Abstract
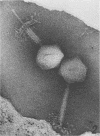
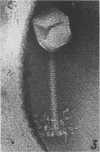
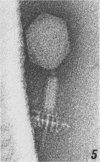
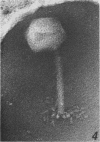
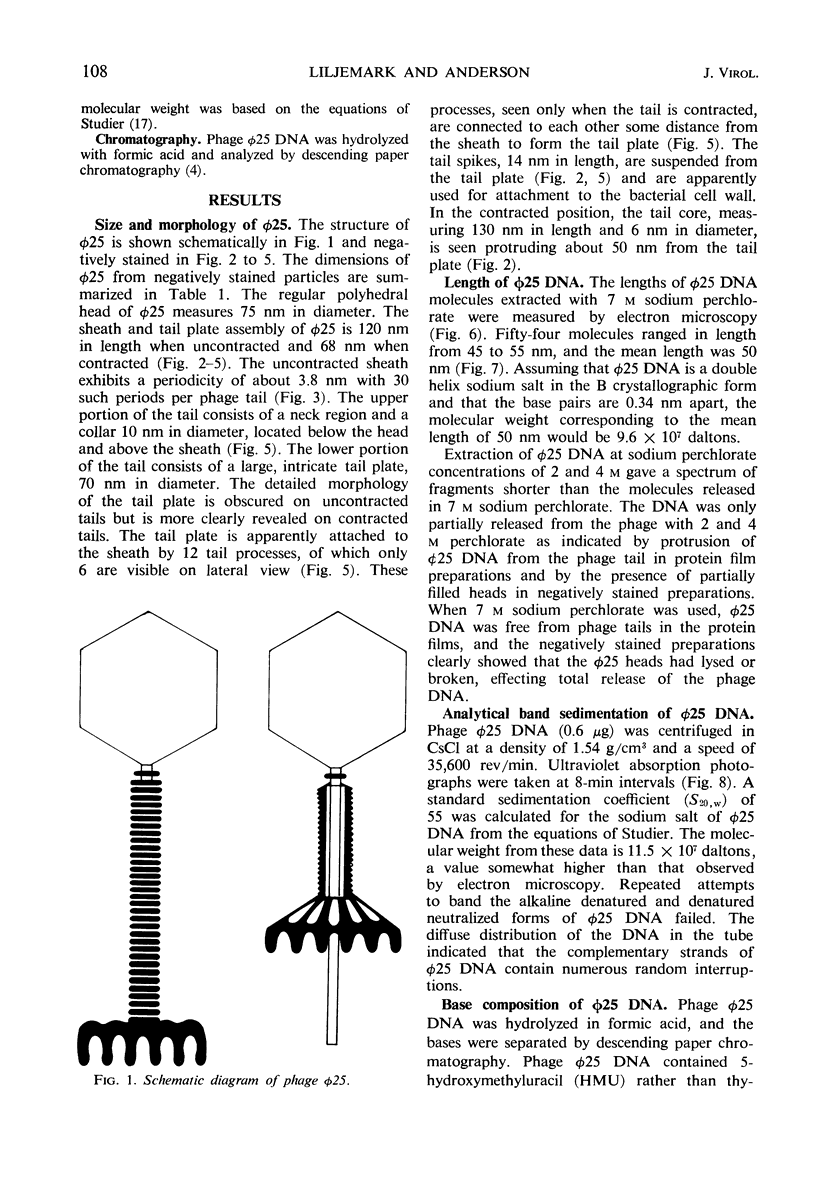
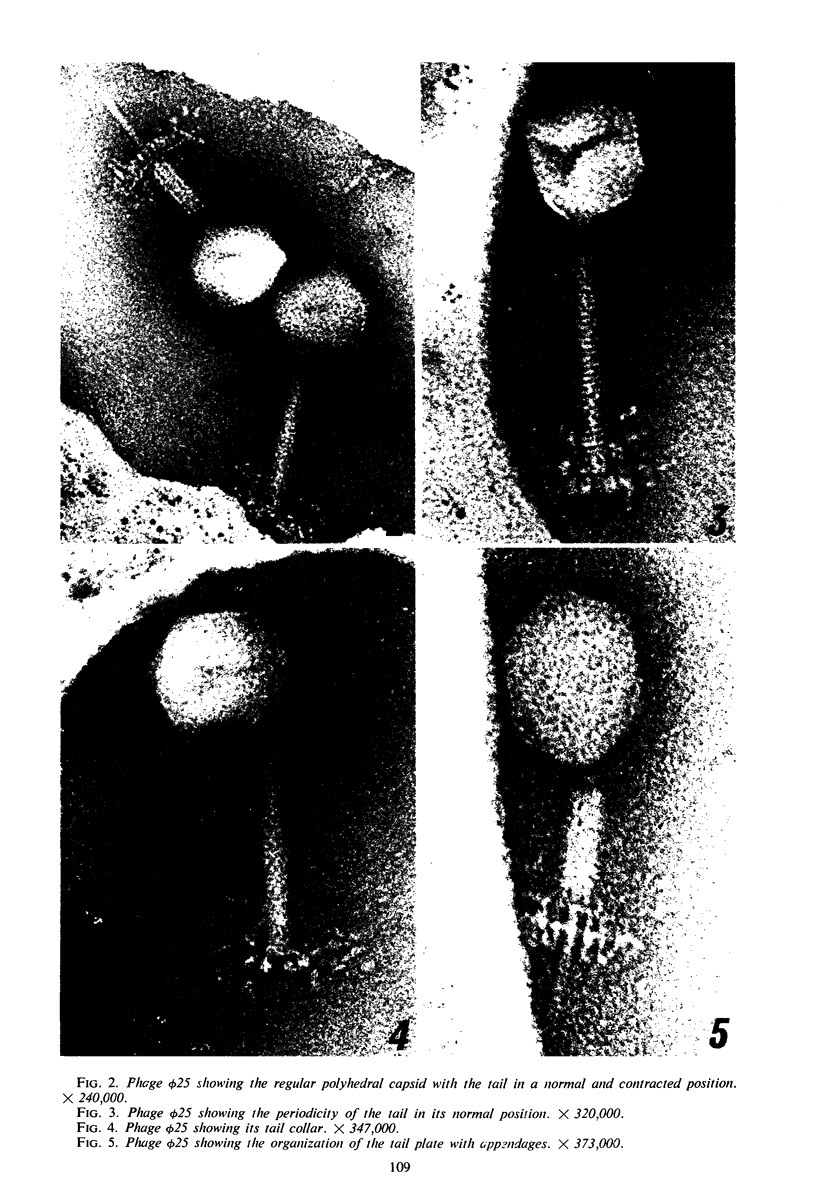
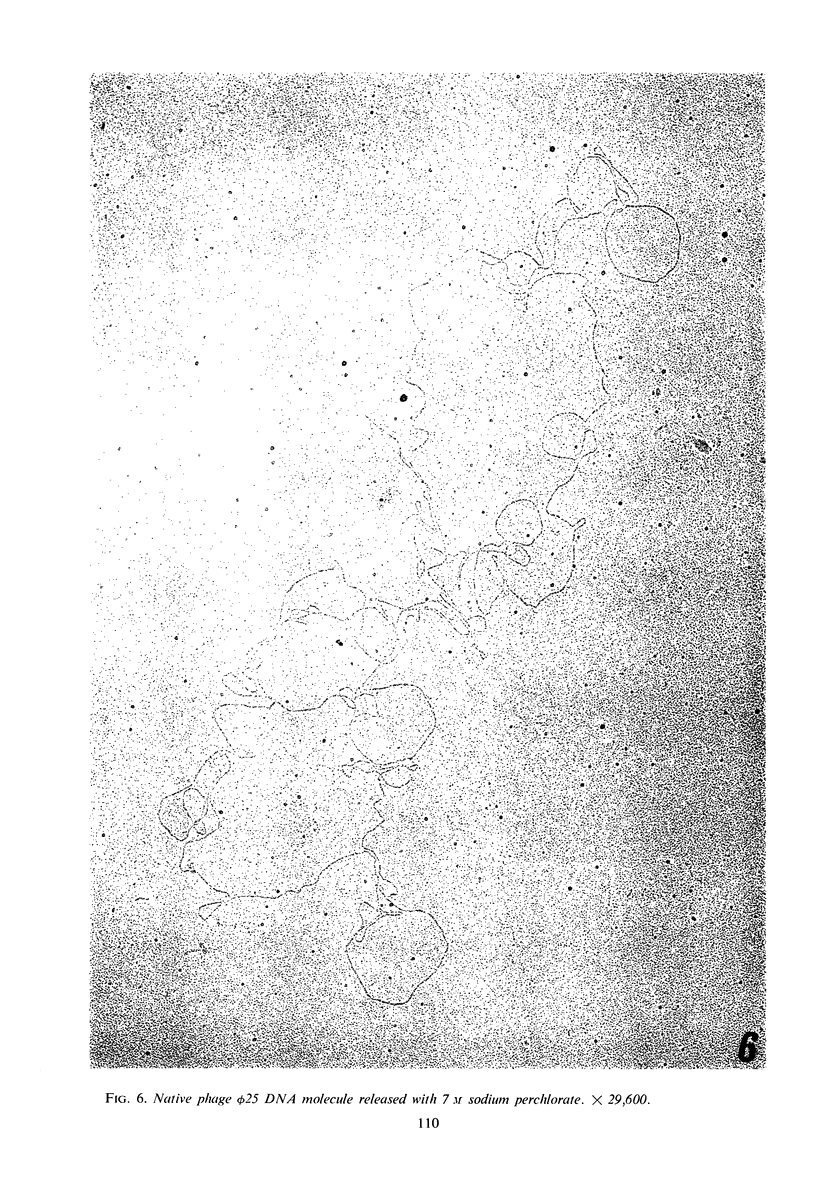
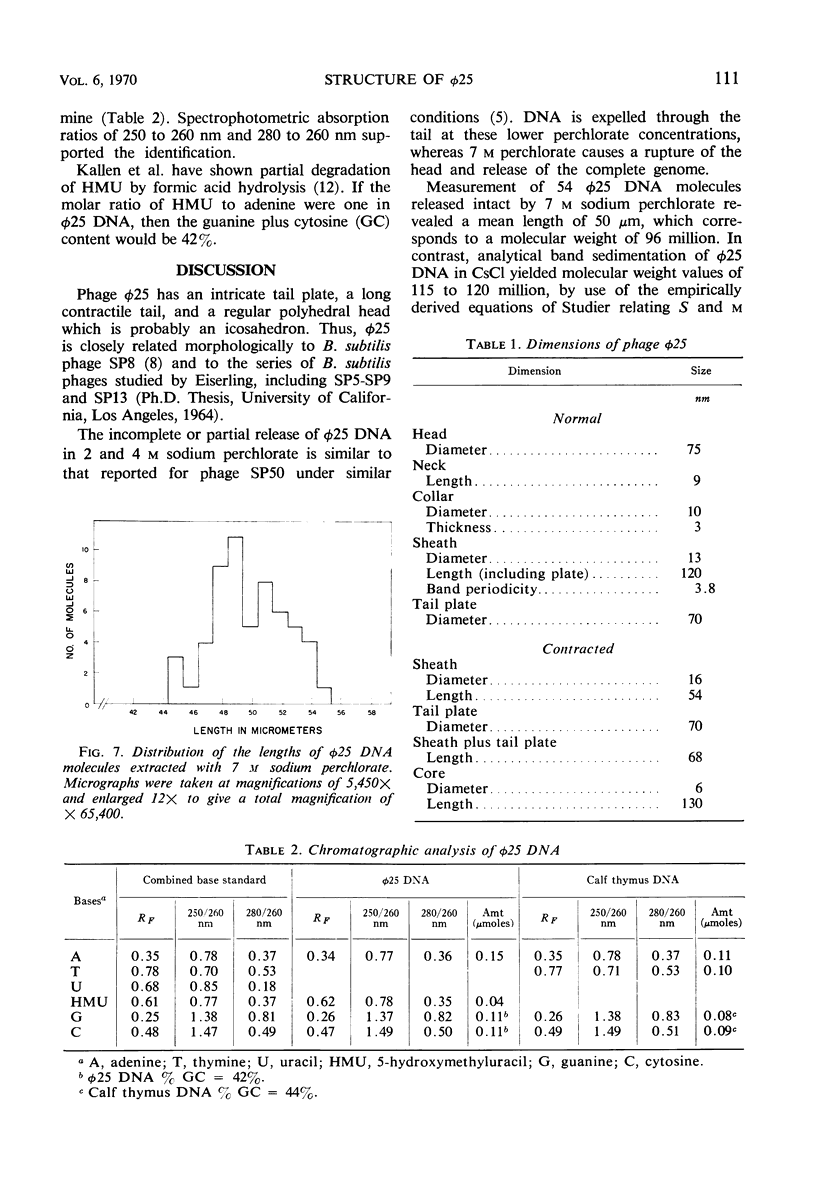
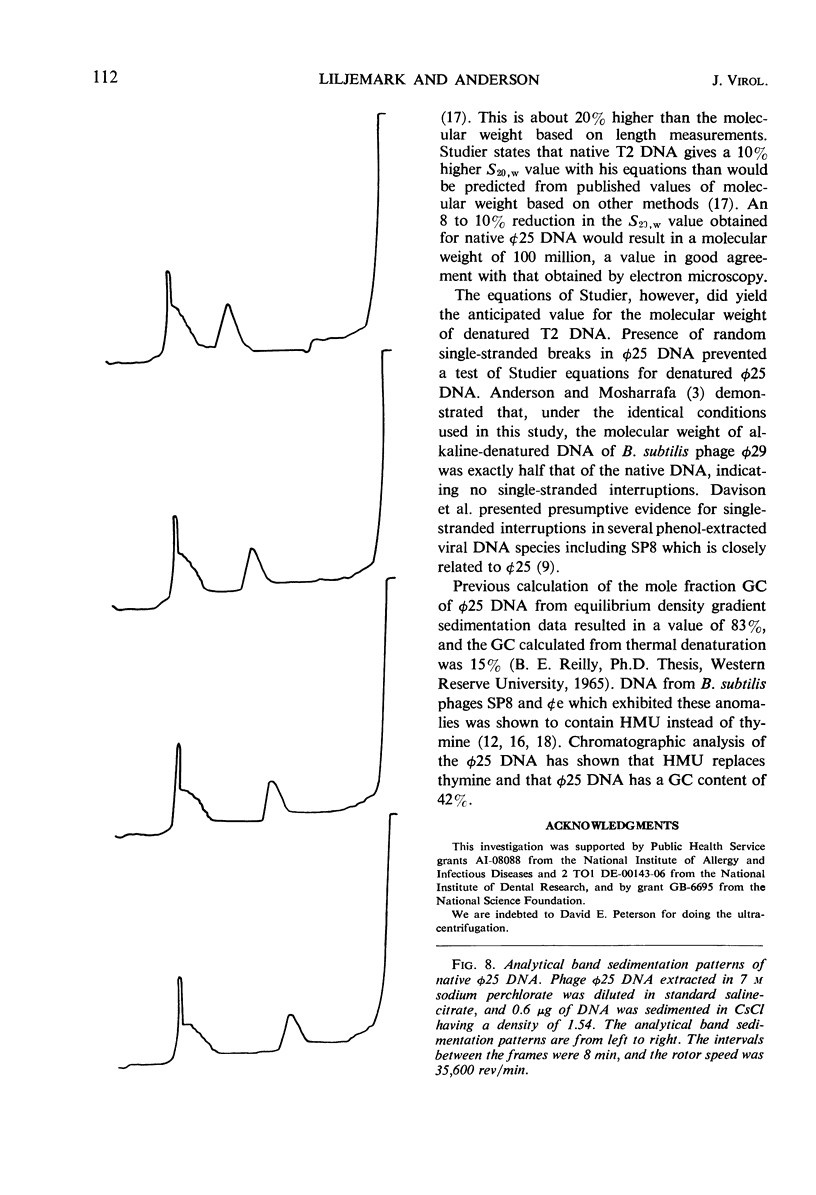
The structure of Bacillus subtilis bacteriophage φ25 and φ25 deoxyribonucleic acid (DNA) were studied by electron microscopy. The head of φ25 is a regular polyhedron measuring 75 nm in diameter. The uncontracted tail of φ25 is 130 nm in length and includes a large, complex tail plate. Phage φ25 DNA is double-stranded and has a molecular weight of approximately 100 million as determined by electron microscopic length measurements and analytical band sedimentation in CsCl. The complementary strands of φ25 DNA contain numerous random interruptions. Chemical analysis of φ25 DNA demonstrated that 5-hydroxymethyluracil replaces thymine and that the DNA has a mole per cent (guanine plus cytosine) of 42.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. L., Hickman D. D., Reilly B. E. Structure of Bacillus subtilis bacteriophage phi 29 and the length of phi 29 deoxyribonucleic acid. J Bacteriol. 1966 May;91(5):2081–2089. doi: 10.1128/jb.91.5.2081-2089.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. L., Mosharrafa E. T. Physical and biological properties of phage phi 29 deoxyribonucleic acid. J Virol. 1968 Oct;2(10):1185–1190. doi: 10.1128/jvi.2.10.1185-1190.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRENNER S., HORNE R. W. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959 Jul;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- Biswal N., Kleinschmidt A. K., Spatz H. C., Trautner T. A. Physical properties of the DNA of bacteriophage SP50. Mol Gen Genet. 1967;100(1):39–55. doi: 10.1007/BF00425774. [DOI] [PubMed] [Google Scholar]
- Bruner R., Vinograd J. The evaluation of standard sedimentation coefficients of sodium RNA and sodium DNA from sedimentation velocity data in concentrated NaCl and CsCl solutions. Biochim Biophys Acta. 1965 Sep 6;108(1):18–29. doi: 10.1016/0005-2787(65)90104-8. [DOI] [PubMed] [Google Scholar]
- DAVISON P. F., FREIFELDER D., HOLLOWAY B. W. INTERRUPTIONS IN THE POLYNUCLEOTIDE STRANDS IN BACTERIOPHAGE DNA. J Mol Biol. 1964 Jan;8:1–10. doi: 10.1016/s0022-2836(64)80142-x. [DOI] [PubMed] [Google Scholar]
- DAVISON P. F. THE STRUCTURE OF BACTERIOPHAGE SP8. Virology. 1963 Oct;21:146–151. doi: 10.1016/0042-6822(63)90250-2. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Effect of NaCIO-4 on bacteriophage: release of DNA and evidence for population heterogeneity. Virology. 1966 Apr;28(4):742–750. doi: 10.1016/0042-6822(66)90258-3. [DOI] [PubMed] [Google Scholar]
- KALLEN R. G., SIMON M., MARMUR J. The new occurrence of a new pyrimidine base replacing thymine in a bacteriophage DNA:5-hydroxymethyl uracil. J Mol Biol. 1962 Aug;5:248–250. doi: 10.1016/s0022-2836(62)80087-4. [DOI] [PubMed] [Google Scholar]
- KLEINSCHMIDT A. K., LANG D., JACHERTS D., ZAHN R. K. [Preparation and length measurements of the total desoxyribonucleic acid content of T2 bacteriophages]. Biochim Biophys Acta. 1962 Dec 31;61:857–864. [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- REILLY B. E., SPIZIZEN J. BACTERIOPHAGE DEOXYRIBONUCLEATE INFECTION OF COMPETENT BACILLUS SUBTILIS. J Bacteriol. 1965 Mar;89:782–790. doi: 10.1128/jb.89.3.782-790.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscoe D. H., Tucker R. G. The biosynthesis of 5-hydroxymethyldeoxyuridylic acid in bacteriophage-infected Bacillus subtilis. Virology. 1966 May;29(1):157–166. doi: 10.1016/0042-6822(66)90205-4. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]