Abstract
Bone morphogenetic proteins (BMPs) are potent secreted signaling factors that trigger phosphorylation of Smad transcriptional regulators through receptor complex binding at the cell-surface. Resulting changes in target gene expression impact critical cellular responses during development and tissue homeostasis. BMP activity is tightly regulated in time and space by secreted modulators that control BMPs extracellular distribution and availability for receptor binding. Such extracellular regulation is key for BMPs to function as morphogens and/or in the formation of morphogen activity gradients. Here, we review shuttling systems utilized to control the distribution of BMP ligands in tissue of various geometries, developing under different temporal constraints. We discuss the biological advantages for employing specific strategies for BMP shuttling and roles of varied ligand forms.
Introduction
BMPs are a functionally diverse group of secreted signaling factors belonging to the TGF-β superfamily. Originally identified in bone extracts as important inducers of bone deposition [1], BMPs are now recognized to mediate cellular communication between adjacent cells (short range) or cells far apart (long range) [2,3]. Misregulation of BMP signaling is associated with many developmental abnormalities and disease states highlighting the need for this pathway to be tightly regulated, especially within the extracellular space where ligands bind their receptors to initiate signaling.
Active BMP ligands are processed from a proprotein [4]. Secreted dimeric ligands bind to a multi-component signaling complex composed of at least two different types of transmembrane Ser/Thr kinase (Type I and Type II receptors). Different combinations of ligand homo- and heterodimers associate with different combinations of receptors to generate the signaling complex [4,5]. Following ligand binding, the Type I receptor kinase, activated by Type II transphosphorylation, phosphorylates the intracellular R-Smad transducer (Smad1, 5, or 8 in vertebrates, and Mad in Drosophila) [6,7]. The association of pR-Smad with other proteins including the related co-Smad allows accumulation in the nucleus and a direct transcriptional response.
BMPs are notoriously ‘sticky’ molecules [8], they bind their receptors with slow kinetics (reviewed in [9]), and their signal is short lived [3,10,11]. Yet in a number of instances BMPs have been shown to travel over multiple cell diameters to generate morphogen gradients with signaling maintained over long periods. How, then, do BMPs reach and signal to cells afar? The answer appears to be in the many molecules that bind BMPs in the extracellular space and prevent them from receptor-mediated internalization and degradation, increasing their lifetime and activity range. Here, we discuss the importance of extracellular factors and their ability to control the distribution of BMP ligands and their availability for receptor binding. We first review the Short gastrulation (Sog)/Chordin multi-component shuttling system and discuss the evolution of its components. Then, we briefly review other ‘shuttling’ mechanisms that involve different extracellular modulators as well as varied ligand forms.
Shuttling BMPs via Sog/Chordin
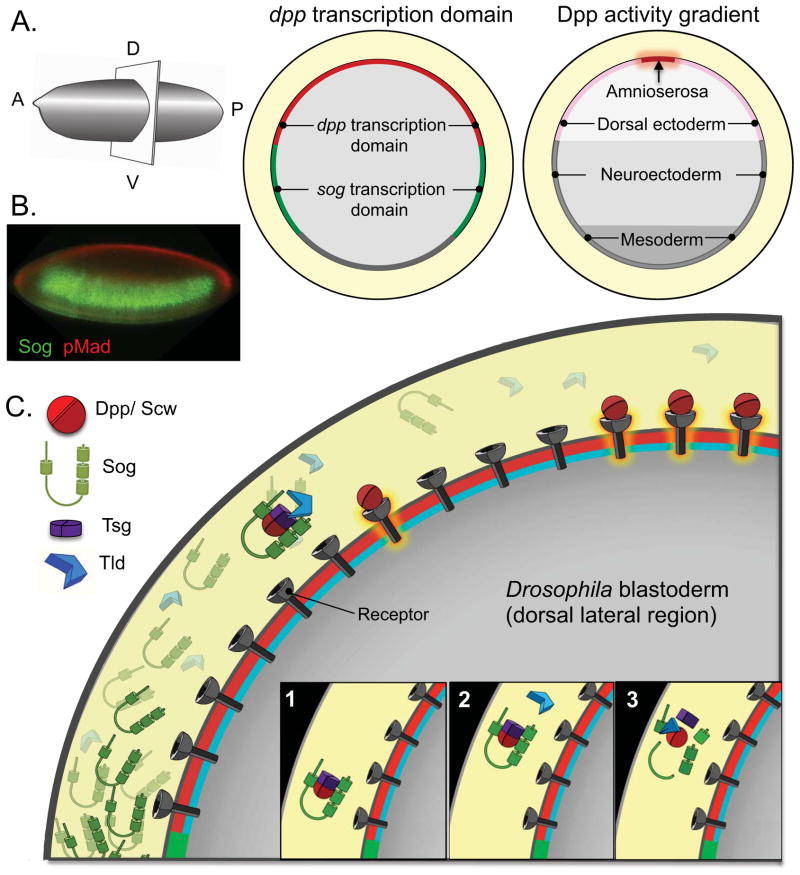
In the early Drosophila embryo, high levels of BMP signaling specify amnioserosa fates at the dorsal midline, lower levels specify dorsal ectoderm in the lateral regions, while neuronal cell fates arise in the absence of signaling [12–16]. Similarly, specification along the D/V axis in frog (Xenopus laevis) and zebrafish (Danio rerio) embryos depends on a gradient of BMP signaling [17–19]. In all cases, positive intracellular feedback sharpens the boundaries of distinct BMP signaling domains and ensures reproducible cell fate allocation and tissue size [16,20].
In the early Drosophila embryo, decapentaplegic (dpp), encoding a BMP2/4 homologue, is uniformly transcribed throughout the dorsal domain, yet a narrow domain of high BMP signaling is seen along the dorsal midline. Dpp secreted into the perivitelline space is rapidly (30 min) concentrated extracellularly at the dorsal midline where a peak of phosphorylated Mad (pMad) is generated [20,21]. This rapid concentration of BMP signaling relies on the spatial redistribution of ligands by several extracellular modulators (Figure 1). The dorsal domain of dpp expression is flanked laterally by ventrally expressed short gastrulation (sog), which encodes a Dpp binding protein [22]. Sog binds Dpp via its Cysteine-rich (CR) von Willebrand factor type C domains to locally inhibit signaling and to prevent movement ventrally. At the same time, this complex protects Dpp from degradation and receptor-mediated internalization, thus, facilitating long-range ligand shuttling of Dpp from the lateral and dorsal domains to the dorsal midline [20–22]. Sog has a short-range negative role on BMP signaling since it inhibits the access of ligands to receptors; at the same time Sog has a long-range positive effect on BMP signaling as it facilitates Dpp movement. In the absence of Sog, BMP signaling levels remain uniformly low with a failure to specify the amnioserosa. Similarly, in the pupal wing Sog abuts and limits the region of BMP signaling where it is also required to create a domain of high BMP signaling in the posterior crossvein (PCV)[23–25]. Dpp is produced by the longitudinal proveins but is moved into the PCV competent zone to create a corridor of high signaling.
Figure 1. Sog-mediated BMP shuttling in the early Drosophila embryos requires several secreted modulators.
(A) Diagrams of dpp and sog transcription domains and cell fate maps are shown in cross-sections of an early Drosophila embryo, oriented with dorsal side up and anterior to the left. (B) Confocal image of a blastoderm stage Drosophila embryo immunostained against pMad (red) and Sog (green). pMad signals are high in the dorsal-most domain (with high level of BMP signaling) but drop sharply below the limit of detection in the dorsal-lateral regions (with low levels of BMP signaling). Sog is produced in ventral-lateral regions and diffuses dorsally in the perivitelline space to form an extracellular gradient with a range controlled by Tlds activities [94]. (C) Cartoon representation of the steps of BMP gradient formation in the early Drosophila embryos. Ventrally secreted Sog makes a complex with BMPs that inhibits BMP signaling from spreading into the lateral domain. This complex also protects BMPs from degradation and receptor binding and internalization and allows them to diffuse. The assembly of Sog-BMPs complex uses collagen IV as a scaffold [38]; binding of Tsg releases the complex from collagen IV and promotes its movement [39]. In the dorsal domain, the complexes will encounter Tld, which cleaves Sog releasing the ligands. Released BMPs have 2 possible fates: they can bind to receptors and signal, or they can be captured by another complex. When the Sog levels are high, as in the lateral domain, the probability of recapture is high, whereas at the midline, BMPs are more likely to bind to receptors and signal. Multiple cycles of complex formation (1), diffusion (2) and destruction by Tld (3) generate a net movement of BMPs from the lateral domain towards the midline. An animated description of this shuttling process is available [49].
In both instances two BMP family members are required for normal patterning raising the possibility that BMP heterodimers are involved. Heterodimers have been reported to exhibit increased signaling ability [21,26–29]. The mechanism underlying these differences in activity is currently under intense investigation [30]. In the early fly embryo a combination of Dpp, Screw (Scw) homodimers and Dpp:Scw heterodimers are thought to pattern the dorsal domain [20,21,31], while in the pupal wing, Dpp and Glass bottom boat (Gbb) are each essential for PCV formation [32,33]. Interestingly, BMP heterodimers have a higher affinity for Sog than the homodimers and thus, are favored for the long-range transport. In fact, Gbb appears required for the movement of Dpp into the PCV competent zone [24]* where BMP signaling induces dpp transcription [25]. Given the widespread expression of gbb, this activation of dpp expression could then trigger the secretion of Dpp:Gbb to enhance of signaling in these cells.
The Sog-BMP shuttling complex requires at least one additional molecule, Twisted Gastrulation (Tsg) in the early embryo, and a related molecule Tsg2/Cv in the pupal wing [21,34–36]. Tsg proteins form complexes with Sog that are more efficient for BMP binding and long-range shuttling. A molecule resembling half-Tsg, Shrew (Srw), is also required for high levels BMP signaling in the early embryo [37]. The assembly of the shuttling complex is aided by collagen IV, which functions as a scaffold for BMP-Sog binding [38]. Binding of Tsg appears to release the BMP-Sog complex from collagen IV and promote its movement [39]**.
Strikingly, BMP shuttling as a strategy is highly conserved across the phyla suggesting an ancient origin for BMP signal modulation using opposing BMP and BMP antagonist gradients. In zebrafish and frog embryos, BMP4 and 7 are expressed and secreted from ventral tissue whereas a specialized dorsal tissue known as Spemann’s organizer secretes the Sog homologue Chordin together with other BMP-binding proteins such as Follistatin, Noggin and Cerberus to modulate BMP activity (reviewed in [17,18]).
Tolloids release BMPs from the shuttle complexes
A key component that helps create the flux is the processing of Sog by BMP-1 family metalloproteases, Tolloid (Tld) and Tolloid-related (Tlr) or Tolkin [23,40–45]. Upon cleavage of Sog, the released BMP ligands can bind receptors, or be re-captured by Sog. Sog concentrations are high in lateral regions of the early embryo and the probability of BMP re-capture is high, whereas in the dorsal most region where Sog levels are low, released ligands are more likely to find receptors. Reiterated cycles of complex assembly, diffusion and destruction produce a physical re-distribution of the BMP ligands with an increase in the domains of high BMP signaling [21,24]. The embryonic and pupal BMP signaling gradients differ in their spatial and temporal constraints, yet in both cases a balance between the inhibitory and positive activities of Sog remains crucial for proper patterning. This balance is kept by Tld in the early embryo and Tlr in the pupal wing. Tld and Tlr cannot substitute for each other and appear to have matched their catalytic properties to the temporal requirements of their respective developmental windows [23]. For example, Tlr processes Sog with at least one order of magnitude slower kinetics than Tld, while the more rapid kinetics of Tld are matched to the rapid development of the embryo.
Mathematical models indicate that lower rates of Tld processing result in sharper signaling distributions and with a greater net transport of BMP ligands away from the Sog/Chordin source [46]. Furthermore, in order for the shuttling model to meet the requirements for scale invariance of Xenopus embryos or robustness of Drosophila embryos, Sog/Chordin must be bound by a BMP ligand for Tlds processing [15,47,48]. In fact, Drosophila Sog cannot be cleaved by Tlds in the absence of BMP, an obligatory co-substrate [23,41]. In contrast, vertebrate Chordin can be processed in the absence of BMPs, albeit the processing rate is enhanced in their presence [42]. An obligatory co-substrate for Sog processing is thought to reflect a BMP-induced conformational modification allowing Sog-BMP, but not Sog alone, to fit into the Tlds catalytic pocket. Identification of Sog processing sites and their comparison with Chordin revealed the molecular basis for BMP-dependent cleavage by Tlds [49]** (Figure 2). Interestingly, aromatic substitutions at Sog processing sites produced a “Chordin-like” Sog that was processed by Tld independently of the BMP co-substrate, and was more prone to Tld-destruction at lower BMP levels. Whether aromatic residues in the Tlds catalytic domain confer substrate selectivity remains to be demonstrated, although high conservation is observed of such residues across phylogenetic lineages (Figure 2).
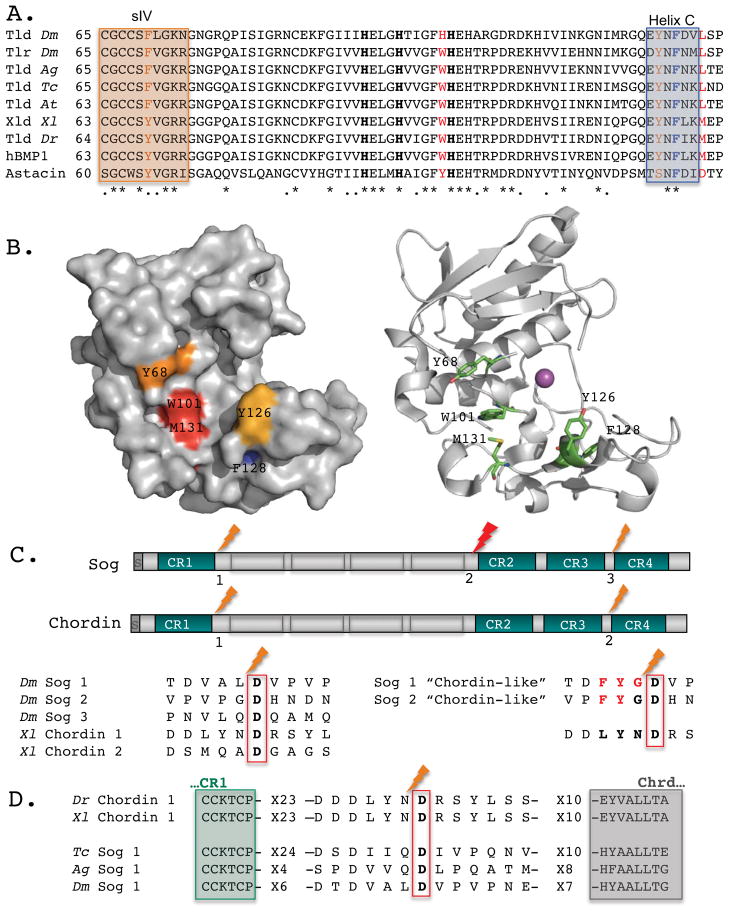
Figure 2. Tlds catalytic domains and their processing sites in Sog/Chordin.
(A) ClustalW alignment reveals highly conserved aromatic residues within the catalytic domain of Tld-type enzymes in Drosophila melanogaster (Dm), Anopheles gambiae (Ag), Tribolium castaneum (Tc), Achaearanea tepidariorum (At), Xenopus laevis (Xl), Danio rerio (Dr), and humans (hBMP-1) as compared with the crayfish astacin. Three zinc-binding His residues are shown in bold. Crystal structure of hBMP-1 catalytic domain indicates that P2 residue of the substrate extends towards Trp101 and Met131, shown in red [95]. Boxes mark the β-sheet strand (sIV) on the upper side of the catalytic pocket and Helix C on the lower side. These motifs contain additional aromatic residues that may come in close proximity to P3 (brown). Helix C also includes F128 (blue), a residue mutated in autosomal recessive osteogenesis imperfecta [96]. (B) A surface diagram of hBMP-1 catalytic domain (left) and a ribbon diagram (right) capture the cavity where P3 and P2 residues bind. The conserved residues discussed are color coded (left) or shown as sticks colored by elements (right) (C, green; O, red; N, blue). The catalytic zinc is shown as a magenta sphere. (C) Drosophila Sog and Xenopus Chordin shared similar organization, with four Cysteine-rich von Willenbrandt factor C domains (CR) separated by four “Chordin-like” (Chrd) motifs. The Tld-processing sites in Sog and Chordin show little conservation besides the S1′ Asp residue, a hallmark of this family of protease. Several residues (P1–P3) are responsible for making Sog dependent on BMP for Tld processing, while Chordin is not. Changes at these positions (shown in red) make Sog a BMP-independent substrate for Tld, “Chordin-like” [49]. (D) Comparison of Tld processing sites, residing between CR1 and Chrd repeats, suggests that Tribolium and Anopheles Sog are BMP-dependent for their processing, resembling the Drosophila Sog.
The impact of the Tld-mediated Sog processing on the profile of BMP gradients in early Drosophila embryos was demonstrated by replacement of endogenous Sog with “Chordin-like” variants [49]. The normal steep BMP signaling gradient in early Drosophila embryos changed to a shallower profile, analogous to that observed in some vertebrate embryos, with accompanying changes in cell fate and tissue size. The resulting expansion of dorsal-most amnioserosa cell fates could be partially rescued with multiple copies of “Chordin-like” Sog that increase BMP transport, but the boundary of the high BMP signaling domain remain diffuse and variable between individuals of the same genotype. Mathematical simulations showed that only a model with a modest increase in “BMP-free-Sog” processing by Tld could fit these experimental data. In contrast, embryos with only one copy of sog have a broader domain of high BMP signaling and expanded amnioserosa, but their BMP signaling gradients remain sharp, step-like. Thus, the acquisition of BMP-dependent Sog processing during evolution appears to facilitate long-range ligand diffusion and formation of robust BMP morphogen gradients required for early Drosophila patterning. Furthermore, Drosophila Sog has evolved to include a third processing site, right before its second CR domain (Figure 2), also BMP-dependent. This additional site appears to be a bottleneck for Tlds-mediated Sog destruction and its processing is highly enhanced by addition of BMPs and Tsgs [49,50].
Sog/Chordin – and evolution of shuttling mechanisms
An intriguing question is why only Drosophila has evolved such an intricate mechanism to ensure formation of BMP gradients while the other phylogenetic lineages did not. A possible explanation lies in the features and the constraints of this system. The Drosophila embryo has already attained its anterior-posterior and D/V axes during oogenesis. The early stages proceed in a syncytium of rapidly dividing nuclei with cells forming only during the last division before the immediate onset of gastrulation. In vertebrate embryos and even in other arthropods, such as spiders (Achaearanea), these early events are more protracted, and formation of BMP gradients occurs over longer time frames and relies on cell-cell communication, as opposed to the limited cellular input and shorter developmental window in early Drosophila embryos. As such, a set of genes arising by duplication and subsequent divergence has been proposed to have enabled BMP-mediated patterning to occur given the cellular constraints imposed by the syncytial nature and rapidity of early development in higher Diptera [51]**. None of these “dedicated” genes are found in the genome of short band insect Tribolium castaneum (Coleoptera) or in Apis mellifera (Hymenoptera). Tribolium has only a Tlr-like protease and lacks the faster acting protease Tld, required in early Drosophila patterning. Similarly Tribolium, as well as frog and fish, have one Tsg, whereas two Tsg-like molecules are required in Drosophila, one for early patterning, one for pupal development, and an additional half-Tsg, Srw, with no known homologue in other systems, also required for early Drosophila patterning. Finally Scw, a Gbb/BMP7-like ligand with no homologue in Tribolium and Apis, fulfills new signaling modes in the higher Diptera while retaining the ability to function in various Gbb-dependent processes in Drosophila [51,52]. Interestingly, the gain of “dedicated” genes in higher Diptera was accompanied by loss of several other BMP modulators including BAMPI and DAN [51].
Phylogenetic analyses have placed the origin of Scw between mosquitos (Culicomorpha) and the higher Diptera, suggesting that the origin of Scw coincides with a reduction in the extraembryonic membranes observed in higher Diptera [51,53]. In lower Diptera and other insects the dorsal ectoderm is expanded and the dorsal midline is subdivided into amnion and serosa lineages, where a broadened domain of higher BMP signaling is observed (reviewed in [54]). Nonetheless, Sog plays a role in dorsal enrichment of Dpp in Tribolium and in aspects of the Dpp signaling profile in Anopheles [55,56], but not in Dpp transport during axis specification in the spider Achaearanea [57]. A preliminary inspection reveals that the putative proximal processing sites in Tribolium and Anopheles Sog are more similar to the Drosophila Sog consensus than to that of vertebrates Chordin (Figure 2). Further biochemical analysis is needed to determine if and in what lineages Tld-mediated processing of Sog requires an obligatory BMP co-substrate. Nonetheless, these comparisons raise the possibility that the BMP-dependence of Sog processing preceded the reduction of extraembryonic membranes observed in higher Diptera and arose earlier as an efficient BMP-mediated transport required for robust patterning. A ‘Chordin-like’ Sog cannot ensure robust Drosophila patterning [49]. Also, Gbb or Tlr, the ancestral BMP pathway components, cannot replace Scw or Tld during early patterning [23,58]*. Thus, acquisition of BMP-dependence for Sog destruction by Tlds may represent a process of canalized development.
Cv-2, an ancient player that helps refine BMP morphogen gradients
If BMP shuttling in the Drosophila PCV competent zone during pupal development constitutes the ancestral function, we expect that some conserved modulators would be used at this stage but not during early development. One such example is Crossveinless-2 (Cv-2) or BMPER in vertebrates, conserved from fly to humans (reviewed in [9]). Named for its role in modulating BMP signaling during PCV formation, Cv-2 is not required for embryonic patterning [25,33,59]. Like Sog, Cv-2 binds BMPs via CR domains (von Willebrandt factor C) and exhibits both BMP agonistic and antagonist activities. Unlike Sog, Cv-2 does not mediate long-range shuttling of BMP ligands and acts locally within the crossvein [9,59]. Cv-2 binds to cell surfaces via heparan sulfate proteoglycans (HSPGs) (Dally) and by associating with the Type I BMP receptor [59,60]. The binding of Cv-2 to Type I receptors can increase the flow of BMPs to the receptors, or sequester them into inactive BMP/Cv-2/receptor complexes depending on BMP-receptor binding affinities. BMP ligands with high receptor affinity (such as Dpp) will be pulled into inactive complexes, while low affinity ligands (such as Gbb) will be recruited into close proximity to the receptors [59]. Since Cv-2 also binds to Sog/Chordin-Tsg complexes, BMPs released from a Sog/Chordin shuttle could be concentrated at the cell surface by binding to Cv-2 (then available for exchange with the receptors) [60]. In an alternative model, the “sink” model, Cv-2 can locally reduce the concentration of soluble complexes by binding to Sog/Chordin with or without BMPs, acting as a sink to increase flow towards Cv-2 expressing cells [61,62]*.
Cv-2 is also a transcriptional target of BMP signaling that helps refine the BMP morphogen gradient in the developing pupal wing and could provide the positive feedback/spatial bi-stability required for sharp, step gradients [59,63]. Molecules like Cv-2 could also buffer signaling noise and attenuate stochastic fluctuations in models of BMP-mediated cell signaling [64]*.
It could all begin with a diverse pool of ligands
In many cases BMPs do not need to be moved over long distances but instead act locally [3]. In the numerous roles for Dpp and Gbb throughout Drosophila development, both local or restricted signaling and long range signaling has been observed [65–73] and our understanding of the relative contributions of each ligand is still evolving. In the wing imaginal disc where Dpp has long been thought to act as the quintessential diffusible morphogen, in fact in the absence of Gbb, Dpp is unable to elicit a response far beyond the cells from which it is produced [74,75]**. Furthermore, the range may depend on particular ligand variants.
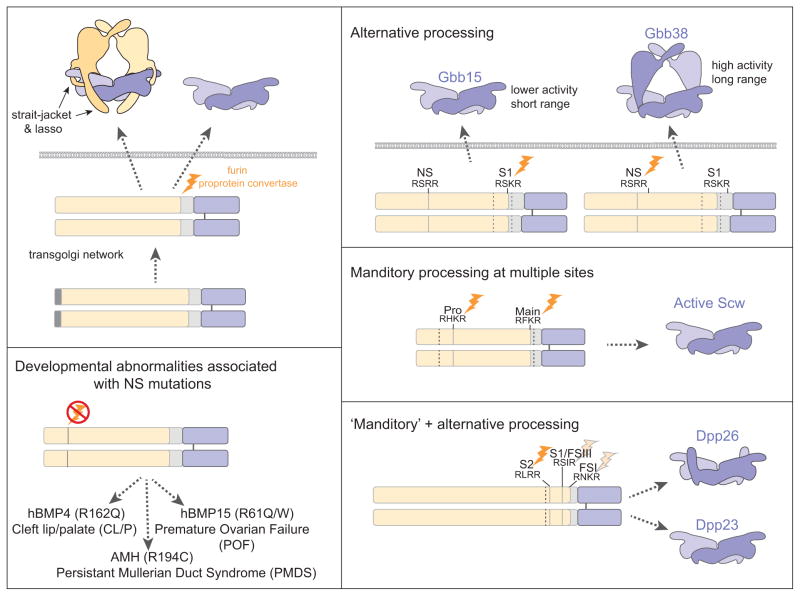
In actuality BMP signaling begins with the production of the dimeric ligand (Figure 3). New studies have revealed that this initial step is likely to be a key point in the regulation of BMP signaling. Sequential proconvertase processing of BMP4 within the linker between prodomain and C-terminal ligand domain influences signaling activity [76,77]. Dpp follows a similar pattern of cleavage as BMP4. A Dpp variant produced by only one cleavage rescues loss of dpp signaling between adjacent cell layers in the embryonic midgut but not in imaginal discs where Dpp is thought to act over longer distances [78,79]*. In the case of Drosophila BMPs, Scw and Gbb, processing at alternative furin cleavage sites also influences activity, but here, one site (NS) resides within the less conserved prodomain [58,75]. Active Gbb ligands significantly different in size (Gbb38 and Gbb15) are produced by cleavage at either the NS site or the conventional S1 site, but this does not hold true for Scw. In vivo analyses indicate that that the large variant, Gbb38, exhibits a long range while Gbb15 does not, and Gbb15 is enriched in tissues where Gbb is known to act at short range [73,75]. Biochemical and structural studies have identified a core or ‘arm’ domain with a conserved fold that is contributed by the prodomain [80]*. NS processing would leave this ‘prodomain core’ or arm domain associated with the cysteine knot ligand domain but remove the ‘strait jacket’ and lasso that interfere with receptor binding and confer latency in proTGF-β [81]**. Significantly, mutations in an NS consensus sequence in human BMP4, BMP15 and AMH are associated with specific developmental abnormalities, cleft lip/palate (CL/P, premature oovarian failure (POF) and persistent Müllerian duct syndrome (PMDS), respectively [75]** (Figure 3).
Figure 3. Alternative furin proconvertase processing generates BMP variants.
(A) TGF-β/BMP superfamily ligands are synthesized as preproproteins and processed by furin proprotein convertases after dimerization. The highly conserved C-terminal domain (blue) defining the family are secreted alone or in complex with the less conserved prodomain (yellow). In many cases prodomain association renders the ligand inactive, as suggested by the ability of the strait jacket and lasso motifs of the TGF-β latent protein structure to interfere with receptor binding [81]. (B, C) An alternative cleavage site within the prodomain of Gbb (NS) and Scw (Pro) is critical for full ligand activity. Differential cleavage of Gbb proprotein at the NS and the conventional S1 site gives rise to two ligand variants with varying activities in vivo (B) [75]. In contrast production of an active Scw ligand requires cleavage at both sites (pro and main) [58]. (D) Point mutations with the putative NS site of hBMP4, hBMP15 and AMH are associated with human developmental abnormalities [75]. (E) Of three furin cleavage sites identified in Dpp, cleavage at S2 is essential with alternative processing that gives rise to two Dpp variants with differentially signaling abilities [78,79].
What function could the added prodomain core serve in a large BMP variant not seen in the conventional small BMP? In vivo studies suggest a difference in signaling range [75]**. This prodomain core could influence binding of antagonists, such as Noggin/Chordin/Sog, and thus, indirectly impact ligand range. Or it could more directly mediate ligand distribution by interacting with extracellular factors. The differential interaction of BMP-5, 7 and GDF-8 prodomains with fibrillin and perlecan has been reported [82]*, although the consequences of such interactions on ligand distribution and activity remain to be investigated. Alternatively, the prodomain core could influence receptor complex formation as the BMP7 prodomain has been shown to impact Type II and Type I receptors interactions [83].
Other modes of moving BMP ligands in the extracellular space
The distribution of BMP ligands is not only affected by the constellation of BMP variants but also by an increasing number of extracellular modulators distinct from the Sog/Chordin family. Of particular interest in the wing primordium are the GPI-linked Drosophila glypicans, Dally and Dally-like protein (Dlp), and two secreted molecules, Pentagone (Pent) and Larval Translucida (Ltl) that appear to provide a ‘BMP shuttle’ function while ensuring tight feedback controls (reviewed in [84]) (Figure 4). The ability of Dally to stabilize Dpp at the cell surface in trans, has led to a model by which BMPs are handed off from one cell to another in a series of glypican-BMP association-dissociations [85]**. Dally and Dlp exhibit different specificities for Dpp and Gbb. With their different spatial distributions one can envision that these glypicans could act to corral different dimer types. Interestingly, HSPGs also function in more confined settings to regulate BMP signaling, such as in synapse development at the larval NMJ, where Dlp and Syndecan both influence the accumulation and distribution of Gbb [86]*.
Figure 4. Extracellular factors + feedback regulate BMPs in the wing disc.
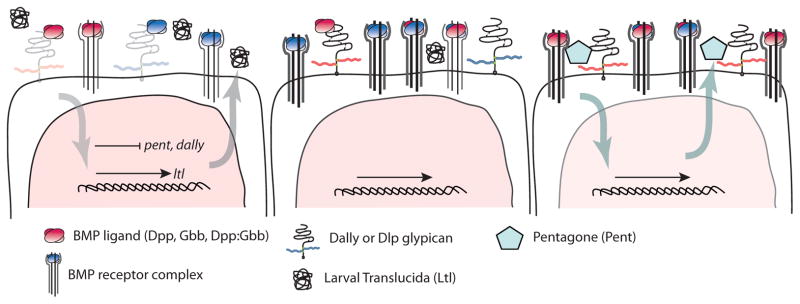
A gradient of BMP signaling (pink nucleus) is established in the medial (left) to lateral (right) wing imaginal disc. Both Dpp and Gbb are essential for gradient formation and wing patterning [74]. Visualizing the spatial distribution of homodimers and putative heterodimers has been impeded by the recognition that tagging either ligand with fluorescence proteins impacts activity most likely due to its effect on protein-ligand interactions that could alter movement [75]. Nevertheless differential interactions between glypicans (Dally and Dlp) and BMPs (Dpp and Gbb) could affect their spatial distribution. Ltl is abundant in the medial disc to facilitates signaling while Pent is present in the lateral region and is necessary in those cells for the breadth of signaling, while it hinders signaling in the medial cells [87,88]. BMP signaling-dependent transcriptional regulation is responsible for the spatial distribution of these BMP modulators. High levels of BMP signaling in the medial cells (left) result in a down regulation of dally, pent and the type I receptor tkv but an up regulation of ltl (see [84]). Low levels of signaling in the lateral cells enable high levels of Pent and the glypicans.
In contrast to cell-bound Dally and Dlp, Pent and Ltl are secreted. Their expression is regulated by BMP signaling, pent, negatively, and ltl, positively. Pent acts to broaden the domain of BMP signaling and Dally is the likely mediator of Pent’s influence on BMP signaling [87]*. Ltl, on the other hand is expressed in the medial domain of the wing disc and acts to limit signaling [88]*. Ltl acts similarly to Cv-2 and it too, may act through glypicans, as ltl has been shown to genetically interact with dlp. While the dynamics of these interactions are not yet known, it is clear that this extracellular control of ligand distribution and availability is reinforced by feedback controls, a reoccurring theme in many BMP-controlled processes [84,89]**. Another completely different mode of regulation occurring during PCV formation involves a vitellogenin-like protein, Cv-d, that makes its way from the fat body through the hemolymph to the pupal wing to bind BMPs and enable signaling [90]*.
Perspectives
Many factors that regulate the availability of BMP ligands in the extracellular space have being identified. Some of them work through complex mechanisms such as Sog/Chordin-mediated shuttling, others through mechanisms that appear less elaborate but serve the same general purpose to protect BMPs from receptor-mediated internalization and turnover, and allow them to reach the cells in need of signal. Do the different molecules and mechanisms co-opted for ‘shuttling’ BMPs in the early Drosophila embryo vs the developing wing reflect fundamental differences in the cellular environment and time over which a BMP signaling gradient is built and maintained to accomplish its task in development? Sog-mediated BMP shuttling may be necessary for the rapid generation of a steep gradient with a high amplitude while a ‘capture and release’ mechanism with multiple feedback controls is better suited for coordinating tissue growth and longer term maintenance of graded signaling [89,91]**. In light of the role of Dally and Dlp as modulators of BMP signaling in the wing disc, it is interesting that in the early embryo a translational block is in place that prevents HSPG synthesis and thus, eliminates this point of regulation from the mix of molecules available for interaction during early BMP shuttling [92].
As the molecular details of the various BMP shuttling systems emerge we are beginning to understand how they have been exploited for diversified patterning during evolution. However, a number of issues remain unresolved. For example when did signaling by BMP heterodimers appear during evolution? Heterodimers seem to pattern early embryos in insects such as Drosophila, but not Tribolium or Apis mellifera. More importantly, how do heterodimers signal synergistically? Are two Type I BMP receptors needed? Two Type I receptors are also required at the Drosophila NMJ even though only one ligand, Gbb, mediates signaling [72,93]. How do Sog and BMPs have access to collagen IV scaffolding surfaces? The sites for Dpp binding were mapped to residues within NC1 domain, in a region involved in multimerization and formation of the characteristic chicken-wire structure [38]. Such residues would be presumably inaccessible unless some NC1 domain(s) are removed to expose the scaffolding surfaces. Also, Tlds are secreted enzymes yet Tld acts cell autonomously in the early Drosophila embryos [20]. Moving Tld enzymatic activity from a soluble phase to the cell surfaces is expected to greatly enhance the efficiency of proteolytic activity, but the way this is achieved remains a mystery. The early Drosophila embryos utilizes positive feedback for sharpening BMP morphogen gradients [20]. What are the molecular determinants for such positive feedback? How does a system achieve a fine balance between perfecting elaborate BMP shuttle complexes and recruiting multiple feedback mechanisms to modulate BMP signaling in time and space? Mathematical models have been instrumental in describing the dynamics of BMP tissue distribution as well as the dynamics of BMP reception at the level of a single cell. As mathematical models become more comprehensive, the field will increasingly engage them to describe and predict the many layers of regulation of BMP signaling.
Acknowledgments
We apologize to authors whose work was not cited due to space constraints. This work was supported in part by the Intramural Research Program at NIH. K.A.W. acknowledges support from NIH RO1GM068118 and a Cali Family Development Grant from The Center for Research in FOP and Related Disorders in the Department of Orthopedic Surgery at The Perelman School of Medicine of The University of Pennsylvania.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 2.Hogan BL. Bone morphogenetic proteins in development. Curr Opin Genet Dev. 1996;6:432–438. doi: 10.1016/s0959-437x(96)80064-5. [DOI] [PubMed] [Google Scholar]
- 3.Ramel MC, Hill CS. Spatial regulation of BMP activity. FEBS Lett. 2012;586:1929–1941. doi: 10.1016/j.febslet.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 4.Massague J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- 5.Ehrlich M, Gutman O, Knaus P, Henis YI. Oligomeric interactions of TGF-beta and BMP receptors. FEBS Lett. 2012;586:1885–1896. doi: 10.1016/j.febslet.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 6.Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 7.Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 8.Urist MR, Huo YK, Brownell AG, Hohl WM, Buyske J, Lietze A, Tempst P, Hunkapiller M, DeLange RJ. Purification of bovine bone morphogenetic protein by hydroxyapatite chromatography. Proc Natl Acad Sci U S A. 1984;81:371–375. doi: 10.1073/pnas.81.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umulis D, O’Connor MB, Blair SS. The extracellular regulation of bone morphogenetic protein signaling. Development. 2009;136:3715–3728. doi: 10.1242/dev.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connor MB, Umulis D, Othmer HG, Blair SS. Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development. 2006;133:183–193. doi: 10.1242/dev.02214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tucker JA, Mintzer KA, Mullins MC. The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev Cell. 2008;14:108–119. doi: 10.1016/j.devcel.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson EL, Anderson KV. Decapentaplegic acts as a morphogen to organize dorsal-ventral pattern in the Drosophila embryo. Cell. 1992;71:451–461. doi: 10.1016/0092-8674(92)90514-d. [DOI] [PubMed] [Google Scholar]
- 13.Wharton KA, Ray RP, Gelbart WM. An activity gradient of decapentaplegic is necessary for the specification of dorsal pattern elements in the Drosophila embryo. Development. 1993;117:807–822. doi: 10.1242/dev.117.2.807. [DOI] [PubMed] [Google Scholar]
- 14.Sutherland DJ, Li M, Liu XQ, Stefancsik R, Raftery LA. Stepwise formation of a SMAD activity gradient during dorsal-ventral patterning of the Drosophila embryo. Development. 2003;130:5705–5716. doi: 10.1242/dev.00801. [DOI] [PubMed] [Google Scholar]
- 15.Mizutani CM, Meyer N, Roelink H, Bier E. Threshold-dependent BMP-mediated repression: a model for a conserved mechanism that patterns the neuroectoderm. PLoS Biol. 2006;4:e313. doi: 10.1371/journal.pbio.0040313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorfman R, Shilo BZ. Biphasic activation of the BMP pathway patterns the Drosophila embryonic dorsal region. Development. 2001;128:965–972. doi: 10.1242/dev.128.6.965. [DOI] [PubMed] [Google Scholar]
- 17.De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Little SC, Mullins MC. Extracellular modulation of BMP activity in patterning the dorsoventral axis. Birth Defects Res C Embryo Today. 2006;78:224–242. doi: 10.1002/bdrc.20079. [DOI] [PubMed] [Google Scholar]
- 19.Raible DW. Development of the neural crest: achieving specificity in regulatory pathways. Curr Opin Cell Biol. 2006;18:698–703. doi: 10.1016/j.ceb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Wang YC, Ferguson EL. Spatial bistability of Dpp-receptor interactions during Drosophila dorsal-ventral patterning. Nature. 2005;434:229–234. doi: 10.1038/nature03318. [DOI] [PubMed] [Google Scholar]
- 21.Shimmi O, Umulis D, Othmer H, O’Connor MB. Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo. Cell. 2005;120:873–886. doi: 10.1016/j.cell.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francois V, Solloway M, O’Neill JW, Emery J, Bier E. Dorsal-ventral patterning of the Drosophila embryo depends on a putative negative growth factor encoded by the short gastrulation gene. Genes Dev. 1994;8:2602–2616. doi: 10.1101/gad.8.21.2602. [DOI] [PubMed] [Google Scholar]
- 23.Serpe M, Ralston A, Blair SS, O’Connor MB. Matching catalytic activity to developmental function: tolloid-related processes Sog in order to help specify the posterior crossvein in the Drosophila wing. Development. 2005;132:2645–2656. doi: 10.1242/dev.01838. [DOI] [PubMed] [Google Scholar]
- 24*.Matsuda S, Shimmi O. Directional transport and active retention of Dpp/BMP create wing vein patterns in Drosophila. Dev Biol. 2012;366:153–162. doi: 10.1016/j.ydbio.2012.04.009. Shows that Dpp is actively retained in the longitudinal veins by the BMP type I receptor and a positive feedback mechanisms and that Dpp movement in the PCV region requires Gbb. [DOI] [PubMed] [Google Scholar]
- 25.Ralston A, Blair SS. Long-range Dpp signaling is regulated to restrict BMP signaling to a crossvein competent zone. Dev Biol. 2005;280:187–200. doi: 10.1016/j.ydbio.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Israel DI, Nove J, Kerns KM, Kaufman RJ, Rosen V, Cox KA, Wozney JM. Heterodimeric bone morphogenetic proteins show enhanced activity in vitro and in vivo. Growth Factors. 1996;13:291–300. doi: 10.3109/08977199609003229. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki A, Kaneko E, Maeda J, Ueno N. Mesoderm induction by BMP-4 and -7 heterodimers. Biochem Biophys Res Commun. 1997;232:153–156. doi: 10.1006/bbrc.1997.6219. [DOI] [PubMed] [Google Scholar]
- 28.Zhu W, Kim J, Cheng C, Rawlins BA, Boachie-Adjei O, Crystal RG, Hidaka C. Noggin regulation of bone morphogenetic protein (BMP) 2/7 heterodimer activity in vitro. Bone. 2006;39:61–71. doi: 10.1016/j.bone.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Little SC, Mullins MC. Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat Cell Biol. 2009;11:637–643. doi: 10.1038/ncb1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isaacs MJ, Kawakami Y, Allendorph GP, Yoon BH, Izpisua Belmonte JC, Choe S. Bone morphogenetic protein-2 and -6 heterodimer illustrates the nature of ligand-receptor assembly. Mol Endocrinol. 2010;24:1469–1477. doi: 10.1210/me.2009-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arora K, Levine MS, O’Connor MB. The screw gene encodes a ubiquitously expressed member of the TGF-beta family required for specification of dorsal cell fates in the Drosophila embryo. Genes Dev. 1994;8:2588–2601. doi: 10.1101/gad.8.21.2588. [DOI] [PubMed] [Google Scholar]
- 32.Ray RP, Wharton KA. Context-dependent relationships between the BMPs gbb and dpp during development of the Drosophila wing imaginal disk. Development. 2001;128:3913–3925. doi: 10.1242/dev.128.20.3913. [DOI] [PubMed] [Google Scholar]
- 33.Conley CA, Silburn R, Singer MA, Ralston A, Rohwer-Nutter D, Olson DJ, Gelbart W, Blair SS. Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development. 2000;127:3947–3959. doi: 10.1242/dev.127.18.3947. [DOI] [PubMed] [Google Scholar]
- 34.Chang C, Holtzman DA, Chau S, Chickering T, Woolf EA, Holmgren LM, Bodorova J, Gearing DP, Holmes WE, Brivanlou AH. Twisted gastrulation can function as a BMP antagonist. Nature. 2001;410:483–487. doi: 10.1038/35068583. [DOI] [PubMed] [Google Scholar]
- 35.Ross JJ, Shimmi O, Vilmos P, Petryk A, Kim H, Gaudenz K, Hermanson S, Ekker SC, O’Connor MB, Marsh JL. Twisted gastrulation is a conserved extracellular BMP antagonist. Nature. 2001;410:479–483. doi: 10.1038/35068578. [DOI] [PubMed] [Google Scholar]
- 36.Scott IC, Blitz IL, Pappano WN, Maas SA, Cho KW, Greenspan DS. Homologues of Twisted gastrulation are extracellular cofactors in antagonism of BMP signalling. Nature. 2001;410:475–478. doi: 10.1038/35068572. [DOI] [PubMed] [Google Scholar]
- 37.Bonds M, Sands J, Poulson W, Harvey C, Von Ohlen T. Genetic screen for regulators of ind expression identifies shrew as encoding a novel twisted gastrulation-like protein involved in Dpp signaling. Dev Dyn. 2007;236:3524–3531. doi: 10.1002/dvdy.21360. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Harris RE, Bayston LJ, Ashe HL. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455:72–77. doi: 10.1038/nature07214. [DOI] [PubMed] [Google Scholar]
- 39**.Sawala A, Sutcliffe C, Ashe HL. Multistep molecular mechanism for bone morphogenetic protein extracellular transport in the Drosophila embryo. Proc Natl Acad Sci U S A. 2012;109:11222–11227. doi: 10.1073/pnas.1202781109. Proposes multistep mechanism for the function of Collagen IV as a scaffold in formation of Dpp/Scw-Sog intermediate complexes and their release by Tsg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimell MJ, Ferguson EL, Childs SR, O’Connor MB. The Drosophila dorsal-ventral patterning gene tolloid is related to human bone morphogenetic protein 1. Cell. 1991;67:469–481. doi: 10.1016/0092-8674(91)90522-z. [DOI] [PubMed] [Google Scholar]
- 41.Marques G, Musacchio M, Shimell MJ, Wunnenberg-Stapleton K, Cho KW, O’Connor MB. Production of a DPP activity gradient in the early Drosophila embryo through the opposing actions of the SOG and TLD proteins. Cell. 1997;91:417–426. doi: 10.1016/s0092-8674(00)80425-0. [DOI] [PubMed] [Google Scholar]
- 42.Piccolo S, Agius E, Lu B, Goodman S, Dale L, De Robertis EM. Cleavage of Chordin by Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell. 1997;91:407–416. doi: 10.1016/s0092-8674(00)80424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashe HL, Levine M. Local inhibition and long-range enhancement of Dpp signal transduction by Sog. Nature. 1999;398:427–431. doi: 10.1038/18892. [DOI] [PubMed] [Google Scholar]
- 44.Finelli AL, Xie T, Bossie CA, Blackman RK, Padgett RW. The tolkin gene is a tolloid/BMP-1 homologue that is essential for Drosophila development. Genetics. 1995;141:271–281. doi: 10.1093/genetics/141.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen T, Jamal J, Shimell MJ, Arora K, O’Connor MB. Characterization of tolloid-related-1: a BMP-1-like product that is required during larval and pupal stages of Drosophila development. Dev Biol. 1994;166:569–586. doi: 10.1006/dbio.1994.1338. [DOI] [PubMed] [Google Scholar]
- 46.Umulis DM, Shimmi O, O’Connor MB, Othmer HG. Organism-scale modeling of early Drosophila patterning via bone morphogenetic proteins. Dev Cell. 2010;18:260–274. doi: 10.1016/j.devcel.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eldar A, Dorfman R, Weiss D, Ashe H, Shilo BZ, Barkai N. Robustness of the BMP morphogen gradient in Drosophila embryonic patterning. Nature. 2002;419:304–308. doi: 10.1038/nature01061. [DOI] [PubMed] [Google Scholar]
- 48.Ben-Zvi D, Shilo BZ, Fainsod A, Barkai N. Scaling of the BMP activation gradient in Xenopus embryos. Nature. 2008;453:1205–1211. doi: 10.1038/nature07059. [DOI] [PubMed] [Google Scholar]
- 49**.Peluso CE, Umulis D, Kim YJ, O’Connor MB, Serpe M. Shaping BMP morphogen gradients through enzyme-substrate interactions. Dev Cell. 2011;21:375–383. doi: 10.1016/j.devcel.2011.06.025. Characterizes Sog processing sites and shows how evolutionary changes in proteolytic control of Sog have influenced morphogen gradient formation and function in the Drosophila embryo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimmi O, O’Connor MB. Physical properties of Tld, Sog, Tsg and Dpp protein interactions are predicted to help create a sharp boundary in Bmp signals during dorsoventral patterning of the Drosophila embryo. Development. 2003;130:4673–4682. doi: 10.1242/dev.00684. [DOI] [PubMed] [Google Scholar]
- 51**.Van der Zee M, da Fonseca RN, Roth S. TGFbeta signaling in Tribolium: vertebrate-like components in a beetle. Dev Genes Evol. 2008;218:203–213. doi: 10.1007/s00427-007-0179-7. Comprehensive analysis of the TGF-β-signaling components present in the genome of the beetle Tribolium castaneum. [DOI] [PubMed] [Google Scholar]
- 52.Fritsch C, Lanfear R, Ray RP. Rapid evolution of a novel signalling mechanism by concerted duplication and divergence of a BMP ligand and its extracellular modulators. Dev Genes Evol. 2010;220:235–250. doi: 10.1007/s00427-010-0341-5. [DOI] [PubMed] [Google Scholar]
- 53.Stauber M, Prell A, Schmidt-Ott U. A single Hox3 gene with composite bicoid and zerknullt expression characteristics in non-Cyclorrhaphan flies. Proc Natl Acad Sci U S A. 2002;99:274–279. doi: 10.1073/pnas.012292899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Podos SD, Ferguson EL. Morphogen gradients: new insights from DPP. Trends Genet. 1999;15:396–402. doi: 10.1016/s0168-9525(99)01854-5. [DOI] [PubMed] [Google Scholar]
- 55.van der Zee M, Stockhammer O, von Levetzow C, Nunes da Fonseca R, Roth S. Sog/Chordin is required for ventral-to-dorsal Dpp/BMP transport and head formation in a short germ insect. Proc Natl Acad Sci U S A. 2006;103:16307–16312. doi: 10.1073/pnas.0605154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goltsev Y, Fuse N, Frasch M, Zinzen RP, Lanzaro G, Levine M. Evolution of the dorsal-ventral patterning network in the mosquito, Anopheles gambiae. Development. 2007;134:2415–2424. doi: 10.1242/dev.02863. [DOI] [PubMed] [Google Scholar]
- 57.Akiyama-Oda Y, Oda H. Axis specification in the spider embryo: dpp is required for radial-to-axial symmetry transformation and sog for ventral patterning. Development. 2006;133:2347–2357. doi: 10.1242/dev.02400. [DOI] [PubMed] [Google Scholar]
- 58*.Fritsch C, Sawala A, Harris R, Maartens A, Sutcliffe C, Ashe HL, Ray RP. Different requirements for proteolytic processing of bone morphogenetic protein 5/6/7/8 ligands in Drosophila melanogaster. J Biol Chem. 2012;287:5942–5953. doi: 10.1074/jbc.M111.316745. Demonstration of prodomain cleavage sites in Gbb and Scw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serpe M, Umulis D, Ralston A, Chen J, Olson DJ, Avanesov A, Othmer H, O’Connor MB, Blair SS. The BMP-binding protein Crossveinless 2 is a short-range, concentration-dependent, biphasic modulator of BMP signaling in Drosophila. Dev Cell. 2008;14:940–953. doi: 10.1016/j.devcel.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ambrosio AL, Taelman VF, Lee HX, Metzinger CA, Coffinier C, De Robertis EM. Crossveinless-2 Is a BMP feedback inhibitor that binds Chordin/BMP to regulate Xenopus embryonic patterning. Dev Cell. 2008;15:248–260. doi: 10.1016/j.devcel.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zakin L, Metzinger CA, Chang EY, Coffinier C, De Robertis EM. Development of the vertebral morphogenetic field in the mouse: interactions between Crossveinless-2 and Twisted Gastrulation. Dev Biol. 2008;323:6–18. doi: 10.1016/j.ydbio.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62*.Zakin L, Chang EY, Plouhinec JL, De Robertis EM. Crossveinless-2 is required for the relocalization of Chordin protein within the vertebral field in mouse embryos. Dev Biol. 2010;347:204–215. doi: 10.1016/j.ydbio.2010.08.025. Demonstrates a Cv-2-dependent flow of Chordin and argues for a “sink” function for Cv-2, which is essential for axial skeletal development in the mouse embryo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Umulis DM, Serpe M, O’Connor MB, Othmer HG. Robust, bistable patterning of the dorsal surface of the Drosophila embryo. Proc Natl Acad Sci U S A. 2006;103:11613–11618. doi: 10.1073/pnas.0510398103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64*.Karim MS, Buzzard GT, Umulis DM. Secreted, receptor-associated bone morphogenetic protein regulators reduce stochastic noise intrinsic to many extracellular morphogen distributions. J R Soc Interface. 2012;9:1073–1083. doi: 10.1098/rsif.2011.0547. Develops a stochastic model of Dpp signaling and uses it to investigate how a surface-associated BMP-binding protein buffers out signaling noise. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Panganiban GE, Reuter R, Scott MP, Hoffmann FM. A Drosophila growth factor homolog, decapentaplegic, regulates homeotic gene expression within and across germ layers during midgut morphogenesis. Development. 1990;110:1041–1050. doi: 10.1242/dev.110.4.1041. [DOI] [PubMed] [Google Scholar]
- 66.Fernandez BG, Arias AM, Jacinto A. Dpp signalling orchestrates dorsal closure by regulating cell shape changes both in the amnioserosa and in the epidermis. Mech Dev. 2007;124:884–897. doi: 10.1016/j.mod.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 67.Song X, Wong MD, Kawase E, Xi R, Ding BC, McCarthy JJ, Xie T. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development. 2004;131:1353–1364. doi: 10.1242/dev.01026. [DOI] [PubMed] [Google Scholar]
- 68.Xie T, Spradling AC. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94:251–260. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- 69.Rojas-Rios P, Guerrero I, Gonzalez-Reyes A. Cytoneme-mediated delivery of hedgehog regulates the expression of bone morphogenetic proteins to maintain germline stem cells in Drosophila. PLoS Biol. 2012;10:e1001298. doi: 10.1371/journal.pbio.1001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Allan DW, St Pierre SE, Miguel-Aliaga I, Thor S. Specification of neuropeptide cell identity by the integration of retrograde BMP signaling and a combinatorial transcription factor code. Cell. 2003;113:73–86. doi: 10.1016/s0092-8674(03)00204-6. [DOI] [PubMed] [Google Scholar]
- 71.Marques G, Haerry TE, Crotty ML, Xue M, Zhang B, O’Connor MB. Retrograde Gbb signaling through the Bmp type 2 receptor wishful thinking regulates systemic FMRFa expression in Drosophila. Development. 2003;130:5457–5470. doi: 10.1242/dev.00772. [DOI] [PubMed] [Google Scholar]
- 72.McCabe BD, Marques G, Haghighi AP, Fetter RD, Crotty ML, Haerry TE, Goodman CS, O’Connor MB. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron. 2003;39:241–254. doi: 10.1016/s0896-6273(03)00426-4. [DOI] [PubMed] [Google Scholar]
- 73.Ballard SL, Jarolimova J, Wharton KA. Gbb/BMP signaling is required to maintain energy homeostasis in Drosophila. Dev Biol. 2010;337:375–385. doi: 10.1016/j.ydbio.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bangi E, Wharton K. Dpp and Gbb exhibit different effective ranges in the establishment of the BMP activity gradient critical for Drosophila wing patterning. Dev Biol. 2006;295:178–193. doi: 10.1016/j.ydbio.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 75**.Akiyama T, Marques G, Wharton KA. A large bioactive BMP ligand with distinct signaling properties is produced by alternative proconvertase processing. Sci Signal. 2012;5:ra28. doi: 10.1126/scisignal.2002549. Identification of a large, more active Gbb variant produced by cleavage within the prodomain and retaining parts of the prodomain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Degnin C, Jean F, Thomas G, Christian JL. Cleavages within the prodomain direct intracellular trafficking and degradation of mature bone morphogenetic protein-4. Mol Biol Cell. 2004;15:5012–5020. doi: 10.1091/mbc.E04-08-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goldman DC, Hackenmiller R, Nakayama T, Sopory S, Wong C, Kulessa H, Christian JL. Mutation of an upstream cleavage site in the BMP4 prodomain leads to tissue-specific loss of activity. Development. 2006;133:1933–1942. doi: 10.1242/dev.02368. [DOI] [PubMed] [Google Scholar]
- 78*.Kunnapuu J, Bjorkgren I, Shimmi O. The Drosophila DPP signal is produced by cleavage of its proprotein at evolutionary diversified furin-recognition sites. Proc Natl Acad Sci U S A. 2009;106:8501–8506. doi: 10.1073/pnas.0809885106. Demonstrates multiple cleavage sites within Dpp linker, with one being required for activity and the other two responsible for generation of Dpp26 and Dpp23 variants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79*.Sopory S, Kwon S, Wehrli M, Christian JL. Regulation of Dpp activity by tissue-specific cleavage of an upstream site within the prodomain. Dev Biol. 2010;346:102–112. doi: 10.1016/j.ydbio.2010.07.019. Important study demonstrating that differential Dpp cleavage provides a tissue-specific mechanism for regulating Dpp activity and determines signaling range. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80*.Kuhfahl S, Hauburger A, Thieme T, Groppe J, Ihling C, Tomic S, Schutkowski M, Sinz A, Schwarz E. Identification of a core domain within the proregion of bone morphogenetic proteins that interacts with the dimeric, mature domain. Biochem Biophys Res Commun. 2011;408:300–305. doi: 10.1016/j.bbrc.2011.04.021. Identifies protease resistant core within the prodomain of BMPs that can fold independently and interact with the ligand domain. [DOI] [PubMed] [Google Scholar]
- 81**.Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. Latent TGF-beta structure and activation. Nature. 2011;474:343–349. doi: 10.1038/nature10152. First report of TGF-β/BMP family proprotein structure highlighting presence of strait jacket and lasso motifs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82*.Sengle G, Ono RN, Sasaki T, Sakai LY. Prodomains of transforming growth factor beta (TGFbeta) superfamily members specify different functions: extracellular matrix interactions and growth factor bioavailability. J Biol Chem. 2011;286:5087–5099. doi: 10.1074/jbc.M110.188615. Prodomains of BMP-5,7 and GDF-8 interact differently with fibrillin and perlecan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sengle G, Ono RN, Lyons KM, Bachinger HP, Sakai LY. A new model for growth factor activation: type II receptors compete with the prodomain for BMP-7. J Mol Biol. 2008;381:1025–1039. doi: 10.1016/j.jmb.2008.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raftery LA, Umulis DM. Regulation of BMP activity and range in Drosophila wing development. Curr Opin Cell Biol. 2012;24:158–165. doi: 10.1016/j.ceb.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85**.Dejima K, Kanai MI, Akiyama T, Levings DC, Nakato H. Novel contact-dependent bone morphogenetic protein (BMP) signaling mediated by heparan sulfate proteoglycans. J Biol Chem. 2011;286:17103–17111. doi: 10.1074/jbc.M110.208082. Describes contact-dependent interactions critical for BMP signaling suggesting importance of HSPGs in trans-signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86*.Dani N, Nahm M, Lee S, Broadie K. A targeted glycan-related gene screen reveals heparan sulfate proteoglycan sulfation regulates WNT and BMP trans-synaptic signaling. PLoS Genet. 2012;8:e1003031. doi: 10.1371/journal.pgen.1003031. Shows that HSPGs limit the Gbb abundance in the NMJ synaptic cleft and therefore influence trans-synaptic signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87*.Vuilleumier R, Springhorn A, Patterson L, Koidl S, Hammerschmidt M, Affolter M, Pyrowolakis G. Control of Dpp morphogen signalling by a secreted feedback regulator. Nat Cell Biol. 2010;12:611–617. doi: 10.1038/ncb2064. Identifies Pent as a secreted protein, target of BMP activity, which interacts with the Dally to control Dpp distribution in the imaginal disc. [DOI] [PubMed] [Google Scholar]
- 88*.Szuperak M, Salah S, Meyer EJ, Nagarajan U, Ikmi A, Gibson MC. Feedback regulation of Drosophila BMP signaling by the novel extracellular protein larval translucida. Development. 2011;138:715–724. doi: 10.1242/dev.059477. Identifies Ltl as a secreted protein, target of BMP activity, that antagonizes BMP signaling in the extracellular space. [DOI] [PubMed] [Google Scholar]
- 89**.Ben-Zvi D, Pyrowolakis G, Barkai N, Shilo BZ. Expansion-repression mechanism for scaling the Dpp activation gradient in Drosophila wing imaginal discs. Curr Biol. 2011;21:1391–1396. doi: 10.1016/j.cub.2011.07.015. Describes an expansion-repression feedback mechanism for scaling the Dpp gradient in the wing imaginal disc. [DOI] [PubMed] [Google Scholar]
- 90*.Chen J, Honeyager SM, Schleede J, Avanesov A, Laughon A, Blair SS. Crossveinless d is a vitellogenin-like lipoprotein that binds BMPs and HSPGs, and is required for normal BMP signaling in the Drosophila wing. Development. 2012;139:2170–2176. doi: 10.1242/dev.073817. Describes a new circulatory long-range BMP shuttle which affects the range of BMP movement in the pupal wing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91**.Wartlick O, Mumcu P, Kicheva A, Bittig T, Seum C, Julicher F, Gonzalez-Gaitan M. Dynamics of Dpp signaling and proliferation control. Science. 2011;331:1154–1159. doi: 10.1126/science.1200037. Shows that Dpp concentration and signaling gradients scale with tissue size during development and proposes that cells control growth by computing the relative temporal variation in Dpp activity. [DOI] [PubMed] [Google Scholar]
- 92.Bornemann DJ, Park S, Phin S, Warrior R. A translational block to HSPG synthesis permits BMP signaling in the early Drosophila embryo. Development. 2008;135:1039–1047. doi: 10.1242/dev.017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhaes TR, Goodman CS. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron. 2002;33:545–558. doi: 10.1016/s0896-6273(02)00589-5. [DOI] [PubMed] [Google Scholar]
- 94.Srinivasan S, Rashka KE, Bier E. Creation of a Sog morphogen gradient in the Drosophila embryo. Dev Cell. 2002;2:91–101. doi: 10.1016/s1534-5807(01)00097-1. [DOI] [PubMed] [Google Scholar]
- 95.Mac Sweeney A, Gil-Parrado S, Vinzenz D, Bernardi A, Hein A, Bodendorf U, Erbel P, Logel C, Gerhartz B. Structural basis for the substrate specificity of bone morphogenetic protein 1/tolloid-like metalloproteases. J Mol Biol. 2008;384:228–239. doi: 10.1016/j.jmb.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 96.Martinez-Glez V, Valencia M, Caparros-Martin JA, Aglan M, Temtamy S, Tenorio J, Pulido V, Lindert U, Rohrbach M, Eyre D, et al. Identification of a mutation causing deficient BMP1/mTLD proteolytic activity in autosomal recessive osteogenesis imperfecta. Hum Mutat. 2012;33:343–350. doi: 10.1002/humu.21647. [DOI] [PMC free article] [PubMed] [Google Scholar]