Abstract
Laccases are copper-containing enzymes involved in the degradation of lignocellulosic materials and used in the treatment of phenol-containing wastewater. In this study we investigated the effect of culture conditions, i.e. submerged or semi-solid, and copper supplementation on laccase production by Trametes pubescens grown on coffee husk, soybean pod husk, or cedar sawdust. The highest specific laccase activity was achieved when the culture was conducted under submerged conditions supplemented with copper (5 mM), and using coffee husk as substrate. The crude extracts presented two laccase isoforms with molecular mass of 120 (Lac1) and 60 kDa (Lac2). Regardless of the substrate, enzymatic crude extract and purified fractions behaved similarly at different temperatures and pHs, most of them presented the maximum activity at 55 °C and a pH range between 2 and 3. In addition, they showed similar stability and electro-chemical properties. At optimal culture conditions laccase activity was 7.69±0.28 U mg-1 of protein for the crude extract, and 0.08±0.001 and 2.86±0.05 U mg-1 of protein for Lac1 and Lac2, respectively. In summary, these results show the potential of coffee husk as an important and economical growth medium to produce laccase, offering a new alternative use for this common agro-industrial byproduct.
Introduction
Lignin, cellulose, and hemicellulose are the major compounds present in plant residues. Among them, cellulose and hemicellulose can be decomposed by a large number of aerobic and anaerobic microorganisms through the action of hydrolytic enzymes [1]. Conversely, lignin biodegradation occurs at a lower rate than plant cell wall polysaccharides [2]. Certain fungi, mostly belonging to Basidiomycetes, such as white- and brown-rot fungi, are known to be able to degrade lignin from lignocellulosic biomass [3]. The extracellular enzymatic system responsible for lignin degradation consists of lignin peroxidase (LiP, E.C. 1.11.1.14), manganese-dependent peroxidase (MnP, E.C. 1.11.1.13), and laccase (para-benzene-diol: oxygen oxidoreductase, EC 1.10.3.2I) [2,3]. While LiP catalyzes the oxidation of non-phenolic aromatic compounds such as veratryl alcohol, and MnP mainly oxidizes phenolic compounds [2], laccase catalyzes the oxidation of phenolic substrates with the concomitant reduction of oxygen to water [4]. However, the number of substrates that laccase can oxidize might be extended by using low molecular mass mediators (i.e. hydroxybenzotriazole, violuric acid, 2,2',6,6'-tetramethylpiperidine-1-oxyl), which are oxidized by laccase to organic radicals intermediates that in turn act as redox mediators [5]. In general, laccases are glycosylated and copper-containing enzymes with a molecular mass between 60 and 80 kDa, and an isoelectric point (pI) between 3.0 to 6.0 [6].
The role of laccases in lignin and phenolic compound degradation has been evaluated in a large number of biotechnological applications such as dye degradation, bioremediation of some toxic chemical wastes (e.g. chlorinated aromatic compounds, polycyclic aromatic hydrocarbons, nitroaromatics, and pesticides), and biosensor developments [3,7]. In addition, laccases have been used in food industry for wine and beer stabilization, fruit juice processing, and different food-related biosensors [8].
Use of industrial and agricultural wastes for laccase production by white-rot fungi is an effective way to reduce the cost of production [9,10]. In addition, laccase-mediated delignification allows to increase the nutritional value of agro-industrial byproducts for animal feed or soil fertilizer. To accomplish the transformation of these agro-industrial wastes, submerged and solid state cultures have been conducted (Table 1). Submerged fermentations are commonly used in industrial process; although they can present drawbacks due to physical space, and energetic and water requirements [11]. On the other hand, solid state cultures present several advantages like mimicking the natural habitat in which the microorganism grows, reduced water activity that reduce microbial contaminations, and limited water consumption and equipment size. Also, it has been reported that solid-state cultures present higher volumetric yield, less energy requirements, and higher end-product stability and concentration than submerged cultures [11,12]. Nevertheless, there are important issues related to heat and mass transfer that must be overcome in order to scale up a solid-state culture, in addition to accrued estimation of the biomass and recovery of the end product [12]. Semi-solid culture has been studied as an alternative to address some of the drawbacks presented by submerged and solid-state cultures. The increment of water activity in this type of culture allows to improve the nutrient and end-product availability, and culture control [13]. It has been established that nature and moisture of the agro-industrial waste used for a solid-state culture are critical factors [12]. The evaluation of these parameters among other process conditions, i.e. culture temperature, pH, and aeration, has encouraged the screening of several agro-industrial wastes for the production of different enzymes. In spite that several studies have been reported for the production of laccase in semi-solid culture using Phanerochaete chrysosporium, few studies are referred to its production in semi-solid culture using Trametes pubescens [14–16].
Table 1. Agro-industrial byproducts used as substrates for laccase production.
| Agro-industrial byproduct | Culture | Organism | Activity (U g-1 or UL-1) | Substrate | Reference |
|---|---|---|---|---|---|
| Sugarcane bagasse | SF | Pleurotus ostreatus | 112±2.8 | ABTS | [56] |
| SF | Phanerochaete chrysosporium | 25.2±1.5 | ABTS | [56] | |
| SF | Pycnoporus cinnabarinus | 2 | ABTS | [57] | |
| SmF | T . versicolor | 410 | ABTS | [58] | |
| Coffee pulp | SF | Ganoderma sp . | 142 | ABTS | [59] |
| SF | Pleurotus ostreatus | 1 | SGZ | [60] | |
| SF | Pleurotus pulmonarius | 1.2 | SGZ | [60] | |
| Wheat bran | SF | Pleurotus sp. IE137 | ~145 | SGZ | [61] |
| SF | Pleurotus pulmonarius CCB19 | 20000 | SGZ | [62] | |
| SmF | Pleurotus ostreatus 1804 | 8033 | ABTS | [63] | |
| SmF | Ganoderma lucidum 447 | 97340±9460 | ABTS | [64] | |
| Banana peels | SF | Ganoderma sp . | 974 | ABTS | [59] |
| SF | Pleurotus florida | 5.4 | ABTS | [65] | |
| SF | Lentinula edodes 122 | 390.31 | ABTS | [66] | |
| SF | Trametes hirsuta BT 2566 | 3550 | ABTS | [67] | |
| Mandarin peels | SmF | Ganoderma lucidum 447 | 3145±370 | ABTS | [64] |
| SF | Pleurotus florida | 3.1 | ABTS | [65] | |
| SmF | Ganoderma lucidum 447 | 35980±3616 | ABTS | [64] | |
| SmF | Trametes pubescens | 100 | ABTS | [68] | |
| Cantaloupe peels | SmF | Trametes pubescens IBB 663 | 1084±92 | ABTS | [67] |
| Soy bran | SF | Pleurotus florida | 4.0 | ABTS | [65] |
| Vinasse + Cotton stalk extract | SmF | Ganoderma lucidum 447 | 93840±9566 | ABTS | [64] |
| Tomato pomace | SmF | Coriolus versicolor ATCC 200801 | 4406 | SGZ | [69] |
| Grape stalks | SmF | Funaliatrogii ATCC 200800 | 4880 | SGZ | [70] |
| Barley straw | SF | Pleurotus ostreatus | 15 | ABTS | [70] |
| Grape seeds | SF | Trametes versicolor | 35 | ABTS | [71] |
| SmF | Trametes versicolor | ~800 | ABTS | [71] | |
| Wheat straw | SmF | Trametes versicolor | ~500 | ABTS | [71] |
| SmF | Trametes versicolor | ~500 | ABTS | [66] | |
| Reed grass | SF | Trametes hirsuta BT 2566 | 22550 | ABTS | [72] |
| Bean stalk | SF | Lentinula edodes 122 | 548.67 | SGZ | [67] |
| Barley bran | SF | Trametes pubescens IBB 663 | 162±20 | ABTS | [72] |
| Barley bran + Cu+2 | SF | Lentinula edodes 122 | 257.69 | SGZ | [66] |
| Apple peels | SF | Trametes hirsuta BT 2566 | 15740 | ABTS | [67] |
| SF | Trametes pubescens IBB 663 | 188±20* | ABTS | [67] | |
| Tree leaves | SmF | Trametes pubescens IBB 663 | 1680±190 | ABTS | [67] |
| SF | Trametes pubescens IBB 663 | 280±23* | ABTS | [67] | |
| Olive mill wastewater | SmF | Trametes pubescens IBB 663 | 834±61 | ABTS | [67] |
| SF | Trametes pubescens IBB 663 | 205±25* | ABTS | [67] | |
| Wood shavings | SmF | Trametes pubescens IBB 663 | 630±54 | ABTS | [73] |
| SmF | Panus tigrinus CBS 577.79 | 4600±98 | 2,6-DMP | [73] | |
| SF | Panus tigrinus CBS 577.79 | 1309±20* | 2,6-DMP | [74] | |
| SmF | Trametes versicolor CBS 100.29 | 451 | ABTS | [75] | |
| Wood shavings + Cu+2 | SF | Ceriporiopsissubvermispora | ~0.5 | N.I. | [75] |
| SF | Ganoderma sp . | ~500 | ABTS | [59] | |
| SF | Trametestrogii MYA 28-11 | 901 | ABTS | [76] |
SF: Solid state fermentation; SmF: Submerged fermentation
*Laccase activity expressed as UL- 1 for these solid state cultures
SGZ: Syringaldazine; 2,6-DMP: 2,6-dimethoxyphenol; N.I.: No indicated
Agriculture is one of the major economic activities in Colombia. Thus, there is a large production of agro-industrial byproducts, and a concern about their disposal. In this study, coffee husk, soybean pod husk, and cedar sawdust were evaluated as growth substrates for laccase production by T . pubescens CBS 696.94 under submerged (SmC) or semisolid cultures (SSC). The effect of Cu+2 supplementation and culture method on laccase production was also evaluated. The different crude extracts were purified, and the obtained laccase fractions were partially characterized. Results showed coffee husk as an alternative for laccase production regardless to the culture mode. This probably owe to its content of caffeine and tannins that might act as laccase inducer. In addition, the dependence of the laccase production yield and the culture mode showed to be substrate dependent.
Materials and Methods
Microorganism and agro-industrial substrates
Laccase was produced by using the white-rot fungus T . pubescens CBS 696.94. Soybean pod and coffee husk were obtained from Corporación Colombiana de Investigación Agropecuaria (CORPOICA) and a local coffee-processing company, respectively. The cedar sawdust was obtained from a local market in Bogota (Colombia). The different byproducts were treated with a 0.5% v/v hypochlorite solution for 5 h. After that, they were rinsed with distilled water until obtaining a neutral pH. Finally, the byproducts were oven dried at 60 °C until constant weight. The soybean pod and coffee husk were crushed to an average particle size of about 0.8 x 0.5 mm, while cedar sawdust was sieved. The portion retained by a No. 100 sieve (opening 0.15 mm) was used for laccase production.
Fungal culture, maintenance, and laccase production
T . pubescens was cultured on malt extract agar (MEA) plates for 10 days at 30 °C. Colonized agar plugs were used for subculture maintenance every 15 days. Independent cultures, using each of the mentioned byproducts, were conducted to produce laccase under SmC or SSC conditions. Each culture was conducted in 1000 mL Erlenmeyer containing 15 g of the desired dry byproduct and 150 or 50 mL of basal medium for SmC or SSC, respectively [basal medium composition per liter: 0.5 g glucose, 2 g KH2PO4, 0.25 g MgSO4·7H2O, 0.9 g (NH4)2SO4, 0.1 g CaCl2 and 0.5 g KCl, in a citrate buffer 20 mM, pH 4.5, supplemented with 0.5 g L-1 thiamine [17]]. Each culture flask was inoculated with three 10-mm plugs of active fungus cultured on MEA, and incubated for 25 days at 30°C without agitation. Culture supernatant samples of 0.5 mL were withdrawn to monitor laccase activity. The effect of Cu+2 on laccase production was evaluated within a 0 to 5 mM concentration range (supplied as copper sulphate). The copper solution was added to 4-days-old cultures. A control was prepared by growing the fungus in the basal medium without any agro-industrial waste under the described culture conditions. All the assays were performed in triplicate.
Laccase purification and characterization
Laccase purification and characterization was conducted using the crude extract that showed the highest enzyme activity for each substrate under the evaluated culture conditions (SmC or SSC). The crude extract was obtained by vacuum filtration through paper Whatman No. 1 followed by centrifugation at 4 °C and 4000 rpm for 15 min, serial vacuum filtration through 0.45 and 0.22 µm polyether sulphone membranes (Pall Corp, Port Washington, NY USA), and ultrafiltration (UF) through a 10 kDa cut-off membrane of regenerated cellulose (Millipore, Billerica, MA, USA). The retentate (~10 mL) was analyzed by low-pressure chromatography with a fraction collector (BioLogicTM, LPC, Biorad, NJ, US) using a Q-Sepharose column (Pharmacia Biotech, 1.0 x 10 cm glass chromatography column filled with 6 mL media) equilibrated with 50 mM sodium acetate buffer pH 5.0. Elution was performed by a linear NaCl gradient (~20 mL) from 0 to 0.5 M. All procedures were carried out at 4 °C. Fractions corresponding to protein peaks were collected and analyzed by sodium dodecyl sulfate-polyacrylamide electrophoresis gel (SDS-PAGE) under non-reducing conditions and without heat denaturation. Staining of the electrophoresis gel was done with 5 mM 2,2’-azino-bis(3-ethylbenzothiazoline-6) sulphonic acid (ABTS) in succinic acid (25 mM, pH 4.5) to detect laccase activity. Fractions that revealed the same protein profile and laccase activity were combined. Samples of the combine fractions were analyzed by SDS-PAGE under mentioned conditions. As a negative control were used a sample of the ultrafiltrated fraction which did not reveal laccase activity along the previous purification step. Bands that revealed laccase activity were identified as Lac1 and Lac2. These bands were excised from the electrophoresis gel and analyzed through nanoLC-MS/MS (Applied Biomics, Hayward, CA, USA). Peptides and protein sequences were aligned by using Clustal Omega [18] at EBI web server, and Blast at NCBI web server. Alignments figures were generated using CLC Sequence Viewer (CLC bio, Denmark).
Enzyme activity was determined spectroscopically by using either 2,2’-azino-bis(3-ethylbenzothiazoline-6) sulphonic acid (ABTS, ε420 = 36mM-1 cm-1, Sigma-Aldrich) or 4-hydroxy-3,5-dimethoxybenzaldehyde (Syringaldazine, ε530 = 65mM-1 cm-1, Sigma-Aldrich) [19–21]. ABTS activity assay was carried out as previously described [21,22]. Briefly, 850 µL of 5 mM ABTS in succinic acid (25 mM, pH 4.5) were mixed with 150 µL of the respective dilution of culture extract and the absorbance was at 420 nm. Syringaldazine activity assay was carried out following the substrate manufacturer’s instructions (Sigma-Aldrich). One unit (U) was defined as the amount of enzyme required to oxidize 1 µmol of substrate (ABTS or syringaldazine) per minute. Specific activity was expressed as U mg−1 of protein as determined by Lowry assay [23].
Laccase production during the fungus culture was monitored using syringaldazine, which has demonstrated to be a specific substrate for detecting extracellular laccase in the absence of hydrogen peroxide [24]. Absence of hydrogen peroxide was confirmed by incubation with catalase. Crude extracts and purified fractions were analyzed by SDS-PAGE without reduction by β-mercaptoethanol and heat denaturation. Proteins were visualized by silver staining, while laccases were identified by placing the SDS-PAGE gel in an ABTS solution for 5 min. Temperature and pH effect on laccase activity was determined for the UF retentate and the purified fractions by using ABTS. The temperature effect on laccase activity was evaluated at 30, 40, 50, 55, 60 and 70 °C at pH 5.0, and the pH effect on laccase activity was evaluated at 2, 2.5, 3, 4, 5, 6, 6.5 and 7 at 55 °C. Laccase stability was evaluated by using ABTS at optimal pH and temperature for 48 h.
Cyclic voltammetry analysis
Cyclic voltammetry (CV) analysis was performed on a computer-controlled potentiostat (Autolab Potentiostat/Galvanostat PGSTAT 128N, Metrohm, USA). Data were acquired by using the software Nova version 1.6. A conventional three-electrode system was used to carry out the cyclic voltammograms. The used electrodes were graphite-working electrode with a surface area of 0.23 cm2, Ag/AgCl KCl saturated reference electrode (Metrohm, USA), and platinum wire counter electrode. Before running each experiment, the surface of the graphite electrode was polished with alumina and thoroughly rinsed with distilled water. CV analysis was done to the crude extract and purified laccase fractions that presented the highest activity for each substrate. For the test, 20 µL of sample were added on the polished surface of the working electrode, and allowed to dry for 60 min at room temperature. Each experiment was performed in triplicate using either a solution 1:1 of anhydride ethanol: buffer acetate (100 mM, pH 5.0) and syringaldazine to a final concentration of 0.2 mM; or a solution of 100 mM acetate buffer (pH 3.0) and 0.5 mM ABTS. Cyclic voltammograms were carried out in 30 mL of working solution with continuous agitation. Voltage was scanned at 100 mV s-1 between -0.2 and 1 V vs. Ag|AgCl|KClsat for syringaldazine assays while for ABTS assays voltage was scanned at 250 mV s-1 between -0.2 and 1.2 V vs. Ag|AgCl|KClsat. Assays were conducted in a Faraday cage at room temperature (20-22°C). Temperature and pH in the reaction solution was monitored with the Unitrode with Pt 1000 (Metrohm, USA).
Statistical analysis
All experiments were conducted by triplicate. Data are presented as the mean ± standard deviation (SD). Statistical comparisons were conducted by using either t-test or Duncan’s test for one-factor design by using StatGraphics Centurion® v. 16.1.11 (2011). Statistical differences were established for p < 0.05.
Results
SmC and SSC production of laccase by T. pubescens
Coffee husk, soybean pod husk, and cedar sawdust were used as substrate for the production of laccase by T . pubescens under SmC or SSC conditions, while syringaldazine was used to monitor laccase production during the fungus culture under the studied conditions. Hydrogen peroxide was not detected in any of the evaluated samples, which discard the interference of peroxidases in the syringaldazine assay. Laccase activity and glucose concentration time course for T . pubescens grown on coffee husk under SmC and SSC at different copper concentrations are presented in Figure 1. Although, a similar time course was observed for laccase activity and glucose concentration of soybean pod husk and cedar sawdust cultures, differences on the day of maximum lacasse activity as well as in the maximum activity value were observed among the agro-industrial byproducts (Table 2). In general, it was noticed that laccase activity was detectable around the seventh and fourth day of culture for SmC and SSC, respectively. After the eighth culture day, glucose was depleted from the culture medium and laccase activity increased sharply.
Figure 1. Laccase activity profiles during T . pubescens CBS 696.94 culture under SmC (a) and SSC (b) on coffee husk.
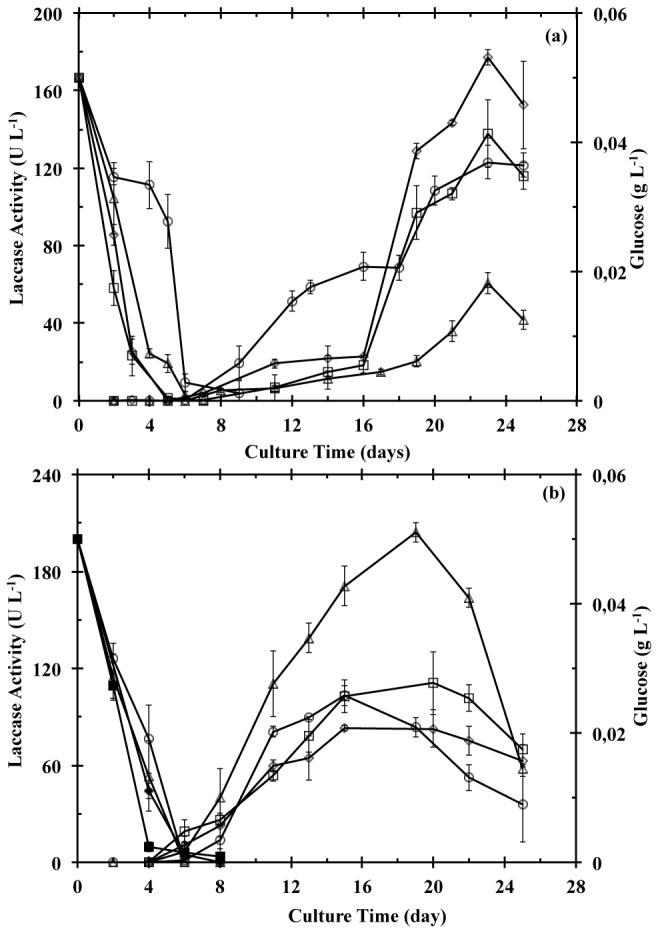
Culture media was enriched with different copper concentrations: 0 (Δ), 0.5 (□), 2 (○) and 5 (◊) mM, and laccase activity was determined by using syringaldazine as substrate. Filled symbols refer to the glucose concentration at the respective copper concentration. All the assays were performed in triplicate.
Table 2. Effect of substrate, copper concentration and culture mode on laccase activity (UL-1).
Syringaldazine was used for monitoring laccase activity.
| Substrate | SmC | SSC |
|---|---|---|
| Soybean Pod Husk + 0 mM Cu+2 | 4.46 ± 1.38 (23)** | 12.55 ± 0.52 (13)** |
| Soybean Pod Husk + 0.5 mM Cu+2 | 84.93 ± 8.46 (23)† | 20.28 ± 3.87 (21) |
| Soybean Pod Husk + 2 mM Cu+2 | 57.61 ± 3.70 (19) | 137.85 ± 0.71 (18)† |
| Soybean Pod Husk + 5 mM Cu+2 | 44.92 ± 4.87 (23) | 29.00 ± 4.07 (18) |
| Coffee Husk + 0 mM Cu+2 | 61.84 ± 1.76 (23)** | 204.00 ± 5.96 (19)**,† |
| Coffee Husk + 0.5 mM Cu+2 | 137.63 ± 5.68 (23) | 110.83 ± 9.40 (20)a,b |
| Coffee Husk + 2 mM Cu+2 | 123.04 ± 3.73 (23)b | 102.78 ± 8.14 (15)b |
| Coffee Husk + 5 mM Cu+2 | 177.23 ± 3.72 (23)† | 82.66 ± 8.82 (15) |
| Cedar Sawdust + 0 mM Cu+2 | 13.55 ± 0.95 (17)* | 10.82 ± 0.53 (15)* |
| Cedar Sawdust + 0.5 mM Cu+2 | 22.72 ± 0.84 (17) | 19.02 ± 0.02 (13) |
| Cedar Sawdust + 2 mM Cu+2 | 28.97 ± 1.27 (12)a | 26.38 ± 0.68 (8) |
| Cedar Sawdust + 5 mM Cu+2 | 29.36 ± 1.53 (13) a,b,† | 30.73 ± 1.52 (12)b,† |
Data in parenthesis refers to the day where was observed the maximum laccase activity
* No Statistical Difference, ** Statistical Difference. (Evaluation of substrate effect on laccase activity for both culture modes, submerged (SmC) and semisolid (SSC), no copper induction considered)
Equal letters inside one agro-industrial waste group refers to No Statistical Difference; no letters refers to Statistical Difference inside the group. (Evaluation of copper effect on laccase activity for both SmC and SSC)
† Maximum laccase activity for the used agro-industrial wastes either in SmC or SSC culture.
The substrate source seemed to be the most important factor for laccase production (Table 2). T . pubescens culture on coffee husk allowed the maximum laccase activities for both SmC (177.23±3.72 U L-1) and SSC (204.00±5.96 U L-1) at copper concentrations of 5 and 0 µM, respectively. Laccase activity in SSC and SmC using coffee husk as substrate was between 1.47- and 6.63-fold higher than that observed for cultures with soybean pod husk and cedar sawdust. Laccase activity obtained in the control culture, without agro-industrial waste, presented a maximum laccase activity of 2.22 ± 0.88 U L-1 after 14 days of culture, while the glucose was depleted on the seventh day of culture.
Copper supplementation effect on laccase activity was evaluated for both SmC and SSC. Although in a non-linear mode, the laccase activity was increased by the presence of copper with all substrates under SmC or SSC except with coffee husks under SSC (Table 2). In addition, the required copper concentration to obtain the highest laccase activity varied depending on the used agro-industrial waste. For example, laccase production was favored in cultures with soybean pod husk at copper concentrations of 0.5 mM and 2.0 mM for SmC and SSC, respectively, while for cultures with coffee husk the highest laccase activity levels for SmC and SSC was observed at copper concentrations of 5 mM and 0 mM, respectively.
Despite that culture mode, i.e. SmC or SSC, presented an effect on laccase production, this is substrate sensitive. In the assays conducted without copper, it was observed that laccase activity was 2.8- and 3.3-fold higher in SSC than in SmC for the soybean pod husk and coffee husk, respectively. However, when cedar sawdust was used, no significant difference in laccase activity between the two culture types was obtained (Table 2). Due to the low volume of liquid media and high laccase activity in SSC when compared to SmC, the SSC presented a higher volumetric yield. For all agro-industrial waste, the SSC presented a shorter time than SmC to reach the highest laccase activity, which suggest a higher productivity under SSC conditions.
These results show that coffee husk under SmC or SSC conditions could be considered as a potential alternative for laccase production by T . pubescens . However, soybean pod husk and cedar sawdust could be also considered as important alternatives for laccase production, especially in comparison with synthetic culture media [10]. Finally, the optimal copper concentration to obtain the highest laccase activity depends on the agro-industrial byproduct used for the fungus culture.
Laccase purification and characterization
Laccase purification was conducted using the crude extracts that presented the highest activities. For the SmC, crude extracts obtained with soybean pod husk + Cu+2 0.5 mM, coffee husk + Cu+2 5 mM, and cedar sawdust + Cu+2 5 mM were selected; while for SSC crude extracts obtained with soybean pod husk + Cu+2 2 mM, coffee husk + Cu+2 0 mM, and cedar sawdust + Cu+2 5 mM were selected. The electrophoretic analysis of permeates from 0.45 and 0.22 µm filtrations and the UF retentate showed the presence of two proteins with laccase activity, while no activity was observed within the ultrafiltrated sample (Figure 2a). These proteins presented a molecular mass of about 120 kDa and 60 kDa (Figure 2a). The purification of the UF retentate by ionic exchange chromatography confirmed the presence of two different fractions with laccase activity (Figure 2b). The SDS-PAGE analysis of these fractions, under non-reducing conditions, showed that one protein with laccase activity was presented on each fraction (Figure 2b), and were named Lac1 (120 kDa) and Lac2 (60 kDa). Considering that the conditions used to measure the enzyme activity were those suggested for laccase activity, and that H2O2 or manganese were not added during the reaction for measuring the enzyme activity, these results suggest that both proteins are laccases but not peroxidases.
Figure 2. Purification of lacasses produced by submerged culture of T . pubescens using coffee husk as substrate.
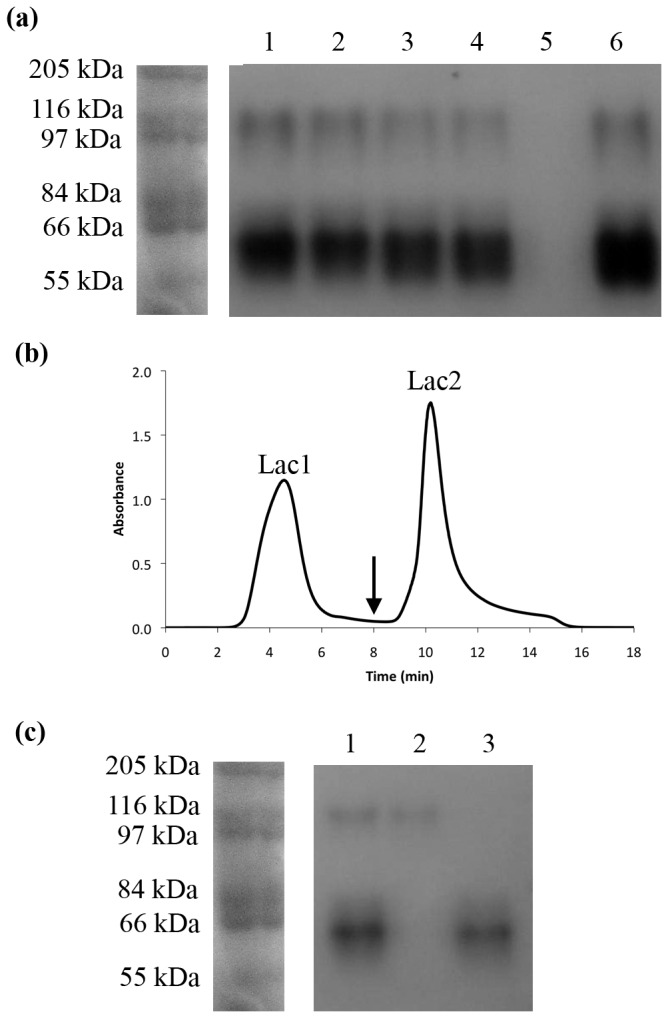
All samples were analyzed by SDS-PAGE under non-reducing conditions and stained with ABTS. No heat denaturation of the samples was conducted. (a) The crude extract (1) was centrifuged and filtered through a Whatman No. 1 filter (2), 0.45 µM membrane (3) 0.22 µm membrane (4), and ultrafiltrated through a 10 kDa cut-off membrane, permeate (5) and retentate (6). (b) UF retentate was purified by anionic exchange chromatography. Lac1 represent the unbound fraction, while Lac2 represent the eluted fraction. The arrow shows the point when protein elution was started. (c) Purified fractions (1) crude extract, (2) Lac1 and (3) Lac2. MW: Molecular Weight. Similar results were observed for crude extracts produced by using soybean pod husk and cedar sawdust.
NanoLC-MS/MS analysis of purified fractions showed that the identified peptides for Lac1 (SAGSTTYNYNDPIFR and RDTVSTGNSGDNVTIR) and Lac2 (SAGSTVYNYDNPIFR and ANPNFGNVGFTGGINSAI), had identity scores between 80 to 100% with laccases (over 100 sequences) available at the non-redundant GenBank dataset. Proteins containing these peptides, different that laccases, were not identified. Similar results were observed when peptides were evaluated at Uniprot. Galhaup et al. [17] described two laccase active fractions from T . pubescens MB 89 (CBS 696.94): Lap1 (UniProtKB/TrEMBL Q8TG93) and Lap2 (UniProtKB/TrEMBL Q8TG94), which share a 70% identity (Figure 3a). Sequence alignment of Lac1 and Lac2 peptides with Lap1 and Lap2 sequences, respectively, showed that the peptides are presented within T . pubescens MB 89 laccase sequences, though some differences were observed for the Lac1 peptide (Figure 3b). This result suggests that the purified fractions are laccase isoenzymes.
Figure 3. Aligment of reported lacasses sequences and Lac1 and Lac2 peptides.
(a) Alignment of Lap1 (UniProtKB/Swiss-Prot Q8TG93) and Lap2 (UniProtKB/Swiss-Prot Q8TG94) from T . pubescens CBS 696.94. (b) Alignment of Lac1 and Lac2 peptides with Lap1 and Lap2, respectively, and tryptic peptides described by Shleev et al 2007. Alignments were carried out using Clustal Omega and figures were using CLC Sequence Viewer. Different residues are colored in red.
Protein concentration and volumetric and specific laccase activities at different purification stages are presented in Table 3. Lac1 presented lower laccase activity than Lac2 in a ratio that ranges from 0.80- to 38-fold depending on the substrate source. On the other hand, laccase activity of the UF retentate from soybean pod husk culture was from 4.5- to 6.4-fold higher than that of the purified fractions, while for cedar sawdust and coffee husk the laccase activity was from 4.6- to 12.0-fold and from 3.3- to 163-fold higher than the purified fractions, respectively (Table 3). The purified Lac2 fractions from coffee husk and cedar sawdust cultures showed the highest specific activity levels (Table 3). The volumetric activities of UF retentates were higher than that obtained with purified fractions (Table 4).
Table 3. Laccase activity during the purification of crude extracts resulted from T . pubescens culture on agro-industrial wastes under submerged (SmC) and semisolid (SSC) cultures.
|
Soybean pod husk
|
Coffee husk
|
Cedar sawdust
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein (mg mL-1) | Volumetric Activity (UL-1) | Specific Activity (U mg-1 protein) | Recovery (%) | Protein (mg mL-1) | Volumetric Activity (UL-1) | Specific Activity (U mg-1 protein) | Recovery (%) | Protein (mg mL-1) | Volumetric Activity (UL-1) | Specific Activity (U mg-1 protein) | Recover (%) | |
| SmC | ||||||||||||
| Crude Extract | 0.869 | 528.88 | 0.608 | 100 | 0.414 | 1527.08 | 3.688 | 100 | 0.431 | 107.35 | 0.249 | 100 |
| 0.45 µm Filtration | 0.736 | 465.49 | 0.632 | 88 | 0.334 | 1178.92 | 3.529 | 77 | 0.444 | 120.53 | 0.271 | 112 |
| UF retentate | 1.010 | 787.31 | 0.779 | 74 | 0.378 | 1952.63 | 5.165 | 64 | 0.510 | 259.05 | 0.507 | 120 |
| Lac1 | 0.494 | 54.71 | 0.110 | 1 | 0.232 | 23.99 | 0.103 | 0.2 | 0.222 | 8.38 | 0.037 | 0.8 |
| Lac2 | 0.310 | 206.61 | 0.666 | 4 | 0.116 | 1072.40 | 9.252 | 7 | 0.040 | 152.34 | 3.837 | 14 |
| SSC | ||||||||||||
| Crude Extract | 1.146 | 1649.66 | 1.439 | 100 | 0.596 | 1549.07 | 2.601 | 100 | 1.065 | 232.03 | 0.217 | 100 |
| 0.45 µm Filtration | 0.776 | 1283.92 | 1.654 | 77 | 0.474 | 1157.36 | 2.439 | 75 | 0.806 | 258.24 | 0.320 | 111 |
| UF retentate | 0.973 | 3350.55 | 3.443 | 101 | 0.428 | 1617.50 | 3.779 | 52 | 1.134 | 511.01 | 0.450 | 110 |
| Lac1 | 0.097 | 88.54 | 0.912 | 2 | 0.104 | 15.14 | 0.145 | 0.3 | 0.042 | 13.15 | 0.313 | 2 |
| Lac2 | 0.168 | 489.88 | 2.915 | 10 | 0.131 | 822.98 | 6.282 | 18 | 0.072 | 161.62 | 2.244 | 23 |
740 ABTS was used to determine laccase activity.
Table 4. Optimum temperatures and pHs for maximum laccase activities in UF retentate, Lac1 and Lac2 obtained from semisolid cultured T . pubescens on agro-industrial wastes.
| Enzymatic Fraction |
Temperature
|
pH
|
||
|---|---|---|---|---|
| Volumetric Activity (UL-1) | Specific Activity (U mg-1 protein) | Volumetric Activity (UL-1) | Specific Activity (U mg-1 protein) | |
| Soybean Pod Husk + 2 mM Cu+2 | 1480.00±11.78 (55) | 1.521±0.012 | 1820.37±30.25 (2-3) | 1.871±0.031 |
| Lac1 | 229.25±9.17 (60) | 2.363±0.095 | 361.35±8.31 (2,3) | 3.725±0.085 |
| Lac2 | 326.38±15.58 (55) | 1.942±0.092 | 287.13±2.74 (2,3) | 1.709±0.016 |
| Coffee Husk + 0 mM Cu+2 | 1137.40±82.89 (50-55) | 3.008±0.219 | 2906.66±10.39 (2-3) | 7.689±0.275 |
| Lac1 | 8.89±0.39 (55) | 0.038±0.002 | 17.79±0.28 (2,3) | 0.077±0.001 |
| Lac2 | 341.94±27.89 (60) | 2.947±0.240 | 331.50±5.26 (2–4) | 2.857±0.045 |
| Cedar Sawdust + 5 mM Cu+2 | 2576.66±12.01 (55) | 5.052±0.024 | 3446.34±104.3 (2-3) | 6.757±0.204 |
| Lac1 | 213.70±4.72 (55) | 0.962±0.021 | 291.17±9.43 (2,3) | 1.311±0.042 |
| Lac2 | 551.66±16.56 (60) | 13.79±0.414 | 551.66±16.56 (5) | 13.79±0.414 |
ABTS was used to determine laccase activity.
Data in parenthesis shows the temperature or pH at which relative laccase activity reached 100%.
Temperature and pH effect on laccase activity was determined for the UF retentate, and Lac1 and Lac2 fractions obtained along the purification of the enzymatic crude extracts that resulted after the SSC of T . pubescens on the selected agro-industrial wastes. Laccase activity for the UF retentate under different temperature and pH conditions is presented in Figure 4 and Table 4. The maximum relative activity of the evaluated UF retentates was observed at 50-55 °C, and at a pH range from 2.0 to 4.0. However, UF retentate from cedar sawdust culture behaved different to the UF retentate from coffee husk and soybean pod husk cultures. In this sense, the UF retentate from cedar sawdust culture presented the lowest enzyme activity at temperatures ≤ 40 °C, but at 70 °C presented the highest enzyme activity, ~90% of its initial value, in comparison to the other two UF retentates (Figure 4).
Figure 4. Effect of temperature (a) and pH (b) on laccase activity for the UF retentate obtained by semisolid culture of T . pubescens on coffee husk (□), soybean pod husk (◊), and cedar sawdust (Δ).
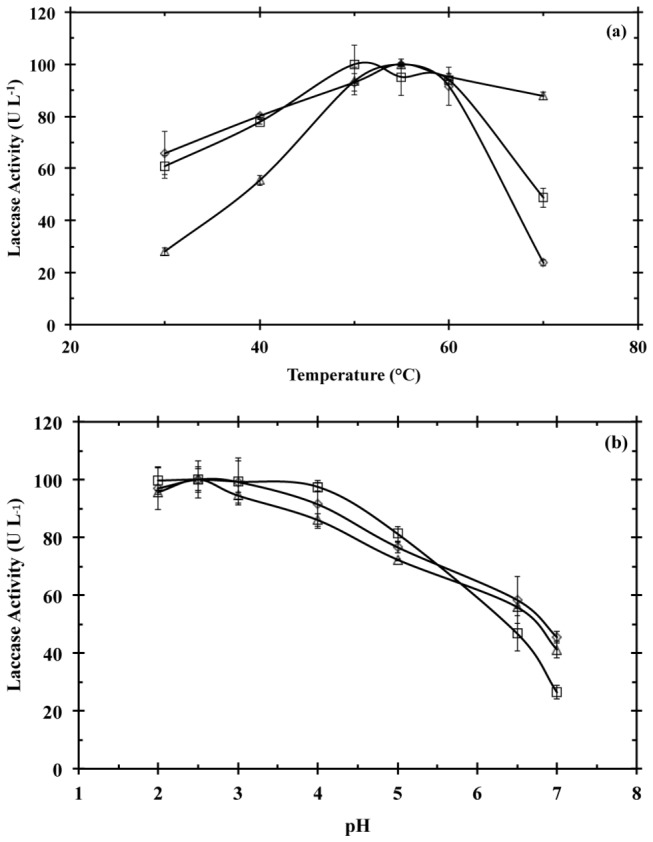
Laccase stability was evaluated by using ABTS. All the assays were performed in triplicate.
The temperature and pH effect on laccase activity for Lac1 and Lac2 is presented in Figure 5. The maximum relative activity for both fractions was presented at a pH from 2.0 to 4.0, regardless of the agro-industrial waste, as observed for the UF retentate. However, at pH > 4.0 Lac1 showed a sharper decrease in its activity than that observed for Lac2. Nevertheless, Lac2 purified from cedar sawdust culture presented a different performance. This fraction showed the maximum activity at pH 5.0, while a sharp drop was observed at pHs above or below this value.
Figure 5. Effect of temperature (■) and pH (□) on enzyme activity for Lac1 (solid line) and Lac2 (dashed line), obtained after the purification of the enzymatic crude extract produced by semisolid culture of T . pubescens on coffee husk (a), soybean pod husk (b) and cedar sawdust (c).
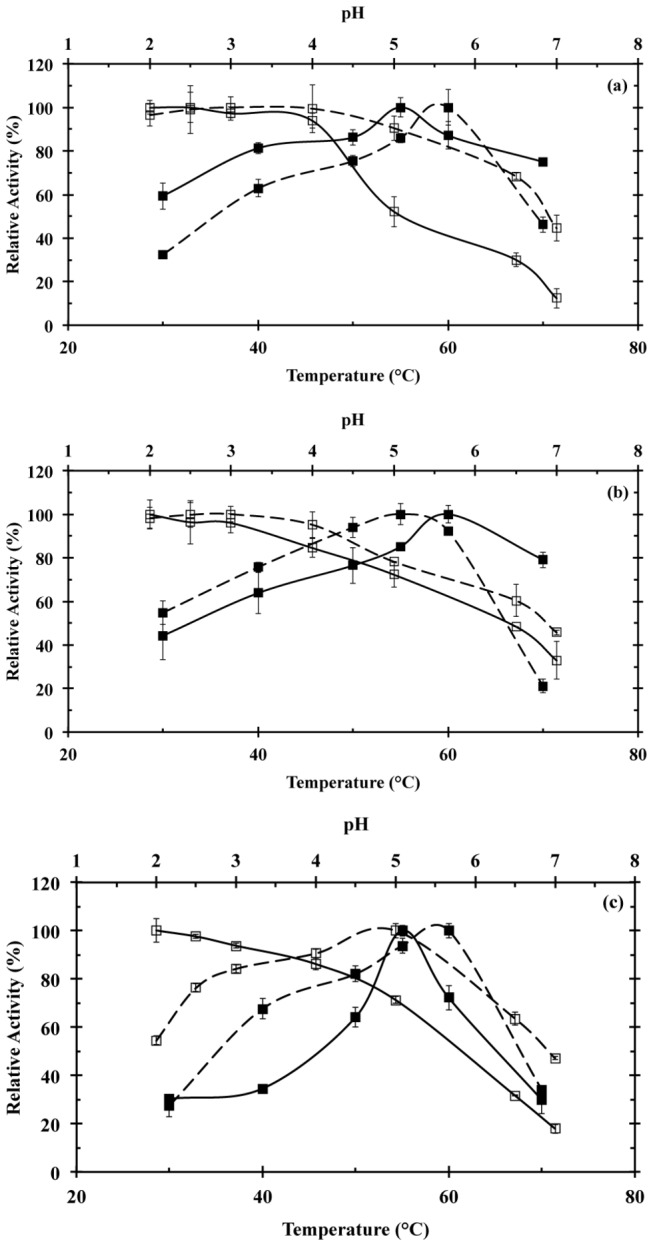
Laccase stability was evaluated by using ABTS. All the assays were performed in triplicate.
Laccase activity profiles showed that Lac1 and Lac2 presented the maximum enzyme activity at 55 and 60 °C, respectively (Table 4). It was observed that Lac1 from coffee husk culture presented a lower decrease in enzyme activity at temperatures ≤ 50 °C or ≥ 60 °C than Lac2. On the contrary, Lac2 from soybean husk or cedar sawdust cultures presented a higher relative laccase activity than Lac1 at temperatures ≤ 55 °C. The purified fractions from soybean pod husk and cedar sawdust cultures presented the lowest relative activity at 30 °C and 65 °C.
Laccase stability
For selected enzymatic extracts and purified fractions (Table 4) a stability test for 48 h at their optimal pH and temperature was conducted. Time course of the relative laccase activity for crude extract and purified fractions from coffee husk and cedar sawdust cultures are presented in Figure 5. Similar results were observed for crude extract and purified fractions from soybean pod husk culture (data no shown).
It was observed that the activity loss depends on the substrate source used to grow T . pubescens . An activity loss of 55% was observed for crude extracts and purified fractions from coffee husk and soybean pod husk cultures, while an activity loss of 70% was observed for the crude extract and purified fractions from cedar sawdust culture. Nevertheless, Lac2 showed the lowest activity reduction, with values of 10, 40 and 55% for coffee husk, soybean pod husk, and cedar sawdust, respectively, while Lac1 presented an activity loss of 34, 50 and 60% for coffee husk, soybean pod husk, and cedar sawdust, respectively. Despite the differences in laccase activity among agro-industrial wastes and between crude extracts and purified fractions, all the profiles showed a sharp decrease during the first 8 h while a reduced activity loss was observed during the last 24 h of the assay (Figure 6).
Figure 6. Laccase stability profiles at optimal pH and temperature conditions for crude extracts (solid line), Lac1 (dashed line), and Lac2 (spotted line) obtained from semisolid culture of T . pubescens on coffee husk (◊) and cedar sawdust (•).
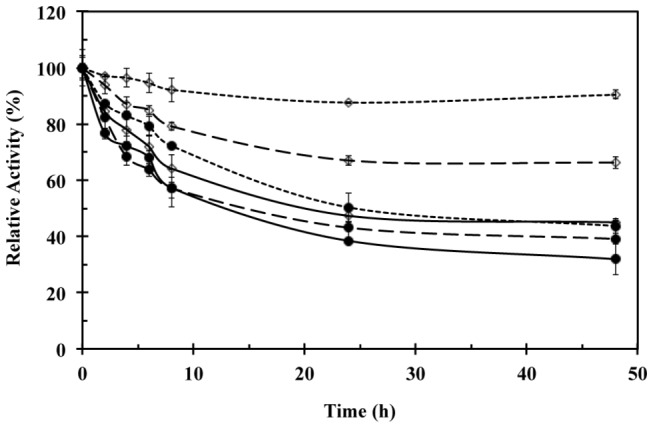
Laccase stability was evaluated by using ABTS. All the assays were performed in triplicate.
Laccase electrochemical characterization
For selected crude extracts and purified fractions a cyclic voltammetry characterization was conducted. For each mediator, syringaldazine or ABTS, similar cyclic voltammograms were obtained for the different crude extracts and purified fractions. Voltammograms of the redox reaction for syringaldazine (Figure 7a) and ABTS (Figure 6b) are presented. Two anionic and cationic peaks were observed when syringaldazine was used as mediator. The first pair of peaks showed a reversible performance, with a mean Ia: Ic ratio of 1.09±0.06, 1.06±0.05, and 0.98±0.02, for the crude extracts, Lac1, and Lac2, respectively. The second pair of peaks showed quasi-reversible performance, and the cathodic peak was presented at a potential of +0.62 V (vs Ag|AgCl), while the anodic peak was presented at a potential of +0.60 V (vs Ag|AgCl). No significant difference on the reduction potential (Eo) for syringaldazine was observed for the crude extracts or the purified fractions, obtaining an Eo, of 0.314±0.008 V (vs Ag|AgCl). However, the peak potential separation (ΔEp) showed a different profile depending on the substrate used for the culture. While not significant difference (p > 0.05) on ΔEp value was observed between crude extract (0.045±0.005 V vs Ag|AgCl), Lac1 (0.046±0.005 V vs Ag|AgCl), and Lac2 (0.028±0.001 V vs Ag|AgCl), from cultures with cedar sawdust and soybean pod husk; the ΔEp was considerably higher for the crude extract (0.083±0.004 V vs Ag|AgCl), Lac1 (0.068±0.003 V vs Ag|AgCl) and Lac2 (0.051±0.005 V vs Ag|AgCl) from the coffee husk culture.
Figure 7. Cyclic voltammograms of (a) syringaldazine at pH 5.0 using the crude extract and purified laccase fractions from semisolid culture of T . pubescens on coffee husk, or (b) ABTS at pH 3.0 using the crude extract and purified laccase fractions from semisolid culture of T . pubescens on soybean pod husk.
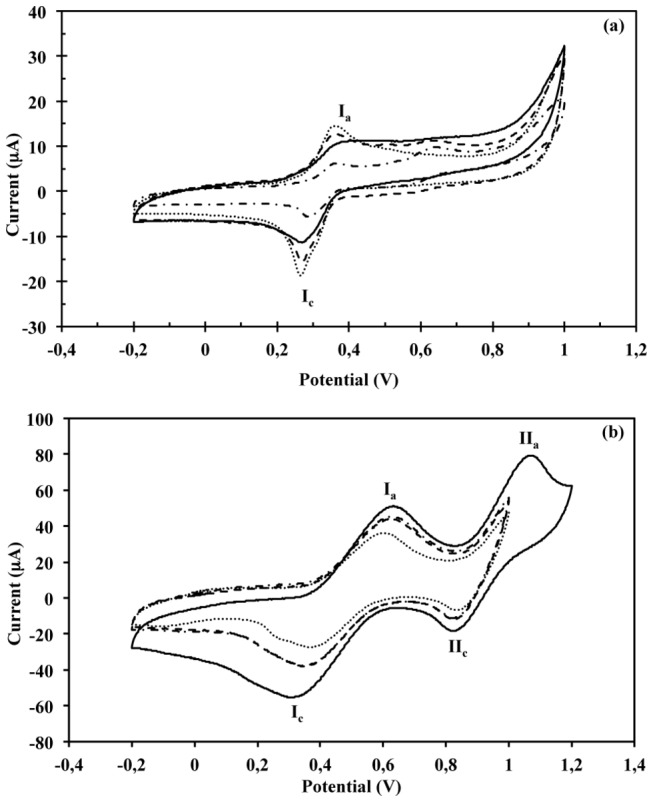
Nomenclature: only mediator (dotted line), crude extract (solid line), Lac1 fraction (dashed line), and Lac2 fraction (dashed and dotted line).
For reactions conducted with ABTS and the different crude extracts and purified fractions, the voltammograms exhibited one pair of cathodic and anodic peaks (Figure 7b), which corresponds to the redox of ABTS/ABTS+ (Ia/Ic) with an Eo, of 0.487±0.006 V (vs Ag|AgCl). This process was reversible presenting a mean Ia: Ic ratio of 0.99±0.02. In addition, a second cathodic peak was presented at +0.83 V (vs Ag|AgCl) when ABTS redox reaction was conducted using the crude extract and the purified fractions. This performance agrees with previous reports in which the laccase activity cannot be observed at potential values of the second reversible oxidation of ABTS, ABTS+/ABTS2+, due to this catalytic process cannot be detected by cyclic voltammetry [25,26]. Nevertheless, voltammograms for laccase crude extract from a soybean pod husk culture presented the second anodic peak, which in addition to the second cathodic peak corresponds to the redox of ABTS+/ABTS2+ (IIa/IIc) with an Eo, of 0.942±0.004 V (vs Ag|AgCl). Contrary to the reactions conducted using syringaldazine as substrate, the ΔEp did not present a significant difference for the crude extracts and purified fractions, 0.248±0.016 V (vs Ag|AgCl).
Discussion
In this study, we evaluated the effect of the culture mode, i.e SmC and SSC, on laccase production by T . pubescens CBS 696.94 using three different agro-industrial wastes (coffee husk, soybean pod husk, and cedar sawdust). Furthermore, the effect of Cu+2 supplementation on laccase production was also evaluated, and the crude extracts and the purified fractions were partially characterized.
Substrate source for laccase production showed to be one of the most important factors for laccase production (Table 2). Cultures with coffee husk as substrate presented the highest laccase production levels. The observed differences in laccase activity for each substrate could be owed to their chemical composition. Coffea arabica is the coffee variety raised in Colombia, and its husk has a reported composition of 23.08% cellulose (20.76% glucose and 1.83% cellobiose), 23.85% hemicellulose (13.56% xylose, 5.23% arabinose, 2.56% acetic acid and 1.95% glucuronic acid); 28.28% total lignin, and 0.71% ashes [27]. In addition, coffee husk contents 1.3% caffeine, 4.5% tannins, and 12.4% pectins [28]. On the other hand, Glycine max is the soybean species cultured in Colombia, and its pod husk composition varies from 5.04 to 6.2% crude protein, 6.08 to 14.3% hemicellulose, 37.15 to 42.08% acid detergent fiber, 44.36 to 60.15% neutral detergent fiber, 8.50 to 8.93% acid detergent lignin, and 20.74 to 21.06% uronic acid [29–31]. Finally, for cedar sawdust the major components are glucose (42.6-44.4%), xylose (4.6-19.5%), cellulose (38.3-41.0%), klason lignin (22.7-27.8%), and acid lignin (4.0-4.3%) [32–34]. Although, lignin is one of the most important compounds associated with laccase production by white-rot fungi, we did not observe a clear correlation between lignin composition percentage and laccase activity. In that sense, the high laccase activity observed for the cultures that use coffee husk could be associated with its content of caffeine and tannins, which have been identified as potent inducers of laccase gene expression [17,35,36]. In fact, caffeine has been used as inducer for laccase production by Rhizoctonia solani, obtaining an increase of 4.1-fold in laccase activity [37]. Similar results were also reported for the production of Coloriopsis gallica laccase [38].
Laccase gene expression is mainly up regulated by Cu2+, although Mn2+, Fe3+, heavy metals, 2,6-dimethoxy-1,4-benzoquinone, H2O2, amphotericin B, syringic acid, tannic acid, Tween 80, soybean oil, aromatic compounds, and microclimatic changes (i.e. lower temperature and osmotic pressure) are also recognized as potent inducers [17,35,36]. In general, Cu2+ supplementation increased the laccase activity in both SmC and SSC. However, Cu2+concentration required to obtain the highest laccase activity varied depending on agro-industrial waste. These differences can be attributed to the copper presented in the agro-industrial waste and the copper availability in the medium. Copper can be found in amounts that range from 15.6 to 18.0 mg kg-1 of coffee husk [39], and from 2.6 to 6.7 mg kg-1 of soybean pod husk (or soybean hull) [39,40]. Despite that no reports indicate the copper content in untreated cedar wood, an average of 1.0 mg kg-1 of wood has been reported for wood used as biomass in six different Europe countries [41]. This allows to consider the cedar sawdust as the substrate with the lowest content of copper. On the other hand, copper adsorption by sawdust and coffee husk has been reported, obtaining a Langmuir based maximum adsorption capacity (Qmax, mg g-1 adsorbent) of 8.45 and 7.5, respectively [42,43]. Although no adsorption studies has been reported using untreated soybean pod husk, Šćiban et al. [44] reported a Qmax for copper adsorption of 0.085 mmol g-1 adsorbent (~ 5.4 mg g-1) using treated soybean straws. Based on this information, it can be expected that a higher amount of exogenous copper would be required for laccase induction on cultures with cedar sawdust than with soybean pod husk and coffee husk due to its lower content of copper. In addition, due to the high copper-adsorption capacity of sawdust, the amount of available copper in the culture would be reduced allowing the laccase production at higher copper concentrations without reaching levels that might be toxic for the fungus. This fact can be observed in the SSC (Table 4). However, for the SmC this behavior is not maintained for the cultures with soybean pod husk and coffee husk (Table 4) where the maximum laccase activity was obtained with a copper concentration of 0.5 and 5 mM, respectively. This could be owed to a lower contact between the fungus and the substrate in the SmC when compared to the SSC which is close related to the transport of nutrients presented in the substrates.
Production of extracellular laccases has been reported in a large number of species of white-rot fungi strains grown on natural substrates (Table 1). Although a laccase activity profile is not clearly observed based on the agro-industrial waste, culture mode, and fungus strain, most cases in which SmC and SSC were conducted using the same substrate and fungus strain, showed a higher laccase activity under SmC conditions. In this study was observed a substrate dependent performance, where the most favorable condition to obtain the highest laccase activity was the SSC. The laccase activities obtained in the present study are comparable, and in some cases higher than those reported for Pleurotus ostreatus, Phanerochaete chrysosporium, Pycnoporus cinnabarinus , T . versicolor and Ganoderma sp. , cultured under SC and SmC conditions, and using different agro-industrial wastes (Table 1). Semi-solid cultures using different agro-industrial wastes have been reported using T . versicolor , Phlebia radiata , T . pubescens , P. ostreatus, and C . unicolor . Rodriguez et al. [16] screened different supports for laccase production by T . versicolor under SSC. They obtained the highest laccase activity (1200 U L-1) when in the culture medium was presented barley bran. In addition, they were able to obtain a laccase activity of 1700 U L-1 when the culture was supplemented with xylidine as an inducer. These results were obtained after 17 or 18 days of culture, and ABTS was used to determine the enzyme activity. Mäkelä et al. [45,46] have studied the expression of the genes involved in laccase production, Pr-lac1 and Pr-lac2, presented in P . radiata , and evaluate laccase production in SSC using milled alder wood ( Alnus incana ) as substrate. They reported a maximum laccase activity of 3 µkat L-1 (~180 U L-1) at the 14th day of culture and using syringaldazine for its detection. Osma et al. [15] have studied the morphological changes and laccase production of strains T . pubescens , P. ostreatus, C . unicolor and T . versicolor grown on wheat bran flakes using SSC. They obtained a maximum laccase production between 1397 and 2778 U L-1 after 10 and 13 days of culture. This enzyme activity is strain dependent, and ABTS was used for its quantification. Our results show a good correlation with reported data. The highest laccase activity was obtained when T . pubescens was grown on coffee husk under SSC without copper (204 U L-1 evaluated with syringaldazine or 1549 U L-1 evaluated with ABTS), which is in some cases higher than reported ones. This shows that under the studied conditions, the production of the laccase crude extract is favored in SSC using coffee husk without the addition of copper, which could favors the cost of the production process.
It has been reported for white rot-fungi the production of multiple laccase isoenzymes, which are encoded by different genes and show different expression profiles, stabilities, substrate affinities, and molecular masses (ranging from 35 to 140 kDa) [36,47–49]. Two isoforms have been identified in T . pubescens strains; two monomeric (Lac1 and Lac2) enzymes of 67 kDa but with different pI (5.3 and 5.1) [50], and two laccase-activity fractions (Lap1 and Lap2) with different pI (> 3.0 and 2.6) from which Lap2 is a monomeric enzyme of 65 kDa [17]. In this study, we identify two laccase isoenzymes from T . pubescens CBS 696.94 that have different molecular masses (120 and 60 kDa) and pI. The purifications results suggest a more acidic pI for Lac1 (≤ 4) than Lac2 (≥ 6), which differs from a previous report for this fungus, in which two fractions with laccase activity were obtained, Lap1 and Lap2, with pI > 3 and of 2.6, respectively [17]. Since in both cases, similar purification protocols were used, and Lac1 and Lac2 were recovered from the unbound and bound fractions, respectively, as Lap1 and Lap2 [17], this difference could be due to the culture conditions used in each case (i.e. the presence of agro-industrial wastes). Nevertheless, the nanoLC-MS/MS analysis showed that peptides of Lac1 and Lac2 were presented in Lap1 (UniProtKB/Swiss-Prot Q8TG93) and Lap2 (UniProtKB/Swiss-Prot Q8TG94), which have a predicted pI of 4.3 and 4.7, respectively. Lac1 and Lac2 peptides were presented in Laccase-2 (UniProtKB/Swiss-Prot Q99046) and Laccase-1 (UniProtKB/Swiss-Prot Q99044) from T . villosa , respectively, with 80 to 100% identity. These T . villosa laccase isoenzymes have a predicted pI of 4.7 (laccase-1) and 6.0 (laccase-2), and a 77% identity among them. In addition, T . villosa laccase-2 is reported as a homodimer protein, while laccase-1 is reported as monomeric protein. Alignment of Lac1-Lap2 and Lac2-Lap1 from T . pubescens CBS 696.94 and T . villosa , showed a 96 and 70% identity, respectively. Finally, similar peptides were identified for Lac1 and Lac2 from T . pubescens (Schumach.) Pilát (Syn.: Coriolus pubescens (Schum. ex Fr.) Quél.) BCB 923-2 (Figure 3b) [51]. These results suggest that the purified fractions correspond to laccase isoenzymes that differ from previously reported ones for this T . pubescens strain.
As mentioned above, laccases isoenzymes present different stabilities and substrate affinities. This behavior was also observed for the laccase isoenzymes purified from T . pubescens , with Lac1 showing lower laccase activity than Lac2, with ABTS as substrate. However, the difference in activity between these isoenzymes depended on the agro-industrial waste used for the T . pubescens growth, suggesting a probable effect of the substrate composition on the laccase physicochemical properties. In addition, it was observed that the total laccase activity, presented in the UF retentates, is higher than the laccase activity of the purified fractions, regardless of the agro-industrial byproduct used for the T . pubescens growth. This suggests a synergistic effect of Lac1 and Lac2, as have been reported for laccases obtained from Trametes and Pleurotus strains [17,52,53].
The maximum relative activity for the UF retentate was presented at a pH from 2.0 to 4.0, regardless of the agro-industrial byproduct, with a monotonical decrease at pHs above this range. The relation between pH and type of electron donor molecule explains this behavior on laccase activity. It has been reported that when the substrate is an organic hydrogen donor, i.e. phenols, the optimal pH ranges from 3.5 to 6.0 while the laccase activity monotonically decreases from pH 2.5 to 7.0, if the substrate is an electron donor like ABTS [54]. Despite that at different pHs, most of the purified laccase fractions presented a similar performance as the UF retentates, the Lac2 fraction obtained from cedar sawdust differed from the previous observation. For this fraction, the maximum activity was observed at pH 5.0, and despite that at acidic pH (≥ 3.0) the relative activity was higher than 80%, at lower pHs (2.0) it decreased almost in a 50%. Nevertheless, at basic pHs the relative laccase activity presented a sharp decreased that is well correlate with the other laccase fractions. We are not able to attribute this particular trend to the presence of multiple laccase isoenzymes in the fraction since the SDS-PAGE revealed only one band when stained with ABTS, and from the NanoLC-MS/MS analysis no difference in the number of peptides and their sequence was detected. We speculate that this particular behavior can be owed to a compound added in the pre-treatment of the cedar wood that might be used for preserving it that might affect the activity of Lac2 and that was not completely removed during the purification.
Temperature stability evaluation showed that Lac1 is the most thermolabile fraction while the laccase activity reduction in the crude extracts did not seem to be the result of an additive effect of the activity loss of the purified fractions. Although there are no reports of laccase stability under the selected conditions, these results agree with previous reports that state that fungal laccases rapidly decrease their activity at temperatures near 60 °C, and thermal stability correlates with the temperature range of the growth of the source organism [55]. In addition, we previously reported for a laccase crude extract obtained from culturing T . pubescens on coffee husk an activity loss of 26% and 10% at 60 and 50 °C, respectively, after 8 h at pH 6.0 using syringaldazine as substrate [48]
Finally, an electrochemical characterization was carried out for selected crude extracts and purified fractions. The results showed a difference between the electrochemical performances of extracts and purified fractions with syringaldazine or ABTS. These differences could be owed to the different mechanisms for electron transfer, as discussed above. However, the overall performance of the obtained cyclic voltammograms for ABTS and syringaldazine agrees with results reported by Fernández-Sánchez et al. [25], who used a saturated calomel reference electrode.
Conclusions
We have shown the potential of coffee husk, soybean pod husk, and cedar sawdust as substrates for the laccase production. In several countries, like Brazil and Colombia, the production of coffee and soybean is of great importance and the generated volume of residues is high. The results showed that semi-solid culture of T . pubescens using coffee husk allows to obtain an important amount of laccase, which could be favored by the phenolic-compounds, i.e. tannins, presented in coffee husk. While supplementation of the culture medium with copper favored the production of laccase in submerged and semi-solid culture using soybean pod husk and cedar sawdust, semi-solid culture using coffee husk, which presented the highest laccase volumetric yield, did not require copper supplementation. This is an economic advantage that should be considered when scaling up the process. In this study, T . pubescens showed its ability to produce two independent laccases regardless of the used substrate. However, a higher laccase activity was observed for crude extracts rather than for purified fractions. This suggests that Lac1 and Lac2 might have a synergistic effect on the final laccase activity rather than an additive effect. Based on this study, the investigation of laccase production by evaluating other agro-industrial wastes, in which phenolic-compounds constitute an important percentage of their composition, is of great interest to avoid the use of inducers that would increase the production cost.
Funding Statement
This work was supported in part by Pontificia Universidad Javeriana (Grant ID 3400) and the Chemical Engineering Department at Universidad de Los Andes. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Vandamme EJ (2009) Agro-industrial residue utilization for industrial biotechnology products. In: Nigam P, Pandey A. Biotechnology for Agro-Industrial Residues Utilisation. Netherlands: Springer Verlag; pp. 3-11. [Google Scholar]
- 2. Martínez AT, Speranza M, Ruiz-Dueñas FJ, Ferreira P, Camarero S et al. (2005) Biodegradation of lignocellulosics: microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int Microbiol 8: 195-204. PubMed: 16200498. [PubMed] [Google Scholar]
- 3. Sánchez O, Sierra R, Alméciga-Díaz CJ (2011) Delignification process of agro-industrial aastes an alternative to obtain fermentable carbohydrates for producing fuel. In: Manzanera M. Alternative Fuel. Intech: 111-154. [Google Scholar]
- 4. Wesenberg D, Kyriakides I, Agathos SN (2003) White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol Adv 22: 161-187. doi:10.1016/j.biotechadv.2003.08.011. PubMed: 14623049. [DOI] [PubMed] [Google Scholar]
- 5. Morozova OV, Shumakovich GP, Shleev SV, Yaropolov YI (2007) Laccase-mediator systems and their applications: A review. Appl Biochem Microbiol 43: 523-535. doi:10.1134/S0003683807050055. [PubMed] [Google Scholar]
- 6. Baldrian P (2006) Fungal laccases - occurrence and properties. FEMS Microbiol Rev 30: 215-242. doi:10.1111/j.1574-4976.2005.00010.x. PubMed: 16472305. [DOI] [PubMed] [Google Scholar]
- 7. Rodríguez Couto S, Herrera JLT (2006) Industrial and biotechnological applications of laccases: A review. Biotechnol Adv 24: 500-513. doi:10.1016/j.biotechadv.2006.04.003. PubMed: 16716556. [DOI] [PubMed] [Google Scholar]
- 8. Osma JF, Toca-Herrera JL, Rodríguez-Couto S (2010) Uses of laccases in the food industry. Enzyme Res, 2010: 2010: 918761. PubMed: 21048873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodriguez S (2008) Exploitation of biological wastes for the production of value-added products under solid-state fermentation conditions. Biotechnol J 3: 859-870. doi:10.1002/biot.200800031. PubMed: 18543242. [DOI] [PubMed] [Google Scholar]
- 10. Osma JF, Toca-Herrera JL, Rodríguez-Couto S (2011) Cost analysis in laccase production. J Environ Manage 92: 2907-2912. doi:10.1016/j.jenvman.2011.06.052. PubMed: 21775046. [DOI] [PubMed] [Google Scholar]
- 11. Jain A, Morlok CK, Henson JM (2013) Comparison of solid-state and submerged-state fermentation for the bioprocessing of switchgrass to ethanol and acetate by Clostridium phytofermentans . Appl Microbiol Biotechnol 97: 905-917. doi:10.1007/s00253-012-4511-4. PubMed: 23111595. [DOI] [PubMed] [Google Scholar]
- 12. Singhania RR, Patel AK, Soccol CR, Pandey A (2009) Recent advances in solid-state fermentation. Biochem Eng J 44: 13-18. doi:10.1016/j.bej.2008.10.019. [Google Scholar]
- 13. Economou CN, Makri A, Aggelis G, Pavlou S, Vayenas DV (2010) Semi-solid state fermentation of sweet sorghum for the biotechnological production of single cell oil. Bioresour Technol 101: 1385-1388. doi:10.1016/j.biortech.2009.09.028. PubMed: 19781936. [DOI] [PubMed] [Google Scholar]
- 14. Rodríguez CS, Santoro R, Cameselle C, Sanroman A (1997) Laccase production in semi-solid cultures of Phanerochaete chrysosporium . Biotechnol Lett 19: 995-998. doi:10.1023/A:1018495216946. [Google Scholar]
- 15. Osma JF, Moilanen U, Toca-Herrera JL, Rodríguez-Couto S (2011) Morphology and laccase production of white-rot fungi grown on wheat bran flakes under semi-solid-state fermentation conditions. FEMS Microbiol Lett 318: 27-34. doi:10.1111/j.1574-6968.2011.02234.x. PubMed: 21291496. [DOI] [PubMed] [Google Scholar]
- 16. Couto SR, Gundin M, Lorenzo M, Sanroman MN (2002) Screening of supports and inducers for laccase production by Trametes versicolor in semi-solid-state conditions. Proc Biochem 38: 249-255. doi:10.1016/S0032-9592(02)00087-0. [Google Scholar]
- 17. Galhaup C, Goller S, Peterbauer CK, Strauss J, Haltrich D (2002) Characterization of the major laccase isoenzyme from Trametes pubescens and regulation of its synthesis by metal ions. Microbiology 148: 2159-2169. PubMed: 12101303. [DOI] [PubMed] [Google Scholar]
- 18. Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K et al. (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7: 539 PubMed: 21988835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Childs RE, Bardsley WG (1975) The steady-state kinetics of peroxidase with 2,2'-azino-di-(3-ethyl-benzthiazoline-6-sulphonic acid) as chromogen. Biochem J 145: 93-103. PubMed: 1191252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harkin JM, Obst JR (1973) Syringaldazine, an Effective Reagent for Detecting Laccase and Peroxidase in Fungi. Experientia 29: 382-387. [Google Scholar]
- 21. Nikupaavola ML, Raaska L, Itavaara M (1990) Detection of white-rot fungi by a non-roxic stain. Mycol Res 94: 27-31. doi:10.1016/S0953-7562(09)81260-4. [Google Scholar]
- 22. Gonzalez LF, Sarria V, Sanchez OF (2010) Degradation of chlorophenols by sequential biological-advanced oxidative process using Trametes pubescens and TiO (2)/UV. Bioresour Technol 101: 3493-3499 [DOI] [PubMed]
- 23. Waterborg JH (2009) The Lowry method for protein quantitation. In: Walker J. The Protein Protocols Handbook. Humana Press; pp. 7-10. [Google Scholar]
- 24. Harkin JM, Obst JR (1973) Syringaldazine, an effective reagent for detecting laccase and peroxidase in fungi. Cell Mol Life Sci 29: 381-387. doi:10.1007/BF01926734. [Google Scholar]
- 25. Fernández-Sánchez C, Tzanov T, Gübitz GM, Cavaco-Paulo A (2002) Voltammetric monitoring of laccase-catalysed mediated reactions. Bioelectrochemistry 58: 149-156. doi:10.1016/S1567-5394(02)00119-6. PubMed: 12414320. [DOI] [PubMed] [Google Scholar]
- 26. Bourbonnais R, Leech D, Paice MG (1998) Electrochemical analysis of the interactions of laccase mediators with lignin model compounds. Biochim Biophys Acta 1379: 381–390. doi:10.1016/S0304-4165(97)00117-7. PubMed: 9545600. [DOI] [PubMed] [Google Scholar]
- 27. Ferraz F, Silva S (2009) Characterization of coffee husk biomass for biotechnological purposes. N Biotechnol 25: S256-S256. doi:10.1016/j.nbt.2009.06.573. [Google Scholar]
- 28. Pandey A, Soccol CR, Nigam P, Brand D, Mohan R et al. (2000) Biotechnological potential of coffee pulp and coffee husk for bioprocesses. Biochem Eng J 6: 153-162. doi:10.1016/S1369-703X(00)00084-X. PubMed: 10959086. [DOI] [PubMed] [Google Scholar]
- 29. Sruamsiri S, Silman P (2008) Nutritive composition of soybean by-products and nutrient digestibility of soybean pod husk. Maejo Int J Sci Technol 2: 568-576. [Google Scholar]
- 30. Romkaew J, Nagaya Y, Goto M, Suzuki J, Umezaki T (2008) Pod dehiscence in relation to chemical components of pod shell in soybean. Plant Prod Sci 11: 278-282. doi:10.1626/pps.11.278. [Google Scholar]
- 31. Nakamanee G, Polbumroong T, Phaikaew C (2003) Alternative use of crop waste to increase milk production. Proceedings of the 8th Meeting of the Regional Working Group on Grazing and Feed Resources for Southeast Asia Kuala Lumpur, Malaysia: 66 - 70. [Google Scholar]
- 32. Kurek B, Gaudard F (2000) Oxidation of spruce wood sawdust by MnO(2) plus oxalate: a biochemical investigation. J Agric Food Chem 48: 3058-3062. doi:10.1021/jf000015g. PubMed: 10898665. [DOI] [PubMed] [Google Scholar]
- 33. Weil J, Brewer M, Hendrickson R, Sarikaya A, Ladisc MR (1998) Continuous pH monitoring during pretreatment of yellow poplar wood sawdust by pressure cooking in water. Appl Biochem Biotechnol 70-72: 99-111. doi:10.1007/BF02920127. [Google Scholar]
- 34. Weil J, Sarikaya A, Rau S-L, Goetz J, Ladisch CM et al. (1997) Pretreatment of yellow poplar sawdust by pressure cooking in water. Appl Biochem Biotechnol 68: 21-40. doi:10.1007/BF02785978. [Google Scholar]
- 35. Dekker RF, Barbosa AM, Giese EC, Godoy SD, Covizzi LG (2007) Influence of nutrients on enhancing laccase production by Botryosphaeria rhodina MAMB-05. Int Microbiol 10: 177-185. PubMed: 18075999. [PubMed] [Google Scholar]
- 36. Xiao YZ, Hong YZ, Li JF, Hang J, Tong PG et al. (2006) Cloning of novel laccase isozyme genes from Trametes sp. AH28-2 and analyses of their differential expression. Appl Microbiol Biotechnol 71: 493-501. doi:10.1007/s00253-005-0188-2. PubMed: 16283298. [DOI] [PubMed] [Google Scholar]
- 37. Crowe JD, Olsson S (2001) Induction of laccase activity in Rhizoctonia solani by antagonistic Pseudomonas fluorescens strains and a range of chemical treatments. Appl Environ Microbiol 67: 2088-2094. doi:10.1128/AEM.67.5.2088-2094.2001. PubMed: 11319086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yagüe S, Terrón MC, González T, Zapico E, Bocchini P et al. (2000) Biotreatment of tannin-rich beer-factory wastewater with white-rot basidiomycete Coriolopsis gallica monitored by pyrolysis/gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom 14: 905-910. doi:10.1002/(SICI)1097-0231(20000530)14:10. PubMed: 10825255. [DOI] [PubMed] [Google Scholar]
- 39. Gowda NKS, Ramana JV, Prasad CS, Singh K (2004) Micronutrient content of certain tropical conventional and unconventional feed resources of southern India. Trop Anim Heatlth Pro 36: 77-94. doi:10.1023/B:TROP.0000009522.30949.1d. PubMed: 14979561. [DOI] [PubMed] [Google Scholar]
- 40. DePeters EJ, Fadel JG, Arana MJ, Ohanesian N, Etchebarne MA et al. (2000) Variability in the chemical composition of seventeen selected by-product feedstuffs used by the California dairy industry. Prof Anim Sci 16: 69-99. [Google Scholar]
- 41. Obernberger I, Thek G (2004) Physical characterisation and chemical composition of densified biomass fuels with regard to their combustion behaviour. Biomass Bioenerg 27: 653-669. doi:10.1016/j.biombioe.2003.07.006. [Google Scholar]
- 42. Larous S, Meniai A-H, Lehocine MB (2005) Experimental study of the removal of copper from aqueous solutions by adsorption using sawdust. Desalination 185: 483–490. doi:10.1016/j.desal.2005.03.090. [Google Scholar]
- 43. Oliveira WE, Franca AS, Oliveira LS, Rocha SD (2008) Untreated coffee husks as biosorbents for the removal of heavy metals from aqueous solutions. J Hazard Mater 152: 1073–1081. doi:10.1016/j.jhazmat.2007.07.085. PubMed: 17804159. [DOI] [PubMed] [Google Scholar]
- 44. Šciban M, Klašnja M, Škrbic B (2008) Adsorption of copper ions from water by modified agricultural by-products. Desalination 229: 170–180. doi:10.1016/j.desal.2007.08.017. [Google Scholar]
- 45. Mäkelä MR, Hildén KS, Hakala TK, Hatakka A, Lundell TK (2006) Expression and molecular properties of a new laccase of the white rot fungus Phlebia radiata grown on wood. Curr Genet 50: 323-333. doi:10.1007/s00294-006-0090-1. PubMed: 16927090. [DOI] [PubMed] [Google Scholar]
- 46. Mäkelä MR, Lundell T, Hatakka A, Hildén K (2013) Effect of copper, nutrient nitrogen, and wood-supplement on the production of lignin-modifying enzymes by the white-rot fungus Phlebia radiata . Fungal Biol 117: 62-70. doi:10.1016/j.funbio.2012.11.006. PubMed: 23332834. [DOI] [PubMed] [Google Scholar]
- 47. Dwivedi UN, Singha P, Pandeya VP, Kumara A (2011) Structure–function relationship among bacterial, fungal and plant laccases. J Mol Catal B Enzym 68: 117-128. doi:10.1016/j.molcatb.2010.11.002. [Google Scholar]
- 48. Gaitan IJ, Medina SC, González JC, Rodríguez A, Espejo ÁJ et al. (2011) Evaluation of toxicity and degradation of a chlorophenol mixture by the laccase produced by Trametes pubescens . Bioresour Technol 102: 3632–3635. doi:10.1016/j.biortech.2010.11.040. PubMed: 21115244. [DOI] [PubMed] [Google Scholar]
- 49. Karp SG, Faraco V, Amore A, Birolo L, Giangrande C et al. (2012) Characterization of laccase isoforms produced by Pleurotus ostreatus in solid state fermentation of sugarcane bagasse. Bioresour Technol 114: 735-739. doi:10.1016/j.biortech.2012.03.058. PubMed: 22487128. [DOI] [PubMed] [Google Scholar]
- 50. Nikitina OV, Shleev SV, Gorshina ES, Rusinova TV, Serezhenkov VA et al. (2005) Isolation and purification of enzymes from ligninolytic complex of the basidial fungus Trametes pubescens (Schumach.) Pilat and study of their properties. Biochemistry (Mosc) 70: 1274-1279. doi:10.1007/s10541-005-0259-0. [DOI] [PubMed] [Google Scholar]
- 51. Shleev S, Nikitina O, Christenson A, Reimann CT, Yaropolov AI et al. (2007) Characterization of two new multiforms of Trametes pubescens laccase. Bioorg Chem 35: 35-49. doi:10.1016/j.bioorg.2006.08.001. PubMed: 16989887. [DOI] [PubMed] [Google Scholar]
- 52. Xiao YZ, Chen Q, Hang J, Shi YY, Wu J et al. (2004) Selective induction, purification and characterization of a laccase isozyme from the basidiomycete Trametes sp. AH28-2. Mycologia 96: 26-35. doi:10.2307/3761984. PubMed: 21148825. [PubMed] [Google Scholar]
- 53. Muñoz C, Guillén F, Martínez AT, Martínez MJ (1997) Laccase isoenzymes of Pleurotus eryngii: characterization, catalytic properties, and participation in activation of molecular oxygen and Mn2+ oxidation. Appl Environ Microbiol 63: 2166-2174. PubMed: 9172335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Morozova OV, Shumakovich GP, Gorbacheva MA, Shleev SV, Yaropolov AI (2007) "Blue" laccases. Biochemistry (Mosc) 72: 1136-1150. doi:10.1134/S0006297907100112. [DOI] [PubMed] [Google Scholar]
- 55. Hildén K, Hakala TK, Lundell T (2009) Thermotolerant and thermostable laccases. Biotechnol Lett 31: 1117-1128. doi:10.1007/s10529-009-9998-0. PubMed: 19360388. [DOI] [PubMed] [Google Scholar]
- 56. Verma P, Madamwar D (2002) Production of ligninolytic enzymes for dye decolorization by cocultivation of white-rot fungi Pleurotus ostreatus and Phanerochaete chrysosporium under solid-state fermentation. Appl Biochem Biotechnol 102-103: 109-118. doi:10.1385/ABAB:102-103:1-6:109. PubMed: 12396115. [DOI] [PubMed] [Google Scholar]
- 57. Meza JC, Lomascolo A, Casalot L, Sigoillot J-C, Auria R (2005) Laccase production by Pycnoporus cinnabarinus grown on sugar-cane bagasse: Influence of ethanol vapours as inducer. Proc Biochem 40: 3365–3371. doi:10.1016/j.procbio.2005.03.004. [Google Scholar]
- 58. Hossain SM, Anantharaman N (2006) Activity enhancement of ligninolytic enzymes of Trametes versicolor with bagasse powder. Afr J Biotechnol 5: 189-194. [Google Scholar]
- 59. Revankar MS, Desai KM, Lele SS (2007) Solid-state fermentation for enhanced production of laccase using indigenously isolated Ganoderma sp . Appl Biochem Biotechnol 143: 16-26. doi:10.1007/s12010-007-0029-0. PubMed: 18025593. [DOI] [PubMed] [Google Scholar]
- 60. Velazquez-Cedeno MA, Mata G, Savoie J-M (2002) Waste-reducing cultivation of Pleurotus ostreatus and Pleurotus pulmonarius on coffee pulp: changes in the production of some lignocellulolytic enzymes. World J Microbiol Biotechnol 18: 201–207. doi:10.1023/A:1014999616381. [Google Scholar]
- 61. Mata G, Murrieta D, Andreu LI (2005) Changes in lignocellulolytic enzyme activities in six Pleurotus spp. strains cultivated on coffee pulp in confrontation with Trichoderma spp. World J Microbiol Biotechnol 21: 143–150. doi:10.1007/s11274-004-3041-3. [Google Scholar]
- 62. de Souza DF, Tychanowicz GK, de Souza CG, Peralta RM (2006) Co-production of ligninolytic enzymes by Pleurotus pulmonarius on wheat bran solid state cultures. J Basic Microbiol 46: 126-134. doi:10.1002/jobm.200510014. PubMed: 16598826. [DOI] [PubMed] [Google Scholar]
- 63. Prasad KK, Mohan SV, Bhaskar YV, Ramanaiah SV, Babu VL et al. (2005) Laccase production using Pleurotus ostreatus 1804 immobilized on PUF cubes in batch and packed bed reactors: influence of culture conditions. J Microbiol 43: 301-307. PubMed: 15995650. [PubMed] [Google Scholar]
- 64. Songulashvili G, Elisashvili V, Wasser SP, Nevo E, Hadar Y (2007) Basidiomycetes laccase and manganese peroxidase activity in submerged fermentation of food industry wastes. Enzyme Microb Technol 41: 57–61. doi:10.1016/j.enzmictec.2006.11.024. [Google Scholar]
- 65. Sathishkumar P, Murugesan K, Palvannan T (2010) Production of laccase from Pleurotus florida using agro-wastes and efficient decolorization of Reactive blue 198. J Basic Microbiol 50: 360-367. doi:10.1002/jobm.200900407. PubMed: 20586068. [DOI] [PubMed] [Google Scholar]
- 66. Moldes D, Gallego PP, Rodríguez Couto S, Sanromán A (2003) Grape seeds: the best lignocellulosic waste to produce laccase by solid state cultures of Trametes hirsuta . Biotechnol Lett 25: 491-495. doi:10.1023/A:1022660230653. PubMed: 12882277. [DOI] [PubMed] [Google Scholar]
- 67. Elisashvili V, Kachlishvili E, Penninckx M (2008) Effect of growth substrate, method of fermentation, and nitrogen source on lignocellulose-degrading enzymes production by white-rot basidiomycetes. J Ind Microbiol Biotechnol 35: 1531-1538. doi:10.1007/s10295-008-0454-2. PubMed: 18716810. [DOI] [PubMed] [Google Scholar]
- 68. Osma JF, Saravia V, Herrera JL, Couto SR (2007) Mandarin peelings: the best carbon source to produce laccase by static cultures of Trametes pubescens . Chemosphere 67: 1677-1680. doi:10.1016/j.chemosphere.2006.11.051. PubMed: 17234250. [DOI] [PubMed] [Google Scholar]
- 69. Kahraman SS, Gurdal IH (2002) Effect of synthetic and natural culture media on laccase production by white rot fungi. Bioresour Technol 82: 215-217. doi:10.1016/S0960-8524(01)00193-6. PubMed: 11991068. [DOI] [PubMed] [Google Scholar]
- 70. Iandolo D, Piscitelli A, Sannia G, Faraco V (2011) Enzyme Production by Solid Substrate Fermentation of Pleurotus ostreatus and Trametes versicolor on Tomato Pomace. Appl Biochem Biotechnol 163: 40-51. doi:10.1007/s12010-010-9014-0. PubMed: 20582639. [DOI] [PubMed] [Google Scholar]
- 71. Moldes D, Lorenzo M, Sanromán MA (2004) Different proportions of laccase isoenzymes produced by submerged cultures of Trametes versicolor grown on lignocellulosic wastes. Biotechnol Lett 26: 327-330. doi:10.1023/B:BILE.0000015452.40213.bf. PubMed: 15055770. [DOI] [PubMed] [Google Scholar]
- 72. Philippoussis A, Diamantopoulou P, Papadopoulou K, Lakhtar H, Roussos S et al. (2010) Biomass, laccase and endoglucanase production by Lentinula edodes during solid state fermentation of reed grass, bean stalks and wheat straw residues. World J Microbiol Biotechnol 27: 285-297. [Google Scholar]
- 73. Fenice M, Giovannozzi Sermanni G, Federici F, D’Annibale A (2003) Submerged and solid-state production of laccase and Mn-peroxidase by Panus tigrinus on olive mill wastewater-based media. J Biotechnol 100: 77-85. doi:10.1016/S0168-1656(02)00241-9. PubMed: 12413788. [DOI] [PubMed] [Google Scholar]
- 74. Rodriguez-Couto S, GundIn M, Lorenzo M, Sanromán MÁ (2002) Screening of supports and inducers for laccase production by Trametes versicolor in semi-solid-state conditions. Proc Biochem 38: 249-255. doi:10.1016/S0032-9592(02)00087-0. [Google Scholar]
- 75. Fackler K, Gradinger C, Schmutzer M, Tavzes C, Burgert I et al. (2007) Biotechnological wood modification with selective white-rot fungi and its molecular mechanisms. Food Technol Biotechnol 45: 269–276. [Google Scholar]
- 76. Levin L, Herrmann C, Papinutti VL (2008) Optimization of lignocellulolytic enzyme production by the white-rot fungus Trametes trogii in solid-state fermentation using response surface methodology. Biochem Eng J 39: 207-214. doi:10.1016/j.bej.2007.09.004. [Google Scholar]



