/choline and lactate/choline) in subcortical grey matter
Skin breaks (β= −0.00069, 95% CI: −0.0012 – −0.00018, p=0.008) were significantly associated with subcortical grey matter NAA/choline values in the initial model. After extending the model to add medical confounders, number of skin breaks was still highly significant (p=0.009). The number of surgeries was again analysed in a separate model and this time revealed an independent effect for having one surgery (β= −0.076, 95% CI: −0.123 – −0.030, p=0.001), but not two or more (β= 0.31, p=0.17), with the effect of skin breaks being still persistent (p=0.001). Thus, surgeries and fentanyl were added to the final model, which then revealed a significant effect for Snap-II (β= 0.0018, 95% CI: 0.00075 – 0.0030, p=0.001), one surgery (β= −0.077, 95%CI: −0.123 – −0.033, p=0.001), hypotension (β= −0.053, 95% CI: −0.088 – −0.018, p=0.003) and skin breaks (β= −0.00061, 95% CI: −0.0016 – −0.00019, p=0.004). Finally, the number of skin breaks from birth to scan 1 and from scan 1 to scan 2 were analysed using the final model which showed that skin breaks during both time periods were significantly associated with lower NAA/choline (β= −0.00058, 95% CI: −0.001– −0.00012, p=0.013 and β= −0.00063, 95% CI: −0.0012 – −0.00011, p=0.018 respectively, Table 4).
Lactate/choline in subcortical grey matter was analysed using the same initial model, but skin breaks were not significantly associated with this measure (p=0.73).
Discussion
Our results demonstrate that higher numbers of skin breaks, which we have used as a surrogate marker for early neonatal pain-related stress, are significantly associated with reduced white matter and subcortical grey matter maturation. The independent association of neonatal procedural pain on early brain development persisted after comprehensively controlling for multiple confounding clinical factors such as infection, illness severity or analgesic medication. Further, early skin breaks (i.e. before scan 1) had a stronger impact on white matter maturation compared to pain exposure later (i.e. between scan 1 and scan 2), while subcortical areas were affected by exposure to pain during both time periods.
Changes in water diffusion in preterm infants with white matter injury have been associated with impaired myelination due to diminished differentiation of precursor oligodendrocytes.32 The white matter FA values in the current study were driven more by the axial λ1 than by the radial λ2 and λ3. Axial diffusivity (i.e. water diffusion along the longitudinal axes parallel to white matter fibres) is believed to reflect the integrity of the axon and its internal components.33 Thus, the imaging findings point to a pain-associated impairment in axonal development. These findings contrast with our previous studies of white matter injury23, 34, where the primary abnormality is in radial diffusivity, indicating a glial abnormality and further supports an independent association of procedural pain and impaired brain development.
Interestingly, only early, but not later pain exposure was a significant predictor of abnormal white matter microstructure. In subcortical grey matter, early and late skin breaks were associated with reduced NAA/choline suggesting that, either subcortical areas are vulnerable for longer periods of time to the effects of pain or that the effects in the subcortical areas are more immediate than in white matter. However, it is important to note that infants underwent more skin breaks before scan 1 than between scan 1 and scan 2 which might contribute to the weaker effect of the later pain. Together with the diffusion abnormalities, these data suggest that the primary mechanism of abnormal brain development may be impaired development of subcortical neurons (lower NAA/choline) with secondary axonal changes measured in the white matter. In studies of term newborns with encephalopathy, abnormalities in NAA, reflecting neuronal integrity and metabolism, follow elevations in lactate that reflect impaired cerebral oxidative metabolism35, 36. The scans in this study were obtained at a time that elevations in lactate may have already resolved. Thus, the reduction in NAA/choline supports the hypothesis of a long-term effect of pain-related stress on neuronal maturation.
A potential mechanism explaining the reduced white and subcortical grey matter may be that activation of neuronal networks due to painful stimuli leads to high stimulation of physiological immature neurons, which are susceptible to overstimulation and excitotoxic damage.12 Slater and colleagues4 showed significantly larger neuronal activity in response to a noxious procedure in preterm infants compared to age-matched term infants, which may be an indicator of increased stimulation of vulnerable neurons. Pain-induced overstimulation may thus lead to neuronal damage and subsequently to a reduction of axonal connections in preterm infants, which is in line with other studies suggesting alterations in cortical connectivity in children born preterm.37, 38 Our findings are consistent with a report on 8 full term infants (7 asphyxiated), showing that tissue-damaging procedures on day two of life were associated with altered brain metabolites on MRSI on day four.39 In preterm infants, the brain may be particularly vulnerable to excitation during the very early development when neural networks are highly immature and thalamo-cortical connections are waiting in the subplate zone,1 explaining the greater effect of early compared to later pain on white matter maturation.
As expected, we found other factors such as exposure to postnatal dexamethasone or hypotension affected brain development, consistent with previous literature.20, 40 Similarly, we found a significant association of NAA/choline and surgery in subcortical grey matter and a trend with FA in white matter, in the same direction as procedural pain. This suggests that despite surgical and post-surgery pain management with anaesthesia and analgesia, a major operation may independently impact brain maturation. This idea is further supported by a study by Fitzgerald and colleagues demonstrating altered sensory thresholds, blunted by prematurity and further by surgery after 11 years implying there is a change in threshold related to pain experience.7 However, the most common reason for surgery in our cohort was patent ductus arteriosus (Table 5), and considering that hypotension was a significant confounder in our analysis, it is possible that the observed effect may be a result of altered cerebral blood flow prior to surgery, or other surgical factors, rather than a consequence of the surgery related pain. In line with this, one surgery had a more negative association with FA and NAA/choline in our models than two or more surgeries, which might be due to the fact that most of the ‘single’ surgeries were patent ductus arteriosus repairs, while ‘two or more’ surgeries included variable types of surgery (e.g. laparotomy, craniotomy, laser eye surgery).
Table 5.
Type and frequency of surgeries performed under general anesthesia while in the Neonatal Intensive Care Unit
| Type | Number of infants undergoing type of surgerya |
|---|---|
| Patent ductus arteriosus | 20 |
| Craniotomy | 3 |
| Laser Eye Surgery | 6 |
| Laparotomy | 6 |
| Hernia Repair | 5 |
| Gastrostomy | 2 |
| Ileostomy reversal | 2 |
| Microlaryngscopy and Bronchoscopy | 2 |
| Central venous line insertion | 2 |
Some infants underwent the same type of surgery twice (e.g. craniotomy) or had two types of surgery under the same anesthesia (counted as one surgery, but surgery types listed here separately).
Previous studies have challenged the effectiveness of opioids in managing procedural pain in this population, at least with the appropriately low doses for avoiding respiratory suppression.41 It is important to note that in the current study, we did not find any evidence that morphine exposure ameliorates the association of pain with our measures of early brain development. Alternative methods to reduce pain such as sucrose, facilitated tucking with non-nutritive sucking, or skin-to-skin care are effective in reducing pain responses in preterm and full term infants.42 However, it is essential to distinguish pain management strategies that reduce both behavioural and physiological indices. For example, sucrose consistently dampens pain behaviours but has variable effects on physiological parameters,43 and does not reduce the cortical pain signal,9 which in turn is an issue for brain vulnerability. Therefore, more research is needed to find effective ways of reducing pain in preterm infants to avoid the negative effect on brain development.
A limitation of clinical studies is that it is difficult to distinguish between the long-term effects of pain and confounding medical factors such as infection or early illness severity, as sicker infants undergo more painful procedures (see Table 1). As it is not possible to conduct randomized controlled trials of the effects of early pain exposure in human neonates, we chose a detailed chart review and comprehensive adjustment for multiple clinical factors in the analysis as a feasible approach to investigate this question. We did not collect other indices of pain-related stress such as cortisol, since cortisol in hospitalised preterm neonates reflects multiple factors such as illness and time spent in the NICU. Further, cortisol levels can be inappropriately low and therefore do not necessarily reflect the degree of pain or stress the infant is exposed to at the time. 28, 44, 45 However, it is important to note that early procedural pain exposure can predict cortisol levels later, long after hospital discharge.46 There could be other confounding factors we have not considered, that may be associated with higher numbers of skin breaks and thereby contribute to altered white and subcortical grey matter maturation. Therefore it is important that our findings on the association between neonatal procedural pain and brain development are consistent with the experimental animal study (the only one to our knowledge) that established a causal connection between early pain and neuronal damage in the brain.12 The strength of our prospective study is that we collected detailed information on the number of procedures for each time window prior to and between the two MRI scans during neonatal hospitalisation, and controlled for multiple clinical factors in our analyses. Our present study is the first, to our knowledge, to show a direct association between early neonatal pain and maturation of neuronal structures in preterm infants. Importantly, pain exposure preceded the imaging changes, thereby suggesting that repeated early procedural pain may be linked with impaired brain development, with a possible primary and early effect on subcortical structures and secondary white matter changes. This discovery may help explain alterations in cortical functioning seen in older preterm children, but it remains for future follow-up studies to show to what extent pain-associated altered brain microstructural maturation can predict cognitive outcome in these children. Our findings challenge interventions for managing procedural pain in preterm infants in the NICU. Current critical care practice guidelines address immediate pain relief in the NICU, however there is a dearth of knowledge as to which pain management strategies protect the brain.
Acknowledgments
We thank the children and their parents who generously participated in this study. This work was funded by grants from the Canadian Institutes for Health Research (CIHR) MOP 79262 to SPM and MOP 86489 to REG. SB holds a Louise and Alan Edwards Foundation Postdoctoral Fellowship and is a member of Pain in Child Health, a strategic training initiative of CIHR. SPM holds a Canada Research Chair (Tier 2) and is a Michael Smith Foundation for Health Research Scholar. REG holds a Senior Scientist award from the Child & Family Research Institute.
Footnotes
Potential Conflicts of Interest
We declare that we have no conflict of interest.
References
- 1.Kostovic I, Judas M. The development of the subplate and thalamocortical connections in the human foetal brain. Acta Paediatr. 2010;99(8):1119–1127. doi: 10.1111/j.1651-2227.2010.01811.x. [DOI] [PubMed] [Google Scholar]
- 2.Anand KJ, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior? Biol Neonate. 2000;77(2):69–82. doi: 10.1159/000014197. [DOI] [PubMed] [Google Scholar]
- 3.Grunau RE, Holsti L, Peters JW. Long-term consequences of pain in human neonates. Semin Fetal Neonatal Med. 2006;11(4):268–275. doi: 10.1016/j.siny.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Slater R, Fabrizi L, Worley A, et al. Premature infants display increased noxious-evoked neuronal activity in the brain compared to healthy age-matched term-born infants. Neuroimage. 2010;52(2):583–589. doi: 10.1016/j.neuroimage.2010.04.253. [DOI] [PubMed] [Google Scholar]
- 5.Bartocci M, Bergqvist LL, Lagercrantz H, Anand KJ. Pain activates cortical areas in the preterm newborn brain. Pain. 2006;122(1–2):109–117. doi: 10.1016/j.pain.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6(7):507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- 7.Walker SM, Franck LS, Fitzgerald M, et al. Long-term impact of neonatal intensive care and surgery on somatosensory perception in children born extremely preterm. Pain. 2009;141(1–2):79–87. doi: 10.1016/j.pain.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Hohmeister J, Kroll A, Wollgarten-Hadamek I, et al. Cerebral processing of pain in school-aged children with neonatal nociceptive input: An exploratory fMRI study. Pain. 2010;150(2):257–267. doi: 10.1016/j.pain.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Slater R, Cornelissen L, Fabrizi L, et al. Oral sucrose as an analgesic drug for procedural pain in newborn infants: A randomised controlled trial. Lancet. 2010;376(9748):1225–1232. doi: 10.1016/S0140-6736(10)61303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozawa M, Kanda K, Hirata M, et al. Influence of repeated painful procedures on prefrontal cortical pain responses in newborns. Acta Paediatr. 2011;100(2):198–203. doi: 10.1111/j.1651-2227.2010.02022.x. [DOI] [PubMed] [Google Scholar]
- 11.Goffaux P, Lafrenaye S, Morin M, et al. Preterm births: Can neonatal pain alter the development of endogenous gating systems? Eur J Pain. 2008;12(7):945–951. doi: 10.1016/j.ejpain.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Anand KJ, Garg S, Rovnaghi CR, et al. Ketamine reduces the cell death following inflammatory pain in newborn rat brain. Pediatr Res. 2007;62(3):283–290. doi: 10.1203/PDR.0b013e3180986d2f. [DOI] [PubMed] [Google Scholar]
- 13.Miller SP, Ferriero DM. From selective vulnerability to connectivity: Insights from newborn brain imaging. Trends Neurosci. 2009;32(9):496–505. doi: 10.1016/j.tins.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grunau RE, Whitfield MF, Petrie-Thomas J, et al. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain. 2009;143(1–2):138–146. doi: 10.1016/j.pain.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124(2):717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- 16.Peterson BS, Vohr B, Staib LH, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284(15):1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 17.Nosarti C, Giouroukou E, Healy E, et al. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain. 2008;131(Pt 1):205–217. doi: 10.1093/brain/awm282. [DOI] [PubMed] [Google Scholar]
- 18.Inder T. Imaging insights of alterations and adaptations in the preterm and late preterm brain. J Pediatr. 2010;156(6):867–868. doi: 10.1016/j.jpeds.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Ment LR, Hirtz D, Huppi PS. Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol. 2009;8(11):1042–1055. doi: 10.1016/S1474-4422(09)70257-1. [DOI] [PubMed] [Google Scholar]
- 20.Chau V, Poskitt KJ, McFadden DE, et al. Effect of chorioamnionitis on brain development and injury in premature newborns. Ann Neurol. 2009;66(2):155–164. doi: 10.1002/ana.21713. [DOI] [PubMed] [Google Scholar]
- 21.Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357(19):1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 22.Mukherjee P, Miller JH, Shimony JS, et al. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR Am J Neuroradiol. 2002;23(9):1445–1456. [PMC free article] [PubMed] [Google Scholar]
- 23.Miller SP, Vigneron DB, Henry RG, et al. Serial quantitative diffusion tensor MRI of the premature brain: Development in newborns with and without injury. J Magn Reson Imaging. 2002;16(6):621–632. doi: 10.1002/jmri.10205. [DOI] [PubMed] [Google Scholar]
- 24.Drobyshevsky A, Song SK, Gamkrelidze G, et al. Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J Neurosci. 2005;25(25):5988–5997. doi: 10.1523/JNEUROSCI.4983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kreis R, Hofmann L, Kuhlmann B, et al. Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 2002;48(6):949–958. doi: 10.1002/mrm.10304. [DOI] [PubMed] [Google Scholar]
- 26.Xu D, Bonifacio SL, Charlton NN, et al. MR spectroscopy of normative premature newborns. J Magn Reson Imaging. 2011;33(2):306–311. doi: 10.1002/jmri.22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138(1):92–100. doi: 10.1067/mpd.2001.109608. [DOI] [PubMed] [Google Scholar]
- 28.Grunau RE, Holsti L, Haley DW, et al. Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain. 2005;113(3):293–300. doi: 10.1016/j.pain.2004.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grunau RE, Oberlander TF, Whitfield MF, et al. Demographic and therapeutic determinants of pain reactivity in very low birth weight neonates at 32 weeks’ postconceptional age. Pediatrics. 2001;107(1):105–112. doi: 10.1542/peds.107.1.105. [DOI] [PubMed] [Google Scholar]
- 30.Grunau RE, Weinberg J, Whitfield MF. Neonatal procedural pain and preterm infant cortisol response to novelty at 8 months. Pediatrics. 2004;114(1):e77–84. doi: 10.1542/peds.114.1.e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holsti L, Grunau RE, Whifield MF, et al. Behavioral responses to pain are heightened after clustered care in preterm infants born between 30 and 32 weeks gestational age. Clin J Pain. 2006;22(9):757–764. doi: 10.1097/01.ajp.0000210921.10912.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volpe JJ, Kinney HC, Jensen FE, Rosenberg PA. The developing oligodendrocyte: Key cellular target in brain injury in the premature infant. Int J Dev Neurosci. 2011;29(4):423–440. doi: 10.1016/j.ijdevneu.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song SK, Sun SW, Ramsbottom MJ, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 34.Adams E, Chau V, Poskitt KJ, et al. Tractography-based quantitation of corticospinal tract development in premature newborns. J Pediatr. 2010;156(6):882–8. 888.e1. doi: 10.1016/j.jpeds.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barkovich AJ, Miller SP, Bartha A, et al. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR Am J Neuroradiol. 2006;27(3):533–547. [PMC free article] [PubMed] [Google Scholar]
- 36.Miller SP, Newton N, Ferriero DM, et al. Predictors of 30-month outcome after perinatal depression: Role of proton MRS and socioeconomic factors. Pediatr Res. 2002;52(1):71–77. doi: 10.1203/00006450-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Smyser CD, Inder TE, Shimony JS, et al. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 2010;20(12):2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doesburg SM, Ribary U, Herdman AT, et al. Altered long-range phase synchronization and cortical activation in children born very preterm. IFMBE Proc. 2010;29(9):250–253. doi: 10.1007/978-3-642-12197-5_57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Angeles DM, Ashwal S, Wycliffe ND, et al. Relationship between opioid therapy, tissue-damaging procedures, and brain metabolites as measured by proton MRS in asphyxiated term neonates. Pediatr Res. 2007;61(5 Pt 1):614–621. doi: 10.1203/pdr.0b013e318045bde9. [DOI] [PubMed] [Google Scholar]
- 40.Murphy BP, Inder TE, Huppi PS, et al. Impaired cerebral cortical gray matter growth after treatment with dexamethasone for neonatal chronic lung disease. Pediatrics. 2001;107(2):217–221. doi: 10.1542/peds.107.2.217. [DOI] [PubMed] [Google Scholar]
- 41.Bellu R, de Waal KA, Zanini R. Opioids for neonates receiving mechanical ventilation. Cochrane Database Syst Rev. 2008;1(1):CD004212. doi: 10.1002/14651858.CD004212.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnston CC, Fernandes AM, Campbell-Yeo M. Pain in neonates is different. Pain. 2011;152(3 Suppl):S65–73. doi: 10.1016/j.pain.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Holsti L, Grunau RE. Considerations for using sucrose to reduce procedural pain in preterm infants. Pediatrics. 2010;125(5):1042–1047. doi: 10.1542/peds.2009-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanna CE, Keith LD, Colasurdo MA, et al. Hypothalamic pituitary adrenal function in the extremely low birth weight infant. J Clin Endocrinol Metab. 1993;76(2):384–387. doi: 10.1210/jcem.76.2.8381799. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez EF, Montman R, Watterberg KL. ACTH and cortisol response to critical illness in term and late preterm newborns. J Perinatol. 2008;28(12):797–802. doi: 10.1038/jp.2008.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grunau RE, Haley DW, Whitfield MF, et al. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J Pediatr. 2007;150(2):151–156. doi: 10.1016/j.jpeds.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]