Abstract
Rationale
An improved understanding of motor dysfunction and recovery after stroke has important clinical implications that may lead to the design of more effective rehabilitation strategies for patients with hemiparesis.
Scope
Transcranial magnetic stimulation (TMS) is a safe and painless tool that has been used in conjunction with other existing diagnostic tools to investigate motor pathophysiology in stroke patients. Since TMS emerged over two decades ago, its application in clinical and basic neuroscience has expanded worldwide. TMS can quantify the corticomotor excitability properties of clinically affected and unaffected muscles, and probe local cortical networks, as well as remote but functionally related areas. This provides novel insight into the physiology of neural circuits underlying motor dysfunction, and brain reorganization during the motor recovery process. This important tool needs to be used with caution by clinical investigators, its limitations need to be understood and the results should be interpreted along with clinical evaluation in this patient population.
Summary
In this review, we provide an overview of the rationale, implementation and limitations of TMS to study stroke motor physiology. This knowledge may be useful to guide future rehabilitation treatments by assessing and promoting functional plasticity.
Keywords: Transcranial magnetic stimulation, stroke rehabilitation, motor recovery
1. Motor Recovery in stroke and the gap in knowledge
Stroke remains a leading cause of serious long-term disability in the United States.[1] This disability is in most cases related to incomplete recovery of motor function on the hemiparetic side.[2] Whether patients are performing at their full potential in the years following stroke is influenced by a host of personal and environmental factors, including age, pre-morbid medical and functional status, motivation, and access to rehabilitation services.[3, 4] However, even with state of the art medical and rehabilitation care, full recovery is often not achieved.[5] Standard rehabilitation interventions typically correlate with modest rather than marked improvements in motor function.[6] This speaks to our limited understanding of the biology of motor dysfunction and recovery. In order to develop ways to optimize rehabilitation interventions and improve function, a more complete understanding of biological restoration is needed. Transcranial magnetic stimulation (TMS), now well into its third decade of experimentation in humans, has proven to be a useful tool to safely and painlessly examine cortical, and corticospinal physiology.[7] This method can complement existing and emerging technologies to enable us to better understand motor dysfunction. When physiologic data are correlated with clinical function, they can provide powerful insight to biological processes that underlie movement dysfunction.[8, 9] In addition, TMS can be a surrogate marker of recovery that is both sensitive and quantitative.[10]
This paper aims to provide an overview of the rationale, implementation, considerations and limitations of TMS for studies in motor recovery following stroke, and to de-mystify this technique for clinicians interested in stroke motor physiology. While TMS can be used to assess non-motor areas of the brain, this paper will focus on the motor systems, using the evoked muscle response as the output measure from corticospinal stimulation.
2. TMS as an investigative tool in motor dysfunction
Since the inception of TMS in 1985, there have been a number of published reports attesting to the safety of the technique in health and disease, within recommended operation guidelines, [11, 12] as well as the putative mechanisms.[13–15] TMS is typically performed in a designated hospital room or university laboratory, the former being preferred for stroke studies for safety reasons. Clinicians and researchers using TMS should have suitably screened patients to exclude for all TMS risk factors (see TMS safety guidelines). TMS alters brain excitability and while single and paired pulse stimulation is considered safe, even in patient populations, there still remains a theoretical risk. Particularly with the development of stronger stimulators (use of higher frequency stimulation protocols, such as theta burst stimulation) and a greater range of patient clinical states being studied (from acute to chronic), new investigations should be carefully considered by the institutional review board (IRB) weighing the risk/benefit. Suitably trained personnel should apply TMS, and adverse event monitoring and safety plan should be put in place. If adverse events occur, they should be carefully documented, as well as the medications and dose, together with other co-morbid and medical history details to aid with data interpretation. For motor studies, surface electromyography (EMG) is used to measure the muscle response in sensitive manner. EMG signal analysis software is used to quantify the response during and after the TMS session. Pragmatic and safety reasons for excluding patients from TMS studies include: (1) Seizure history and presence of metal implants. TMS can induce tensional headaches, neck pain and other symptoms, yet the most clinically significant is considered to be seizure. TMS can and has caused seizures, as the safety guidelines have been developed in healthy subjects even with single and paired pulses. Seizures have been predominantly reported with high-frequency trains of pulses (repetitive TMS, rTMS) and are infrequent when single or paired-pulses are used for investigative purposes.[11] TMS in subjects with brain metal implants or cochlear implants should be avoided due to multiple possible unsafe interactions between the TMS pulse and the implants[16]; (2) Medications. Drugs can be potentially hazardous (depending on the stimulation protocol used – high or low frequency rTMS / number of pulses; as well as the characteristics of the patient), or may substantially influence the MEP response. Of the various medications prescribed to stroke patients, there are none reported where investigative TMS (single and paired-pulse) is contraindicated. Several antidepressants (including imipramine, amitriptyline, doxepine) and neuroleptics (for example: chlorpromazine, clozapine, haloperidol) increase seizure risk by lowering the seizure threshold; and some other drugs can change cortical excitability (such as Dopaminergic and Gabaergic drugs); (3) Inability to sit still for the duration of the experiment and maintain stable arousal; and (4) Inability to generate a motor evoked potential (MEP) using TMS; since the MEP is the outcome measure, the inability to detect an MEP means that the subtle changes are simply not able to be detected. Although it is possible that early after injury there is no MEP, but with evolution of motor recovery a MEP returns, this could be useful in longitudinal studies.
2.1. Machines and coils
A variety of TMS machines and coils are commercially available, with specifications obtainable on the company website of each. An increase in the intensity of stimulator output (from 0–100%, with intensity range varying by manufacturer) will elicit a larger muscle response when the coil is held over the optimal cortical location for a given target muscle. Also a larger diameter coil (circular, figure-8 or coneshaped), and a biphasic pulse shape can all increase the evoked response at a given scalp location.[17] TMS users should note that the response in one person to one machine and set of stimulation parameters is likely to be different from that of another device using the same stimulation parameters, even if the percentage of the stimulator output is the same.
2.2 What is a motor evoked potential (MEP)?
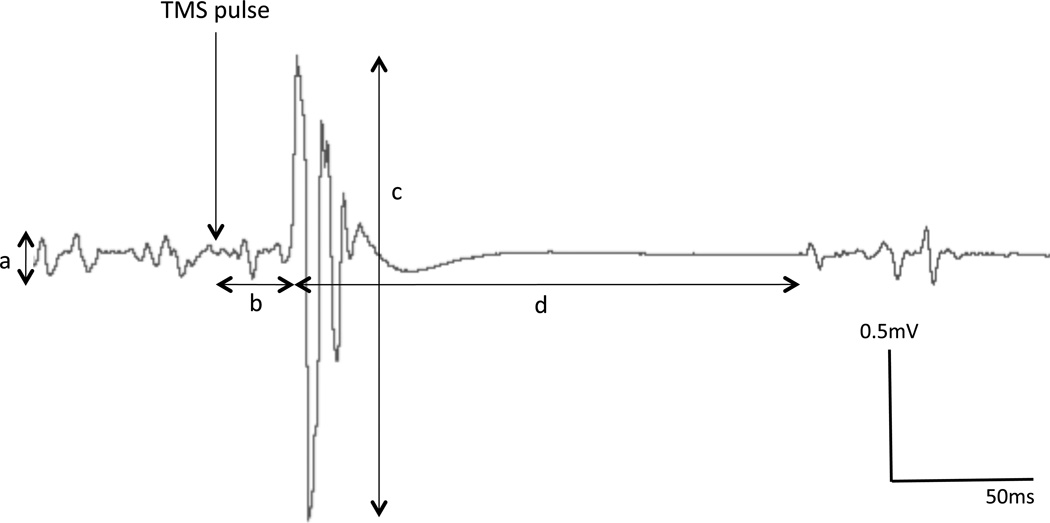
The MEP is the standard measure of motor response to TMS. It is important to have an understanding of the characteristics of the MEP, in order to correctly interpret studies using this as an outcome measure (Figure 1).[18–20] It is an electrical potential difference detected using bipolar surface electromyography over the target muscle. Most commonly for TMS, the intrinsic hand muscles (the first dorsal interosseous, FDI and abductor pollicis brevis, APB muscles) have been studied, since distal muscles more readily evoke a response relative to proximal muscles (in healthy subjects), likely due to the larger cortical representation and lower activation thresholds.[21, 22] Other muscles can also be studied by TMS, since the activation of muscles is detected by surface EMG, and the reliability of the signal is determined by the relative isolation of the targeted muscle. Many muscles have been tested including the face, jaw, neck, arm, forearm, hand, para-spinal muscles, respiratory muscles, thigh, and leg muscles.[23–25] The latency of conduction increases with distance, and not all pathways are precisely understood. The response is typically biphasic comprising a negative deflection (upwards) followed by a positive deflection (downwards), before a return to baseline (if at rest) or short period of EMG silence and return to low-level activity (if during an isometric contraction of the target muscle). The typical measures of the MEP are: resting motor threshold (RMT), latency of onset, peak-to-peak amplitude, area under the curve, and silent period (if performed during contraction), and are explained below. The magnitude of the MEP response (amplitude or area), and the threshold of activation (minimum stimulation intensity to elicit an MEP) are considered corticospinal (or ‘corticomotor’) excitability measures. In lay terms, these parameters may be thought of as the ease with which a response can be elicited and the strength of the response. These measures therefore provide important information on the physiological integrity of the corticospinal pathway from the primary motor cortex, and the areas feeding into it, to the spinal motor neurons, and to the electrical potential in muscle that leads to contraction (excitation-contraction coupling). Unlike the response to a supramaximal peripheral nerve stimulus (influenced only by nerve conduction neuromuscular transmission and muscle excitation), and which generates consistent responses, the MEP is inherently variable.[26–28] This may be in part due to multiple converging inhibitory and excitatory inputs onto corticospinal cells, and alpha motoneurons, and possibly in part due to subtle coil movements as the experimenter balances the small contact area of the coil on the patient’s approximately spherical scalp, thus subtly changing the trajectory of the magnetic pulse. For these reasons, successive pulses yield varying sized responses. This variance should be minimized as much as possible, by maintaining a steady arousal of the patient (no talking/interaction, minimal environmental stimulation, but also regular pauses to ensure the patient does not fall asleep). Also, it is important for the experimenter to maintain consistent positioning of the coil regarding its location, tilt and rotation.
Figure 1. TMS-derived measures of cortical excitability.
Schematic of Motor Evoked Potential characteristics, when a single TMS pulse is recorded from a muscle with a slight contraction. a) Background EMG, b) Latency, c) Peak-to-Peak amplitude, d) Silent Period.
2.3. How is a motor evoked potential generated? TMS physiology
A single pulse of sufficient strength TMS, with the coil held against the scalp approximately over primary motor cortex, can generate a transient current in the cortex in the opposite direction to that in the coil (typically posterior-to-anterior across the pre-central gyrus for monophasic pulse, and both directions for biphasic pulse, resulting in greater neuronal recruitment and larger MEP).
The current is thought to activate horizontal interneurons in cerebral cortex [7, 29, 30] that subsequently depolarizes the corticospinal cell and leads to waves of descending action potentials (‘volleys’ in the TMS literature). These are known as indirect waves, or I-waves, with three predominant waves separated by 1.5ms. This is important, since different interventions may affect different I-waves, and thus give us better insight into the mechanism of action of the interventions. The I-waves depolarize motoneurons in the anterior horn of the spinal cord, relating to the somatotopic organization of the cortical area stimulated.[8, 31] If these motoneurons are sufficiently depolarized (and this is influenced by converging excitatory and inhibitory drive, such as from afferent signals from muscle spindles, as well as pre-synaptic inhibitory mechanisms), then efferent motor unit activity occurs causing a muscle twitch. EMG is more sensitive than observation, because a very small muscle twitch may be insufficient to transfer force through a compliant tendon and overcome inertia of the body part to generate movement. It is difficult to activate a single muscle with TMS, the typical response being multiple adjacent muscles activated in concert. This leads to a net activation that causes a slight ‘jump’ of the body-part in a reproducible direction.[32] This is partly because of the low resolution or relatively large stimulation area (low focality) of conventional TMS coils, and partly because the representation for anatomically adjacent muscles has overlapping projections from primary motor cortex.[33] The fact that many synapses are involved to produce the MEP means that the MEP represents the net effect of multiple neural elements along the pathway. Stated differently, the MEP can be influenced by many physiologic (cortical and spinal) and technical (TMS coil position and movement, electrodes position) factors.[34] Both physiologic and technical aspects should be well controlled so that changes in MEP response can be attributed to real biological changes attributed to recovery or intervention. This means that electrode position should remain fixed in place if the intervention is short term (such as single dose, short time frame), or if the electrodes are to be moved, then a control measure should be done, such as a maximal M-response (that represents the muscle response to a supramaximal peripheral nerve stimulus, uninfluenced by CNS changes) or recording from another muscle not influenced by the intervention if that is possible. The coil should be kept in a consistent location (historically using a cap with surface markings) and the wings of the coil (if Figure-8 coil) should be maintained tangential to the scalp as best as possible.[35] This is part of the skill of the experimenter that takes practice and is quite crucial to obtaining reliable data. Using neuronavigation systems helps (independent of whether an anatomical MRI is used to target specific anatomy), since real-time feedback about coil position is then provided to the experimenter. [36]
Other factors that can modify TMS response and might be a limitation in the result interpretation are previous voluntary activation/movement, age, time of the day, aerobic exercise, attention, genetics, and medication,[37]. These should be suitably controlled for. Since arousal can be a major problem in patients with acute stroke due to their inability to keep the same concentration level during the duration of the TMS session, it may be necessary to conduct the study over two or more sessions, making sure to maximize reliability, and account for changes in baseline due to recovery. At present there are few studies early after stroke, and more work needs to be done in this time-period.
TMS responses are highly variable between subjects (healthy or with neurological diseases).[38, 39] The source of this variability is not well understood but it seems to be multifactorial, and it may limit the interpretation of the results as well as its therapeutic use in patients with disabilities. Other TMS limitations include: low spatial resolution - which can be improved by pairing it with a sophisticated neuronavigation system; the possibility of false negative results due to high threshold in paralyzed muscles or at early stages after stroke; the inability of TMS to explore beyond the corticospinal pathway, when additional pathways may be involved in motor recovery; and the fact that the technique cannot explore the motor tract at subcortical level.[40]
Considering all of the above, what does it mean if you detect a reliable change in MEP? If the data are real, an increase in the MEP means that corticospinal (‘corticomotor’) excitability has increased, and if the MEP has decreased this means that corticomotor excitability has decreased. Again, this means that the net effect of all the inhibitory and excitatory processes feeding into the pyramidal cells and the motoneurons has increased or decreased. For example, if the MEP response is reduced after an intervention, it does not necessarily mean that there is more inhibition, since it could also result from less excitatory or ‘facilitatory’ activity. Further, it does not necessarily mean that something has happened in the brain, since it could also have occurred in the spinal cord. When interpreting the results of TMS studies in stroke patients or other neurological disorders, or when navigating the literature in this field, care should be taken to appreciate that the MEP is a result of many interacting factors. So is the MEP useful? Yes, the MEP can provide valuable information about the state of the corticomotor projection. As well, the MEP can be related to clinical data, or more precise physiology can be probed further using the eloquent paired stimulation paradigms. In addition, the presence of contralateral MEP early after stroke may indicate a better prognosis with a favorable recovery, while the absence of MEP has been related to a poor recovery.[41, 42]
In summary, the MEP is a valuable tool for clinical research, when conclusions are drawn in the context of the reliability of MEP measures and the understanding of the factors that comprise the MEP.
3. The MEP in stroke and its relationship to recovery
TMS outcome measures after stroke vary with the stage of recovery (acute, subacute, chronic) and the degree of motor function.[43] In general, higher motor thresholds, smaller MEP amplitudes, prolonged latencies and longer silent period have been associated with the affected hemisphere in stroke patients, due mainly to loss of cortical-motoneurons, altered membrane excitability in the remaining cells, dispersion of the excitatory volleys onto motoneurons, and compromised conduction and increased cortical inhibition.[21, 44, 45]
3.1. TMS Measures. Single-pulse stimulation
Motor threshold (MT)
When the TMS is applied in the motor cortex, the threshold is defined as the lowest intensity needed to elicit a MEP response of at least 50uV amplitude with the muscle at rest and that has been elicited in 50% of 10 successive trials.[46] This is the most basic measurement of excitability within the corticospinal system. Recently a more efficient method to determine MT has been described by using Bayesian adaptive technique; a method that shortens the MT estimation without compromising accuracy by using fewer pulses than other MT determination methods. [47]
In muscles with lack of motor control – as in stroke patients – the motor threshold is elevated (a greater percentage of maximal stimulator output, MSO, is needed to elicit a response). An absent TMS response (or a weak response - small amplitude, delayed, polyphasic) an be found in paralyzed or severely affected muscles, that is muscle with 0, 1 or 2 out of maximum possible 5 points in Medical Research Council Scale – MRC – for muscle strength, even using high intensities. In order to get some insight into the pathophysioloy of the studied muscle, the active motor threshold can be calculated by asking the patient to do an attempted contraction of the muscle in order to facilitate the evoked response. Lower motor threshold in the affected limb are associated with better motor function in upper extremity in both subacute and chronic patients.[48]
MEP amplitude
The size of the peak-to-peak amplitude of the MEP represents the strength or the integrity of the corticospinal pathway.[18] In stroke patients, with lack of voluntary motor control, the size of the TMS response is smaller than in healthy subjects with normally functioning muscles. Rehabilitation strategies as well as spontaneous motor recovery promote restoration toward the normal values of the MEP. The absence of a MEP response in the early stages after the injury in hemiplegic limbs has been proposed as a negative predictor of motor recovery, while a presence of a response is a positive predictor.[49]
MEP latency
This is the period of time, in milliseconds, from the onset of the stimulation to the start of the motor evoked response. Longer MEP latencies are common in stroke patients compared to healthy subjects, and are more evident in the early stages after the injury.
Silent period (SP)
When a single pulse TMS is applied during a constant muscle contraction; there is an interruption of the electromyographic signal, which is called the silent period. This neurophysiological phenomenon is due to inhibition mechanisms of the motor cortex mediated through the GABABergic system. This suppression of muscle activation or silent period is more pronounced in the acute phase after an injury and it normalizes in conjunction with the improvement in clinical outcomes.[50–52] Prolonged SP duration is found in subacute and chronic patients, although its relationship with motor function has not been well described in the literature.[53] In stroke patients with motor neglect, motor dysfunction appears due to an increased inhibition rather than an alteration of the corticospinal tract. [51]
Motor cortex mapping
Motor cortex mapping can be studied by investigating the MEP responses in the motor cortex area of a target muscle.[54] Map area and volume, center of gravity and optimal site are the parameters that will help to elucidate how the cortex is reorganized after injury and how the restoration of the corticospinal pathway leads to regaining motor function after stroke.[28, 55, 56] An enlargement of the motor area representation and an increase of the MEP have been shown a positive correlation with motor recovery.[33]
3.2. Paired-pulse techniques, what are they, what do they tell us?
Various methods have been developed whereby the principal TMS pulse (the test pulse) may be conditioned by an earlier pulse (the conditioning pulse). The conditioning pulse is delivered the order of one to several hundred milliseconds prior, and generates physiologic activity that influences the test pulse. The conditioning pulse can be in the same cortical location, a different cortical location (using a separate coil) that projects to the primary motor cortex where the test pulse is delivered, or from a peripheral nerve (delivered by peripheral electrical stimulation). The location of the conditioning pulse (same or different location as test pulse), the intensity of stimulation, and the interval between the pulses has a signature effect on the test stimulus response (known as the ‘conditioned MEP’). A change in the conditioned MEP amplitude can be examined during motor recovery after neurologic injury, or in relation to a well-controlled intervention to identify corresponding changes in physiology inter-cortical (across hemispheres) or intra-cortical excitability.[57],[58] Common measures in stroke include: transcallosal inhibition, and intracortical inhibition and facilitation.
Paired-pulse one location (intracortical facilitation and inhibition)
The imbalance between the both hemispheres can be investigated by using a paired pulse with a unique TMS coil.[59, 60] A subthreshold conditioning stimulus (CS) will be elicited from 2 to 7 ms before the suprathreshold test stimulus (TS), at 130% of RMT usually, to examine the short intracortical inhibition (SICI). In the same way, separating both stimuli 7–10 ms, the intracortical facilitation (ICF) will be studied. Lately, numerous studies have adjusted the CS to a pre-established value, for example 0.5 or 1mV to avoid interindividual variability, and to facilitate the comparison pre and post any given intervention. [61] With this paired pulse paradigm the interaction between the affected and unaffected hemisphere can be investigated in stroke patients, where the current thinking establishes that there is an increase of excitability in the healthy hemisphere that is sending inhibitory signals to the affected hemisphere.[62, 63] Patients with hemiparetic stroke usually present exaggerated inhibition within the primary motor cortex.[51]
Several studies support a disruption of interhemispheric excitability balance that occurs with unilateral lesion, thought to contribute to functional motor deficit. A reduction in excessive unaffected hemisphere excitability has been achieved experimentally using non-invasive brain stimulation (low frequency repetitive TMS – rTMS, and cathodal transcranial direct current stimulation –tDCS), as well as increased excitability in the affected hemisphere (high frequency rTMS, or anodal stimulation).[64–66] Functional gain and improved interhemispheric excitability balance has also been demonstrated with behavioral therapy (constraint induce movement therapy), with constraint of the unaffected arm, and force use of the affected arm.
Paired-pulse - two regions (transcallosal inhibition, TCI)
The TCI is quantified as the decrease of the MEP response when paired the TMS single pulse from the contralateral hemisphere with the ipsilateral pulse. This neurophysiological measure is widely used in stroke studies to assess the disinhibition of the ipsilateral motor cortex in the acute stages after the injury. Whether the inhibitory function of the affected hemisphere normalizes or remains decreased in chronic phases is controversial.[67, 68] Several studies have reported reduction in TCI from the intact to the affected hemisphere associated with behavioral gains, measured by Fugl-Meyer Scale score.[69]
4. TMS to index cortical excitability
The current reasoning for use of TMS information is as follows. If we identify specific excitability deficits (deviance from what is established in healthy – preferably aged match – controls), and we know that they are correlated (or at least associated) with a deficit in functional output (as observed in a change in behavior), then by inference we can target interventions that alter specific aspects of physiology (such as pharmacological agents) in order to restore physiology and by consequence improve behavior.[70, 71] Furthermore, we can use TMS to identify the specific physiologic effects of contemporary, well-described interventions, to establish specific aspects of physiology that they are modulating or even to refine and develop therapies to have the desired effect.[72] These might include interventions that act on very specific functional networks, such as behavioral training and therapies (focus network, low potency), or grossly targeted therapies such as CNS drugs or non-invasive brain stimulation, including tDCS or rTMS (low focus, high potency-specificity). This can be taken one step further, to purposefully generate an interaction, and is being tested in many labs presently.
4.1. Optimal TMS study design
Depending on the hypothesis to test a different research study, the method will be chosen. One method is to take a cross-sectional snapshot of neurophysiology in relation to known motor deficits, and use this in conjunction with other clinical data including medical exam and radiologic/stroke related biomarkers. The neurophysiology data are typically compared with normative data, but can also be correlated with specific clinical findings.[39] Another method is the longitudinal study approach, where TMS is used to track the course of recovery during conventional standard of care (or intervention, such as rTMS, transcranial direct current stimulation (tDCS) plasticity enhancing drugs, or a novel training paradigm), acting as a probe to disrupted and changing physiology in the living human. This also complements existing clinical tools. Like most interventions given early after stroke, it can be difficult to determine the degree of change caused by natural recovery versus intervention-induced recovery. Suitable controls need to be built in experimentally such as recording TMS response from a body-part not engaged in the training; this could be the contralateral hand in a unilateral training paradigm. If bilateral effects are suspected from a unilateral intervention, then a proximal contralateral muscle could be chosen, or even a lower limb muscle. The idea is to ensure MEP stability (experimental reliability) in muscle/s that you would not hypothesize to be affected by the intervention. This can be done in addition to having a sham-controlled group that does not receive real intervention.
4.2. Reducing TMS response variability
Muscle responses to brain stimulation are inherently variable (as compared with peripheral nerve stimulation response), which is why recording only one response may be unreliable, and why multiple evoked responses (usually 4 to 20 MEPs) should be reported by a measure of central tendency such as the mean or median. For mapping studies, as few as 2 to 4 stimuli per site may be necessary, while for pre-post intervention comparisons at a single site, 10 to 20 stimuli are collected.[73] This is partly due to the converging influence on spinal motor neurons and cortical pyramidal cells that can change excitability properties from moment to moment. This becomes a challenge for the investigator during a session and between sessions for the collection of reliable data. Experiments that collect many different variables in one session, can take several hours, with has inherent problems with quality of data.
Since arousal and certain aspects of cognition can substantially affect MEP response, it is prudent to try to maintain stable experimental conditions, such that arousal is not affected.[74] There are many interacting factors to be considered, and some remain to be formally tested for an impact on experimental results. However, some ideas for consideration, prior to an experimental session, would include: testing during the same time of day (circadian rhythms), ensuring that patients are not influenced by nicotine or caffeine, not testing immediately after a large meal and waiting prior to the start of an experiment if the patient has rushed to attend the clinic. While recording MEPs during a session, it is important to maintain a steady arousal level by minimizing talking/interaction between the investigator or the patient’s significant other and the patient. While some labs allow the patient to fall asleep, this should be avoided for arousal changes and practical test reasons (especially if in a seated position). A low-level isometric contraction is sometimes performed to stabilize the response.[75] If this is done, biofeedback should be provided regarding contraction level (either force or rectified and smoothed EMG) in order to avoid fluctuations that might influence MEP response. A window of 10–20% maximal voluntary isometric contraction (MVIC) is often used, or greater if patients have difficulty with fine control contraction strength.
4.3. Where does TMS fit with existing clinical tools?
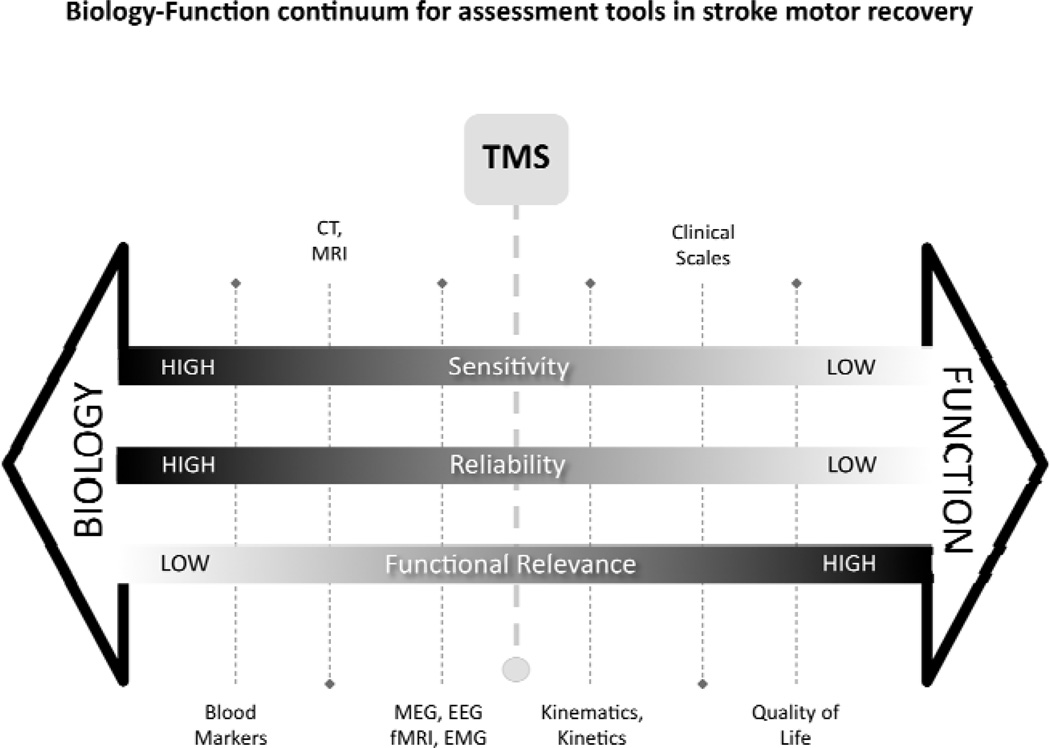
Over the last century, rapid technological advancement in medical investigative tools such as imaging and brain plasticity biomarker analysis (such as brain derived neurotrophic factor – BDNF) has profoundly influenced our understanding of the historically refined, and rigorous clinical information determined at the bedside.[76–78] Yet, there remain large gaps in knowledge about mechanisms underlying specific clinical presentations. Assessment tools in studies of the stroke motor system as with other clinical and investigative tools in medicine can be thought of to lie on a biology-function continuum (Figure 3). Tools that tell us much about function/dysfunction, typically tell us little about biology or mechanism of the dysfunction. Conversely, tools that inform of biology and mechanism may be correlated with function, but themselves do not usually inform of function. For example, a clinical scale such as the Fugl-Meyer Assessment (FMA) informs of voluntary motor control deficit, with the biological cause speculative and non-specific. On the other hand, the anatomical MRI provides a clear indication of the disrupted anatomy, though predictions of influence of voluntary control based on the images are not always accurate. Thus, both types of information are useful and complementary. Moving forward in the field of stroke motor recovery, we need to have a strong understanding of functional deficit as well as the biological mechanism – and similarly for intervention studies in stable or recovering patients, we need to understand the biological and functional effects of well defined interventions, in well characterized patients.[79] Assessment tools or outcome measures can be described in terms of sensitivity (degree of precision to detect a change afforded by a measurement instrument), reliability (confidence to rule-out equipment, technique or rater error when a change is detected), and functional relevance.
Figure 3. The biology-function continuum for assessment tools in stroke motor recovery.
Following stroke, investigative tools and clinical evaluations are selected depending on many factors to inform of clinical status, prognosis, treatment planning, recovery and treatment outcome. The selection of a given tool may compromise on sensitivity, reliability, or functional relevance, but in combination they can provide power insight into function and mechanism. In our opinion, TMS falls in a mid-point, having aspects of biological and functional insight. When used in conjunction with other measures, it can be an important complement to enhance our understanding of motor recovery.
The schematic highlights that when functional relevance is high (such as with ambulatory measures, or upper-limb movement grading), then reliability is low (due to factors such as rater error and patient effort variation) and sensitivity is low (scales are necessarily ordinal, and therefore only gross changes can be detected). The benefit of using assessment tools at this end of the continuum is that change is considered to be meaningful because it has clear personal and socioeconomic benefit, and this becomes highly important for clinical decision-making, as well as for determining intervention efficacy and recovery. However, little can be learned as to why patients recover or respond to interventions the way they do, unless we have more information about biology.[80] The biological assessments typically have the advantage of high precision (sensitivity) and high reliability. While they do not have high functional relevance alone, when combined with functional tools, they can provide powerful insight into mechanism. We suggest that TMS is an important tool that fits towards the middle of the functional biology continuum for assessment tools in stroke motor recovery. This technique assesses the functional biology of the final output from the brain to the muscle. It represents the functional integrity of the pathway used to control voluntary movement, and real-time assessment of its influence at spinal and supraspinal level of networks that feed into it.[45, 81] It has the advantage of being arguably more functionally relevant than other biological measures (such as Computed Tomography – CT – scan, blood analysis), yet more sensitive and less influenced than by human factors than clinical scales. It is acknowledged that while biomarkers may presently not relate directly to specific functional characteristics, this may be possible in the future. Furthermore, the position of individual outcomes on the continuum is a schematic representation only, and may not represent their true position.
TMS has limitations, which are discussed elsewhere in this paper, but it can be an important complement to the other available tools. It may be considered close to functional magnetic resonance imaging (fMRI) or electroencephalograpy (EEG) on the continuum (albeit quite different in information gathered), but it is also close to highly quantitative assessment of limb/body motion, forces, and muscle activity, such as can be captured with rehabilitation robotics.[82, 83]
The importance of these measures should be underscored, since they represent highly quantitative assessment of voluntary motor control, sensitive, reliable and relevant. Of key importance is the sensitivity of the used measure-tool, especially when testing subtle changes in function such as recovery over a small amount of inpatient time, or a short exposure to a novel training intervention. Especially for the latter, it is not prudent to launch into long clinical training studies using gross but relevant clinical outcome measures, without the confidence that a small clinical change can be detected with a small dose. Stated differently, highly sensitive measures of motor control may capture subtle but important changes associated with a short exposure to new interventions being tested (not identified with more coarse and subjective clinical scales). Furthermore, these methods can uncover individual elements of movement generation or appearance that can focus rehabilitation interventions to target specific deficits. Similarly, TMS can uncover functional pathways that cannot be detected clinically, potentially providing biological substrate for TMS-guided rehabilitation interventions.[84] Taken together, sensitive and reliable tools testing output of functional pathways (such as TMS) as well as specific test of anatomy and physiology of motor control (such as fMRI/EEG/MEG), can be powerful complements to understanding motor recovery and intervention in stroke since the types of questions answered by these techniques are different.[85–87] For example, fMRI has excellent spatial resolution, but poor temporal resolution, since it is tracking blood-flow changes secondary to neuronal activity, while EEG has excellent temporal resolution but arguably less spatial resolution.
These techniques can be powerful complements to understanding motor recovery and intervention in stroke, when combined with information from highly sensitive motor control assessment tools such as rehabilitation robots.[82, 88–90]
5. Limitations and caveats
In addition to the limitations stated above, the caveats might be broadly summarized as follows: experimental confounds, the use only of intrinsic hand muscles to represent the upper limb, and the ability to impact only cortical levels.
This represents an exciting time where physiologic findings related to clinical function in stroke, as well as what physiological effects therapies are causing, are slowly being uncovered, providing a better understanding of stroke motor dysfunction physiology in humans, and of motor recovery. This may ultimately lead to targeting specific therapies for specific patients at specific and important time-points post-injury.[91–93] Consumers of this expanding literature and clinicians need to take care in interpreting this literature.
Since the primary determinant of outcome in TMS motor studies is subtle changes in the MEP, the quality and reliability of the MEP is of critical importance. This has experimental implications because the presence and quality of MEPs in stroke patients is diminished. This is important, since reduced corticomotor output in response to TMS is associated with poor motor function, highlighting one aspect of the underlying pathophysiology.[41, 94, 95] But to run more elegant physiologic paradigms such as paired-pulse techniques, then strong changes in a poor quality test pulse (minimal presence and unreliable) are difficult to find or believe. The original TMS physiology studies were often performed in young healthy adults, by experienced investigators. For example, with short-latency intra-cortical inhibition, a strong TMS response for an intrinsic hand muscle of around 0.5–1.5mV might be observed with a supra-threshold TMS pulse at around 120–130% resting motor threshold. A sub-threshold conditioning pulse (preceding pulse) exerting a net inhibitory effect on the test pulse might reduce the amplitude of the test pulse around 50%. When unconditioned (test response alone) and conditioned MEPs are averaged the change is clear and valid, with normalized or ratio data that does not mask effects of a poor quality MEP. However, in a stroke patient, there may be ‘floor’ effects where by a poor MEP response (i.e. barely detectable, 50–100µV) could arguably show only a small raw difference in reduction when conditioned by a test pulse (interestingly, a small reduction from 100-50µV, would have the same 50% normalized reduction as a healthy subject reducing from 1000µV-500µV). These types of difference in altered ability to run protocols in patients as is done in healthy subjects, makes interpretation problematic. That is, the difference between patients and healthy subjects is intriguing, but to examine effects of intervention on a measure that is itself potentially flawed or limited is questionable. The point to be taken from this is not that TMS in stroke patients is questionable, but rather, that data need to be carefully interpreted, even in the most well conducted clinical experiments. There is still the potential to extract rich and meaningful data, and many laboratories successfully do this. The practical implication is that more technically demanding measures of paired-pulse lend themselves well to patients with a higher level of function, where a stronger MEP is more likely seen. Muscles that are completely flaccid are difficult to elicit an MEP, and it would not be reasonable to expect to conduct pair-pulse paradigms in such cases. For this reason, many TMS studies focus on higher-level functioning patients[96–99] (often stable chronic patients), or even the un-affected hemisphere, which can provide rich data and where MEPs are more readily accessible. Another method that has been attempted is to perform a low-level muscle contraction, which facilitates the MEP response (and has the benefit of obtaining a silent period). Other problem is the high motor threshold in patients. Typically to elicit a MEP the stimulator intensity is set to around 120% of motor threshold or 20% of motor stimulator output (MSO) above RMT. If the threshold is 84% or higher, which is often the case for resting state TMS in elderly stroke patients (older subjects have higher RMT),[100] then TMS simply cannot deliver the required experimental intensity. The consequence is that each patient is tested at a different percentage above motor threshold (and at a different part of the slope on the recruitment curve), which makes drawing comparisons between patients less robust.
6. Conclusion
TMS is a remarkable contemporary tool to understand and ultimately promote motor recovery in stroke patients. When studies are properly conducted and results suitably interpreted, TMS information can be a powerful adjunct to existing clinical and investigative information. More work is needed in this area, particularly in the sub-acute phase and we can anticipate insightful data being generated over the coming years.
Figure 2. Diagram of experimental setup used for neuronavigation guided TMS.
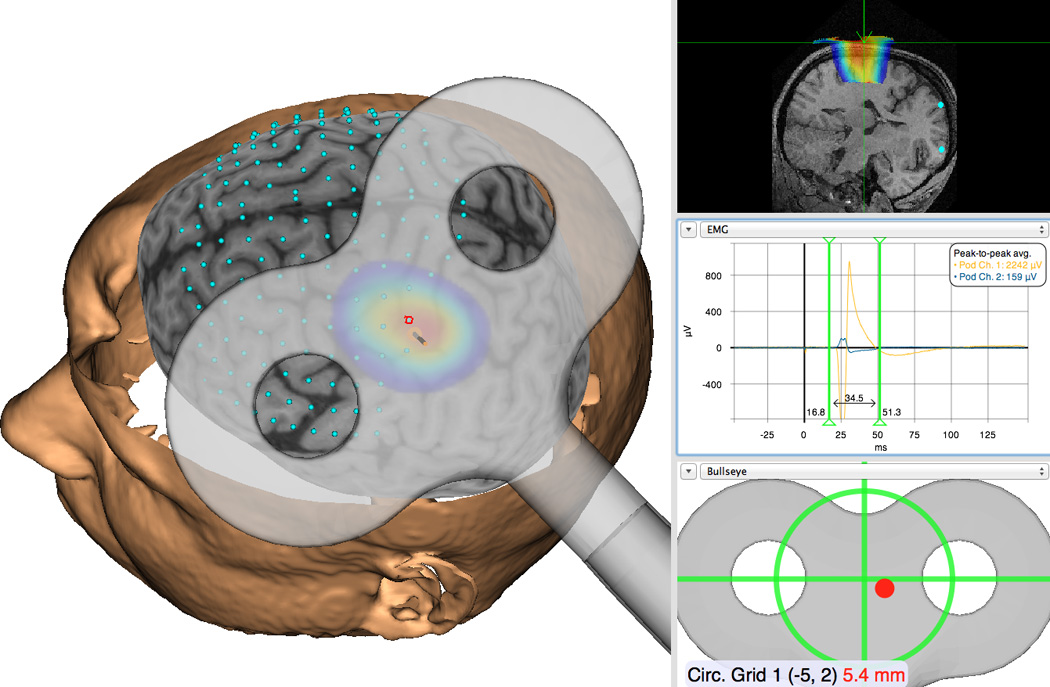
An example of how neuronavigation can be used to guide TMS in stroke studies (with permission). Using either direct anatomical targets or a superimposed reference grid, real-time experimenter feedback is given regarding coil position in relation to the targets during the study. Precise coil position is recorded concurrently with the evoked motor response (MEP) associated with every stimulus.
Acknowledgements
The development of this paper was supported by NIH grant R21HD060999 for DJE and by the New York State Center of Research Excellence in Spinal Cord Injury Grant CO19772 for MC.
Footnotes
Conflict of interest statement: The authors have no conflict of interest in the submission of this manuscript.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young J, Forster A. Review of stroke rehabilitation. BMJ. 2007;334:86–90. doi: 10.1136/bmj.39059.456794.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cramer SCSC. Repairing the human brain after stroke. II. Restorative therapies. Annals of neurology. 2008;63:549–560. doi: 10.1002/ana.21412. [DOI] [PubMed] [Google Scholar]
- 4.Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: Facts and theories. Restorative Neurology & Neuroscience. 2004;22:281–299. [PubMed] [Google Scholar]
- 5.Twitchell TE. The restoration of motor function following hemiplegia in man. Brain. 1951;74:443–480. doi: 10.1093/brain/74.4.443. [DOI] [PubMed] [Google Scholar]
- 6.Marsden J, Greenwood R. Physiotherapy after stroke: define, divide and conquer. J Neurol Neurosurg Psychiatry. 2005;76:465–466. doi: 10.1136/jnnp.2004.053827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thickbroom GW. Transcranial magnetic stimulation and synaptic plasticity: experimental framework and human models. Exp Brain Res. 2007;180:583–593. doi: 10.1007/s00221-007-0991-3. [DOI] [PubMed] [Google Scholar]
- 8.Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. 2003;148:1–16. doi: 10.1007/s00221-002-1234-2. Epub 2002 Nov 2005. [DOI] [PubMed] [Google Scholar]
- 9.Tsai SY, Tchen PH, Chen JD. The relation between motor evoked potential and clinical motor status in stroke patients. Electromyogr Clin Neurophysiol. 1992;32:615–620. [PubMed] [Google Scholar]
- 10.Rossini PM, Calautti C, Pauri F, Baron J-C. Post-stroke plastic reorganisation in the adult brain. The Lancet Neurology. 2003;2:493–502. doi: 10.1016/s1474-4422(03)00485-x. [DOI] [PubMed] [Google Scholar]
- 11.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wassermann E. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 13.Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- 14.Webster, Celnik, Cohen Noninvasive Brain Stimulation in Stroke Rehabilitation. NeuroRX. 2006;3:474–481. doi: 10.1016/j.nurx.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004;115:255–266. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Schrader LM, Stern JM, Fields TA, Nuwer MR, Wilson CL. A lack of effect from transcranial magnetic stimulation (TMS) on the vagus nerve stimulator (VNS) Clin Neurophysiol. 2005;116:2501–2504. doi: 10.1016/j.clinph.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 17.Xu G, Wang M, Chen Y, Yang S, Yan W. The Optimal Design of Magnetic Coil in Transcranial Magnetic Stimulation. Conf Proc IEEE Eng Med Biol Soc. 2005;6:6221–6224. doi: 10.1109/IEMBS.2005.1615917. [DOI] [PubMed] [Google Scholar]
- 18.Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W. Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:97–103. [PubMed] [Google Scholar]
- 19.Harris-Love ML, Cohen LG. Noninvasive cortical stimulation in neurorehabilitation: a review. Arch Phys Med Rehabil. 2006;87:S84–S93. doi: 10.1016/j.apmr.2006.08.330. [DOI] [PubMed] [Google Scholar]
- 20.Reis J, Swayne OB, Vandermeeren Y, Camus M, Dimyan MA, Harris-Love M, Perez MA, Ragert P, Rothwell JC, Cohen LG. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol. 2008;586:325–351. doi: 10.1113/jphysiol.2007.144824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turton A, Wroe S, Trepte N, Fraser C, Lemon RN. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalogr Clin Neurophysiol. 1996;101:316–328. doi: 10.1016/0924-980x(96)95560-5. [DOI] [PubMed] [Google Scholar]
- 22.Byrnes ML, Thickbroom GW, Phillips BA, Wilson SA, Mastaglia FL. Physiological studies of the corticomotor projection to the hand after subcortical stroke. Clinical Neurophysiology. 1999;110:487–498. doi: 10.1016/s1388-2457(98)00044-3. [DOI] [PubMed] [Google Scholar]
- 23.Paradiso GO, Cunic DI, Gunraj CA, Chen R. Representation of facial muscles in human motor cortex. J Physiol. 2005;567:323–336. doi: 10.1113/jphysiol.2005.088542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wheaton LA, Villagra F, Hanley DF, Macko RF, Forrester LW. Reliability of TMS motor evoked potentials in quadriceps of subjects with chronic hemiparesis after stroke. J Neurol Sci. 2009;276:115–117. doi: 10.1016/j.jns.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dishman JD, Greco DS, Burke JR. Motor-evoked potentials recorded from lumbar erector spinae muscles: a study of corticospinal excitability changes associated with spinal manipulation. J Manipulative Physiol Ther. 2008;31:258–270. doi: 10.1016/j.jmpt.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Carroll TJ, Riek S, Carson RG. Reliability of the input-output properties of the cortico-spinal pathway obtained from transcranial magnetic and electrical stimulation. Journal of Neuroscience Methods. 2001;112:193–202. doi: 10.1016/s0165-0270(01)00468-x. [DOI] [PubMed] [Google Scholar]
- 27.Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res. 2000;133:425–430. doi: 10.1007/s002210000432. [DOI] [PubMed] [Google Scholar]
- 28.Weiller C, Ramsay S, Wise R, Friston K, Frackwiak R. Individual patterns of functional reorganization in the human cerebral cortex after capsular infraction. Annals of Neurology. 1993;33:181–189. doi: 10.1002/ana.410330208. [DOI] [PubMed] [Google Scholar]
- 29.Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res. 1997;114:329–338. doi: 10.1007/pl00005641. [DOI] [PubMed] [Google Scholar]
- 30.Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15:333–343. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Swayne OB, Rothwell JC, Ward NS, Greenwood RJ. Stages of motor output reorganization after hemispheric stroke suggested by longitudinal studies of cortical physiology. Cereb Cortex. 2008;18:1909–1922. doi: 10.1093/cercor/bhm218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid Plasticity of Human Cortical Movement Representation Induced by Practice. J Neurophysiol. 1998;79:1117–1123. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- 33.Thickbroom GW, Byrnes ML, Archer SA, Mastaglia FL. Motor outcome after subcortical stroke correlates with the degree of cortical reorganization. Clin Neurophysiol. 2004;115:2144–2150. doi: 10.1016/j.clinph.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Kiers L, Cros D, Chiappa KH, Fang J. Variability of motor potentials evoked by transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1993;89:415–423. doi: 10.1016/0168-5597(93)90115-6. [DOI] [PubMed] [Google Scholar]
- 35.Conforto AB, Z'Graggen WJ, Kohl AS, Rosler KM, Kaelin-Lang A. Impact of coil position and electrophysiological monitoring on determination of motor thresholds to transcranial magnetic stimulation. Clin Neurophysiol. 2004;115:812–819. doi: 10.1016/j.clinph.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 36.Bashir S, Edwards D, Pascual-Leone A. Neuronavigation increases the physiologic and behavioral effects of low-frequency rTMS of primary motor cortex in healthy subjects. Brain Topogr. 24:54–64. doi: 10.1007/s10548-010-0165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ridding MC, Ziemann U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol. 2010;588:2291–2304. doi: 10.1113/jphysiol.2010.190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edwards MJ, Talelli P, Rothwell JC. Clinical applications of transcranial magnetic stimulation in patients with movement disorders. Lancet Neurol. 2008;7:827–840. doi: 10.1016/S1474-4422(08)70190-X. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- 40.Jang SH. A review of motor recovery mechanisms in patients with stroke. NeuroRehabilitation. 2007;22:253–259. [PubMed] [Google Scholar]
- 41.Arac N, Sagduyu A, Binai S, Ertekin C. Prognostic value of transcranial magnetic stimulation in acute stroke. Stroke. 1994;25:2183–2186. doi: 10.1161/01.str.25.11.2183. [DOI] [PubMed] [Google Scholar]
- 42.Rapisarda G, Bastings E, de Noordhout AM, Pennisi G, Delwaide PJ. Can motor recovery in stroke patients be predicted by early transcranial magnetic stimulation? Stroke. 1996;27:2191–2196. doi: 10.1161/01.str.27.12.2191. [DOI] [PubMed] [Google Scholar]
- 43.Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical Synaptogenesis and Motor Map Reorganization Occur during Late, But Not Early, Phase of Motor Skill Learning. J. Neurosci. 2004;24:628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Traversa R, Cicinelli P, Pasqualetti P, Filippi M, Rossini PM. Follow-up of interhemispheric differences of motor evoked potentials from the 'affected' and 'unaffected' hemispheres in human stroke. Brain Res. 1998;803:1–8. doi: 10.1016/s0006-8993(98)00505-8. [DOI] [PubMed] [Google Scholar]
- 45.Trompetto C, Assini A, Buccolieri A, Marchese R, Abbruzzese G. Motor recovery following stroke: a transcranial magnetic stimulation study. Clin Neurophysiol. 2000;111:1860–1867. doi: 10.1016/s1388-2457(00)00419-3. [DOI] [PubMed] [Google Scholar]
- 46.Reid AE, Chiappa KH, Cros D. Motor threshold, facilitation and the silent period in cortical magnetic stimulation. In: Pascual-Leone A, editor. Handbook of Transcranial Magnetic Stimulation. London: Oxford University Press; 2002. pp. 97–111. [Google Scholar]
- 47.Qi F, Wu AD, Schweighofer N. Fast estimation of transcranial magnetic stimulation motor threshold. Brain Stimul. 2011;4:50–57. doi: 10.1016/j.brs.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Brouwer BJ, Schryburt-Brown K. Hand function and motor cortical output poststroke: are they related? Arch Phys Med Rehabil. 2006;87:627–634. doi: 10.1016/j.apmr.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Pennisi G, Rapisarda G, Bella R, Calabrese V, Maertens de Noordhout A, Delwaide PJ. Absence of Response to Early Transcranial Magnetic Stimulation in Ischemic Stroke Patients : Prognostic Value for Hand Motor Recovery. Stroke. 1999;30:2666–2670. doi: 10.1161/01.str.30.12.2666. [DOI] [PubMed] [Google Scholar]
- 50.Kukowski B, Haug B. Quantitative evaluation of the silent period, evoked by transcranial magnetic stimulation during sustained muscle contraction, in normal man and in patients with stroke. Electromyogr Clin Neurophysiol. 1992;32:373–378. [PubMed] [Google Scholar]
- 51.Classen J, Schnitzler A, Binkofski F, Werhahn KJ, Kim YS, Kessler KR, Benecke R. The motor syndrome associated with exaggerated inhibition within the primary motor cortex of patients with hemiparetic. Brain. 1997;120(Pt 4):605–619. doi: 10.1093/brain/120.4.605. [DOI] [PubMed] [Google Scholar]
- 52.Liepert J, Storch P, Fritsch A, Weiller C. Motor cortex disinhibition in acute stroke. Clin Neurophysiol. 2000;111:671–676. doi: 10.1016/s1388-2457(99)00312-0. [DOI] [PubMed] [Google Scholar]
- 53.van Kuijk AA, Pasman JW, Geurts AC, Hendricks HT. How salient is the silent period? The role of the silent period in the prognosis of upper extremity motor recovery after severe stroke. J Clin Neurophysiol. 2005;22:10–24. doi: 10.1097/01.wnp.0000150975.83249.71. [DOI] [PubMed] [Google Scholar]
- 54.Cohen LG, Hallett M. Methodology for non-invasive mapping of human motor cortex with electrical stimulation. Electroencephalogr Clin Neurophysiol. 1988;69:403–411. doi: 10.1016/0013-4694(88)90062-4. [DOI] [PubMed] [Google Scholar]
- 55.Thickbroom GW, Sammut R, Mastaglia FL. Magnetic stimulation mapping of motor cortex: factors contributing to map area. Electroencephalogr Clin Neurophysiol. 1998;109:79–84. doi: 10.1016/s0924-980x(98)00006-x. [DOI] [PubMed] [Google Scholar]
- 56.Traversa R, Cicinelli P, Bassi A, Rossini PM, Bernardi G. Mapping of Motor Cortical Reorganization After Stroke: A Brain Stimulation Study With Focal Magnetic Pulses. Stroke. 1997;28:110–117. doi: 10.1161/01.str.28.1.110. [DOI] [PubMed] [Google Scholar]
- 57.Carvalho TP, Buonomano DV. Differential effects of excitatory and inhibitory plasticity on synaptically driven neuronal input-output functions. Neuron. 2009;61:774–785. doi: 10.1016/j.neuron.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ziemann U. Paired pulse techniques. London: Oxford University Press; 2002. [Google Scholar]
- 60.Chen R, Tam A, Butefisch C, Corwell B, Ziemann U, Rothwell JC, Cohen LG. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol. 1998;80:2870–2881. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- 61.Orth M, Snijders AH, Rothwell JC. The variability of intracortical inhibition and facilitation. Clin Neurophysiol. 2003;114:2362–2369. doi: 10.1016/s1388-2457(03)00243-8. [DOI] [PubMed] [Google Scholar]
- 62.Wassermann EM, Pascual-Leone A, Valls-Sole J, Toro C, Cohen LG, Hallett M. Topography of the inhibitory and excitatory responses to transcranial magnetic stimulation in a hand muscle. Electroencephalogr Clin Neurophysiol. 1993;89:424–433. doi: 10.1016/0168-5597(93)90116-7. [DOI] [PubMed] [Google Scholar]
- 63.Catano A, Houa M, Noel P. Magnetic transcranial stimulation: clinical interest of the silent period in acute and chronic stages of stroke. Electroencephalogr Clin Neurophysiol. 1997;105:290–296. doi: 10.1016/s0924-980x(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 64.Fregni F, Boggio P, Mansur C, Wagner T, Ferreira M, Lima M, Rigonatti S, Marcolin M, Freedman S, Nitsche M, Pascual-Leone A. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16:1551–1555. doi: 10.1097/01.wnr.0000177010.44602.5e. [DOI] [PubMed] [Google Scholar]
- 65.Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. 2007;25:123–129. [PubMed] [Google Scholar]
- 66.Hummel F, Celnik P, Giraux P, Floel A, Wu W-H, Gerloff C, Cohen LG. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- 67.Liepert J. Motor cortex excitability in stroke before and after constraint-induced movement therapy. Cogn Behav Neurol. 2006;19:41–47. doi: 10.1097/00146965-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 68.Wittenberg GF, Bastings EP, Fowlkes AM, Morgan TM, Good DC, Pons TP. Dynamic course of intracortical TMS paired-pulse responses during recovery of motor function after stroke. Neurorehabil Neural Repair. 2007;21:568–573. doi: 10.1177/1545968307302438. [DOI] [PubMed] [Google Scholar]
- 69.Bolognini N, Vallar G, Casati C, Abdul Latif L, El-Nazer R, Williams J, Banco E, Macea DD, Tesio L, Chessa C, Fregni F. Neurophysiological and Behavioral Effects of tDCS Combined With Constraint-Induced Movement Therapy in Poststroke Patients. Neurorehabil Neural Repair. 2011 doi: 10.1177/1545968311411056. [DOI] [PubMed] [Google Scholar]
- 70.Kim Y-H, You SH, Ko M-H, Park J-W, Lee KH, Jang SH, Yoo W-K, Hallett M. Repetitive Transcranial Magnetic Stimulation-Induced Corticomotor Excitability and Associated Motor Skill Acquisition in Chronic Stroke. Stroke. 2006;37:1471–1476. doi: 10.1161/01.STR.0000221233.55497.51. [DOI] [PubMed] [Google Scholar]
- 71.Werhahn K, Conforto A, Kadom N, Hallett M, Cohen L. Contribution of the ipsilateral motor cortex to recovery after chronic stroke. Annals of Neurology. 2003;54:464–472. doi: 10.1002/ana.10686. [DOI] [PubMed] [Google Scholar]
- 72.Capaday C. Neurophysiological methods for studies of the motor system in freely moving human subjects. Journal of Neuroscience Methods. 1997;74:201–218. doi: 10.1016/s0165-0270(97)02250-4. [DOI] [PubMed] [Google Scholar]
- 73.Thickbroom GW, Byrnes ML, Mastaglia FL. Methodology and application of TMS mapping. Electroencephalogr Clin Neurophysiol Suppl. 1999;51:48–54. [PubMed] [Google Scholar]
- 74.Fischer T, Langner R, Birbaumer N, Brocke B. Arousal and attention: self-chosen stimulation optimizes cortical excitability and minimizes compensatory effort. J Cogn Neurosci. 2008;20:1443–1453. doi: 10.1162/jocn.2008.20101. [DOI] [PubMed] [Google Scholar]
- 75.Darling WG, Wolf SL, Butler AJ. Variability of motor potentials evoked by transcranial magnetic stimulation depends on muscle activation. Exp Brain Res. 2006;174:376–385. doi: 10.1007/s00221-006-0468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Di Lazzaro V, Profice P, Pilato F, Dileone M, Florio L, Tonali PA, Angelucci F. BDNF plasma levels in acute stroke. Neurosci Lett. 2007;422:128–130. doi: 10.1016/j.neulet.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 77.Ploughman M, Windle V, MacLellan CL, White N, Dore JJ, Corbett D. Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke. 2009;40:1490–1495. doi: 10.1161/STROKEAHA.108.531806. [DOI] [PubMed] [Google Scholar]
- 78.Qin L, Kim E, Ratan R, Lee FS, Cho S. Genetic variant of BDNF (Val66Met) polymorphism attenuates stroke-induced angiogenic responses by enhancing anti-angiogenic mediator CD36 expression. J Neurosci. 31:775–783. doi: 10.1523/JNEUROSCI.4547-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Edwards DJ. On the understanding and development of modern physical neurorehabilitation methods: robotics and non-invasive brain stimulation. J Neuroeng Rehabil. 2009;6:3. doi: 10.1186/1743-0003-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ward NS, Cohen LG. Mechanisms Underlying Recovery of Motor Function After Stroke. Arch Neurol. 2004;61:1844–1848. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Traversa R, Cicinelli P, Oliveri M, Giuseppina Palmieri M, Filippi MM, Pasqualetti P, Rossini PM. Neurophysiological follow-up of motor cortical output in stroke patients. Clin Neurophysiol. 2000;111:1695–1703. doi: 10.1016/s1388-2457(00)00373-4. [DOI] [PubMed] [Google Scholar]
- 82.Volpe BT, Krebs HI, Hogan N, Edelstein L, Diels C, Aisen M. A novel approach to stroke rehabilitation: Robot-aided sensorimotor stimulation. Neurology. 2000;54:1938–1944. doi: 10.1212/wnl.54.10.1938. [DOI] [PubMed] [Google Scholar]
- 83.Rohrer B, Fasoli S, Krebs HI, Hughes R, Volpe B, Frontera WR, Stein J, Hogan N. Movement smoothness changes during stroke recovery. J Neurosci. 2002;22:8297–8304. doi: 10.1523/JNEUROSCI.22-18-08297.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dylan J, Edwards MC, Thickbroom Gary W, Rykman Avrielle, Pascual-Leone Alvaro, Volpe Bruce T. Corticomotor connectivity without voluntary movement after spinal cord injury: A case report of transcranial magnetic stimulation-guided rehabilitation. in revision. [Google Scholar]
- 85.Stagg CJ, Bachtiar V, O'Shea J, Allman C, Bosnell RA, Kischka U, Matthews PM, Johansen-Berg H. Cortical activation changes underlying stimulation-induced behavioural gains in chronic stroke. Brain. 135:276–284. doi: 10.1093/brain/awr313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Birbaumer N, Murguialday AR, Cohen L. Brain-computer interface in paralysis. Curr Opin Neurol. 2008;21:634–638. doi: 10.1097/WCO.0b013e328315ee2d. [DOI] [PubMed] [Google Scholar]
- 87.Lotze M, Braun C, Birbaumer N, Anders S, Cohen LG. Motor learning elicited by voluntary drive. Brain. 2003;126:866–872. doi: 10.1093/brain/awg079. [DOI] [PubMed] [Google Scholar]
- 88.Fasoli SE, Krebs HI, Stein J, Frontera WR, Hogan N. Effects of robotic therapy on motor impairment and recovery in chronic stroke. Arch Phys Med Rehabil. 2003;84:477–482. doi: 10.1053/apmr.2003.50110. [DOI] [PubMed] [Google Scholar]
- 89.Volpe BT, Huerta PT, Zipse JL, Rykman A, Edwards D, Dipietro L, Hogan N, Krebs HI. Robotic devices as therapeutic and diagnostic tools for stroke recovery. Arch Neurol. 2009;66:1086–1090. doi: 10.1001/archneurol.2009.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Volpe BT, Krebs HI, Hogan N, Edelsteinn L, Diels CM, Aisen ML. Robot training enhanced motor outcome in patients with stroke maintained over 3 years. Neurology. 1999;53:1874. doi: 10.1212/wnl.53.8.1874. [DOI] [PubMed] [Google Scholar]
- 91.Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8:741–754. doi: 10.1016/S1474-4422(09)70150-4. [DOI] [PubMed] [Google Scholar]
- 92.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 93.Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 75:2176–2184. doi: 10.1212/WNL.0b013e318202013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hendricks HT, Zwarts MJ, Plat EF, van Limbeek J. Systematic review for the early prediction of motor and functional outcome after stroke by using motor-evoked potentials. Arch Phys Med Rehabil. 2002;83:1303–1308. doi: 10.1053/apmr.2002.34284. [DOI] [PubMed] [Google Scholar]
- 95.Curra A, Modugno N, Inghilleri M, Manfredi M, Hallett M, Berardelli A. Transcranial magnetic stimulation techniques in clinical investigation. Neurology. 2002;59:1851–1859. doi: 10.1212/01.wnl.0000038744.30298.d4. [DOI] [PubMed] [Google Scholar]
- 96.Bastings EP, Greenberg JP, Good DC. Hand Motor Recovery after Stroke: A Transcranial Magnetic Stimulation Mapping Study of Motor Output Areas and Their Relation to Functional Status. Neurorehabil Neural Repair. 2002;16:275–282. doi: 10.1177/154596802401105207. [DOI] [PubMed] [Google Scholar]
- 97.Alonso-Alonso M, Fregni F, Pascual-Leone A. Brain stimulation in poststroke rehabilitation. Cerebrovasc Dis. 2007;24 Suppl 1:157–166. doi: 10.1159/000107392. [DOI] [PubMed] [Google Scholar]
- 98.Bolognini N, Pascual-Leone A, Fregni F. Using non-invasive brain stimulation to augment motor training-induced plasticity. J Neuroeng Rehabil. 2009;6:8. doi: 10.1186/1743-0003-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brouwer BJBJ, Schryburt-Brown KK. Hand function and motor cortical output poststroke: are they related? Archives of Physical Medicine and Rehabilitation. 2006;87:627–634. doi: 10.1016/j.apmr.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 100.Müller-Dahlhaus JFM, Orekhov Y, Liu Y, Ziemann U. Interindividual variability and age-dependency of motor cortical plasticity induced by paired associative stimulation. Exp Brain Res. May;187(3):467–475. doi: 10.1007/s00221-008-1319-7. Epub 2008 Mar 5. 2008;- 187:- 75. [DOI] [PubMed] [Google Scholar]