Abstract
Background & Aims
The characteristics of nonalcoholic fatty liver disease (NAFLD) in elderly patients are unknown. Therefore, we aim to examine the differences between elderly and non-elderly patients with NAFLD, and to identify determinants of nonalcoholic steatohepatitis (NASH) and advanced fibrosis (bridging fibrosis or cirrhosis) in elderly patients.
Methods
This is a cross-sectional analysis of adult participants who were prospectively enrolled in the NASH Clinical Research Network studies. Participants were included based upon availability of the centrally reviewed liver histology data within one year of enrollment, resulting in 61 elderly (aged ≥ 65 years) and 735 non-elderly (18–64 years) participants. Main outcomes were presence of NASH and advanced fibrosis.
Results
Compared to non-elderly patients with NAFLD, elderly patients had a higher prevalence of NASH (56% versus 74%, P =0.02), and advanced fibrosis (25% versus 44%, P=0.002), respectively. Compared to non-elderly patients with NASH, elderly patients with NASH had higher rates of advanced fibrosis (35% versus 52%, P=0.03), as well as other features of severe liver disease including presence of ballooning degeneration, acidophil bodies, megamitochondria, and Mallory-Denk bodies (P ≤0.05 for each). In multivariable-adjusted logistic regression analyses, independent determinants of NASH in elderly patients included higher AST (odd ratio (OR)= 1.12, P=0.007) and lower platelets (OR= 0.98, P=0.02); and independent determinants of advanced fibrosis included higher AST (OR=1.10, P=0.002), lower ALT value (OR= 0.91, P=0.001) and an increased odds of having low HDL (OR=12.62, P=0.004).
Conclusions
Elderly patients are more likely to have NASH and advanced fibrosis than non-elderly patients with NAFLD. Liver biopsy may be considered in elderly patients and treatment should be initiated in those with NASH and advanced fibrosis.
Keywords: Elderly, Nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), Histology
Introduction
Nonalcoholic fatty liver disease (NAFLD) afflicts one in every three adult Americans and it is the most common cause of elevated serum aminotransferases in the United States (US)1–4. NAFLD is seen in individuals who consume little or no alcohol. It can range from presence of steatosis alone, that is expected to have a non-progressive course, to nonalcoholic steatohepatitis (NASH), the progressive form of NAFLD that can lead to advanced fibrosis, cirrhosis and hepatocellular carcinoma in a subset of patients3, 5–8. Liver biopsy in NASH is typically characterized by steatosis, lobular inflammation, ballooning degeneration with or without peri-sinusoidal fibrosis9, 10.
Children and adolescents with NAFLD may have a different pattern of liver injury than adult patients with NAFLD11–13. This suggests that as an individual grows or ages NAFLD phenotypes may vary. However, there are limited data examining whether we see a different pattern of liver histology in elderly patients with NAFLD. Several groups have now shown that older age is a risk factor for NASH and advanced fibrosis in patients with NAFLD14, 15. Recent studies have suggested that a higher prevalence of NAFLD and more advanced fibrosis may be seen in elderly patients16, 17. However, little is known about the characteristics and histology of NAFLD in elderly patients.
The US population is aging due to the steady rise in life expectancy18–20. In 2010, approximately forty million Americans were older than 65 years. By the year 2030, this age group of Americans is estimated to rise to more than 70 million21. The aging of the American population underscores the importance of studying the characteristics of NAFLD in the elderly patients. Finally, a recent study found that ALT decreases with age, 22 which may cause significant disease to be overlooked in elderly patients if that is the sole determining criterion for a referral to a specialist.
The main aims of this study were to investigate the clinical and histological characteristics of NASH and fibrosis in elderly patients compared to non-elderly patients from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) cohort, and to determine the characteristics associated with NASH in elderly compared to the non-elderly patients. In this study, we hypothesized that elderly patients with NAFLD have more advanced disease, reflected by a higher prevalence of NASH and fibrosis, compared to younger adults.
Methods
Study design and setting
This is a cross-sectional analysis of adult patients with biopsy-proven NAFLD who were enrolled into either the NAFLD Database Study, a prospective cohort study, or the PIVENS (Pioglitazone versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis; Clinical Trial number NCT00063622), a randomized, placebo-controlled, double-masked clinical trial, of the NIDDK sponsored NASH-CRN consortium23, 24.
Participants were enrolled between 2004 through 2008 by one of the eight participating medical centers in the United States: University of California at San Diego (San Diego, CA); Duke University (Durham, NC); Case Western Reserve (Cleveland, OH);; Indiana University (Indianapolis, IN); Saint Louis University (St. Louis, MO); University of California at San Francisco (San Francisco, CA); University of Washington (Seattle, WA); and Virginia Commonwealth University (Richmond, VA). All enrolled patients provided written informed consent before data collection. The clinical protocols, consent forms, and manual of operations were also reviewed and approved by a data safety monitoring board established by the NIDDK specifically for the NASH CRN. In addition the protocol and the informed consent were approved by the Institutional Review Board of each site. STROBE guidelines for cross-sectional studies were followed25.
Patient population
Both the NAFLD Database and PIVENS treatment studies have been published23, 24, 26. Briefly, the inclusion criteria for the NAFLD Database required either histological diagnosis of NAFLD, imaging suggestive of NAFLD, histological diagnosis of cryptogenic cirrhosis, or clinical evidence of cryptogenic cirrhosis. Exclusion criteria included diagnosis of other chronic liver disease or suspected or proven hepatocellular carcinoma, or an average alcohol consumption >20g daily for man, or >10g average for woman, during the 2 years before entry. PIVENS inclusion additionally required patients to have histological evidence of NASH without cirrhosis and the absence of diabetes.
Inclusion criteria for this sub-analysis
To be included in the dataset for the analysis of this study, participants were required to have a biopsy within one year of enrollment that was evaluated through central reading by the NASH CRN Pathology Committee. Subjects were divided into the following groups: (1) patients who were 65 years or older at the time of their biopsy were defined as elderly27, 28 and (2) patients between 18 and 64 years of age were defined as non-elderly.
Co-variates
The following characteristics were examined: demographic factors included age, sex, race (white vs other), and ethnicity (Hispanic vs not); anthropometrics included body mass index (BMI) and waist circumference; and clinical characteristics included hypertension and diabetes. Metabolic syndrome was defined as having 3 of the following 5 factors: impaired fasting glucose (≥ 100 mg/dL), large waist circumference (> 88 cm in women, > 102 cm in men), hypertriglyceridemia (≥ 150 mg/dL), low HDL cholesterol (< 50 mg/dL in women, < 40 mg/dL in men), high blood pressure (HBP) (systolic BP ≥ 130 mmHg or diastolic BP ≥ 85 mmHg). In addition, we also included smoking status (yes/no) and history of coronary heart disease (CHD) (yes/no) as a co-variate. This analysis also included clinical laboratory tests including: aspartate aminotransferase (AST), alanine aminotransferase (ALT), the AST/ALT ratio, gamma glutamyl trans-peptidase (GGT), alkaline phosphatase (ALK), albumin, total protein, prothrombin time, platelet count, total cholesterol, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), triglycerides, hemoglobin A1c (HbA1c), fasting glucose, fasting serum insulin, the homeostasis model assessment of insulin resistance (HOMA-IR) index and titers of antinuclear (ANA), anti-smooth muscle (ASMA), and anti-mitochondrial (AMA) antibodies. Abnormal ALT, AST and alkaline phosphatase were defined as more than > 1 upper limit of normal (ULN) according to local reference ranges. The APRI score was defined as [(AST/ULN)/platelets] multiplied by 100.
Liver histologic assessment section
The NASH CRN Pathology Committee consisted of nine liver pathologists who were blinded to all clinical and identifying data. Biopsies were scored by consensus during pathology committee meetings using the NASH CRN Histologic Scoring System 9.
Briefly, the following variables were recorded and analyzed in this sub-analysis. Steatosis evaluation included the grade of steatosis, location of steatosis and presence (or absence) of microvesicular steatosis. The fibrosis stage was divided into four stages including stage 0: no fibrosis, stage 1a: mild, zone 3, perisinusoidal fibrosis; stage 1b: moderate, zone 3, perisinusoidal fibrosis; stage 1c: portal/periportal fibrosis; stage 2: perisinusoidal and portal/periportal fibrosis; stage 3: bridging fibrosis and stage 4: cirrhosis. The assessment of inflammation included the number of foci of lobular inflammation, the presence of microgranulomas, the presence of large lipogranulomas and the degree of portal inflammation. The liver cell injury assessment included the presence of ballooning degeneration, acidophil bodies, pigmented macrophages and megamitochondria. Other components were the presence of Mallory-Denk bodies (or Mallory Hyaline) and glycogenated nuclei. The histological assessment also included diagnostic classification of NASH and liver biopsies of the participants were classified into one of the three possible categories including not NASH, possible/borderline NASH, and definite NASH.
Primary outcomes
The main outcome variables of this study were the presence of definite NASH and advanced fibrosis defined as either bridging fibrosis or cirrhosis. Secondary outcomes included other histologic variables.
Statistical analysis
We conducted an exploratory analysis of baseline characteristics including demographic, anthropometric, clinical, laboratory measures and histological features. Univariate analyses were performed using this set of characteristics among different study subgroup comparisons of interest: elderly to non-elderly patients with NAFLD to examine the differences in the pattern and severity of liver injury between the two groups; elderly patients with NASH to non-elderly patients with NASH to examine if features of NASH were distinct between the two groups. Finally we developed a logistic regression model to examine the determinants of NASH and advanced fibrosis in elderly patients. Differences between the distributions between subgroups were assessed using Fisher’s exact test for categorical and t-test for continuous features. All histological features were treated as categorical. Univariable results were reported as means and standard deviations or percentages.
Independent predictors of either definite NASH or advanced fibrosis among elderly patients were determined using unadjusted and adjusted multivariable logistic regression29. Odds ratios (OR), 95% confidence intervals (95% CI) and p-values (p) were used to report the results. The candidate set for the multivariable-adjusted models was limited to features that have been linked to NAFLD and based upon biological plausibility and included key demographics (age, race, ethnicity), smoking, h/o CHD, diabetes, components of metabolic syndrome (BMI, hypertriglycerides, low HDL, high blood pressure, insulin resistance (HOMA-IR)) and liver disease biomarkers (ALT, AST, GGT, platelets, ferritin). Ethnicity was not included in the candidate set for the advanced fibrosis model due to multi-collinearity with metabolic traits. The adjusted model was determined from backward stepwise regression using a 0.05 level of significance of definite NASH and advanced fibrosis on the candidate set forcing age, gender and race into the model. Final models were assessed using Hosmer-Lemeshow goodness of fit and the Akaike Information Criterion (AIC)30–33.
All analyses were performed using STATA (version 12) and SAS statistical software (version 9.3)34, 35). Nominal, two-sided P values were used and were considered to be statistically significant if P ≤ 0.05, a priori.
Results
Demographic, clinical and biochemical characteristics in elderly compared to non-elderly patients with NAFLD
Among the 796 patients with biopsy-proven NAFLD who met the inclusion criteria for this study, 61 patients who were aged ≥ 65 years were classified into the elderly patients group, and the remaining 735 patients who were aged between 18 and 65 years were classified into the non-elderly patients group.
The detailed description of the cohort categorized into elderly versus non-elderly patients with NAFLD has been shown in table 1. Compared to non-elderly patients, the elderly patients group with NAFLD had more females and subjects were more likely to be hypertensive. The elderly patients group had a lower mean BMI and smaller waist circumference. Although the elderly patients group had a higher average AST and a lower average ALT, this difference was not statistically significant. The elderly patients group had a higher mean AST/ALT ratio, lower mean platelet count and higher mean APRI score, all of which are suggestive of advanced liver disease.
Table 1.
Demographic, anthropometric and clinical characteristics of patients with NAFLD enrolled in NASH CRN studies by age group
| Characteristics | Age group
|
P† | |
|---|---|---|---|
| Non-elderly (18–64 years old) (N=735) | Elderly (≥ 65 years old) (N=61) | ||
| Demographics & lifestyle: | |||
| Male, n (%) | 295 (40%) | 14 (23%) | 0.01 |
| Age (mean years ± SD) | 47 ± 11 | 68 ± 3 | <0.001 |
| White, n (%) | 593 (84%) | 46 (78%) | 0.20 |
| Hispanic, n (%) | 98 (13%) | 7 (12%) | 0.84 |
| Ever smoked regularly, n (%)‡ | 263 (36%) | 31 (51%) | 0.03 |
| Clinical | |||
| Hypertension, n (%) | 337 (46%) | 42 (69%) | 0.001 |
| Cardiovascular disease (CVD), n (%)‡ | 33 (4%) | 11 (18%) | < 0.001 |
| Type 2 diabetes, n (%) | 174 (24%) | 15 (25%) | 0.88 |
| Metabolic syndrome, n (%) | 451 (61%) | 34 (56%) | 0.41 |
| Anthropometric (mean ± SD) | |||
| Body mass index (kg/m2) | 35 ± 6 | 32 ± 5 | < 0.001 |
| Waist circumference (cm) | 109 ± 14 | 103 ± 12 | < 0.001 |
| Hepatology panel§ (mean ± SD) | |||
| AST (U/L) | 54 ± 36 | 61 ± 44 | 0.20 |
| ALT (U/L) | 76 ± 52 | 67 ± 44 | 0.16 |
| AST/ALT ratio | 0.8 ± 0.4 | 1.0 ± 0.4 | < 0.001 |
| Alkaline phosphatase (ALK) (U/L) | 87 ± 33 | 94 ± 37 | 0.13 |
| GGT (U/L) | 70 ± 78 | 77 ± 75 | 0.53 |
| Albumin (g/dL) | 4.2 ± 0.4 | 4.2 ± 0.4 | 0.20 |
| Bilirubin, total (mg/dL) | 0.8 ± 0.4 | 0.8 ± 0.4 | 0.26 |
| Bilirubin, direct (mg/dL) | 0.1 ± 0.1 | 0.2 ± 0.2 | 0.03 |
| International normalized ratio | 1.0 ± 0.2 | 1.0 ± 0.1 | 0.18 |
| Other laboratory studies§ (mean ±SD) | |||
| Platelet count (1000/mm3) | 245 ± 71 | 218 ± 66 | 0.004 |
| Total cholesterol (mg/dL) | 195 ± 42 | 199 ± 44 | 0.48 |
| HDL cholesterol (mg/dL) | 43 ± 12 | 47 ± 11 | 0.01 |
| LDL cholesterol (mg/dL) | 119 ± 36 | 122 ± 38 | 0.65 |
| Triglycerides (mg/dL) | 178 ± 131 | 159 ± 79 | 0.08 |
| HbA1c (%) | 6.0 ± 1.1 | 6.0 ± 1.0 | 0.70 |
| Fasting serum glucose (mg/dL) | 103 ± 34 | 107 ± 27 | 0.31 |
| Fasting serum insulin (lU/mL) | 23 ± 19 | 19 ± 13 | 0.07 |
| HOMA-IR (mg/dL × lU/mL/405) | 6.0 ± 6.0 | 5.5 ± 4.4 | 0.34 |
| Ferritin (ng/mL) | 235 ± 264 | 326 ± 454 | 0.13 |
| APRI score‡ | 0.5 ± 0.4 | 0.7 ± 0.6 | 0.04 |
Participants enrolled in the NASH CRN with a biopsy within 1 year of enrollment.
P values (2-sided) determined from either a Fisher’s exact test for categorical variables or t-test for continuous variables
Ever smoked regularly defined as smoking at least 1 cigarette per day for a year; 4 non-elderly patients were missing values.
Cardiovascular disease defined as ever diagnosed with cerebrovascular or coronary heart disease.
APRI defined as (AST/ULN)/platelets × 100
Abbreviations: AST: aspartate aminotransferase, ALT: alanine aminotransferase, GGT: gamma glutamyl trans-peptidase, HDL: high density lipoprotein, LDL: low density lipoprotein, HbA1c: hemoglobin A1c, HOMA-IR: the homeostasis model assessment of insulin resistance index
Histologic characteristics in elderly compared to non-elderly patients with NAFLD
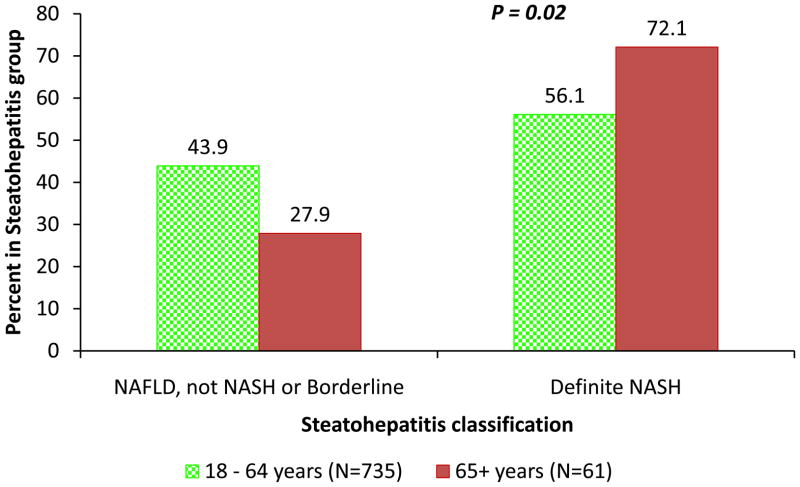
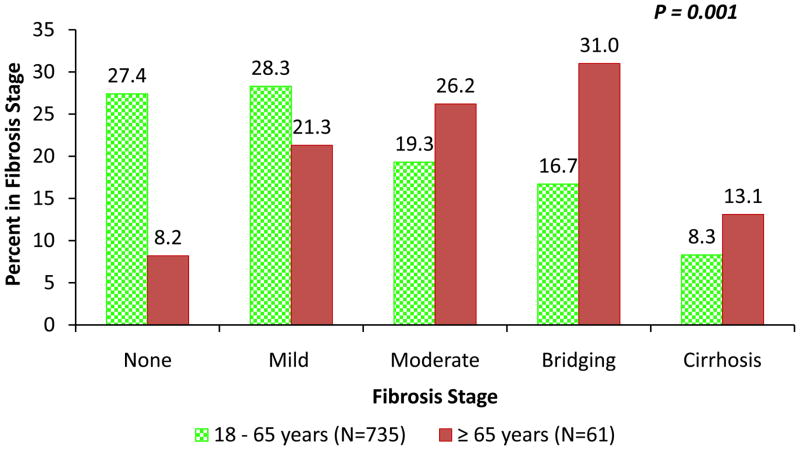
Table 2 presents the comparison of the detailed histological features in elderly and non-elderly patients with NAFLD. Compared to non-elderly patients with NAFLD, the elderly had a higher prevalence of NASH (56% versus 74%, p-value P=0.02) (figure 1), advanced fibrosis (25% versus 44%, P=0.002) (figure 2) and azonal-distribution of steatosis (27% versus 43%, P=0.01) (Table 2).
Table 2.
Histological features of patients with NAFLD comparing elderly to non-elderly patients
| Histological Feature* | Age Group
|
P† | |
|---|---|---|---|
| Non-elderly (N=735) No. (%) | Elderly (N=61) No. (%) | ||
| Steatosis: | |||
| Grade | 0.20 | ||
| 0–1 (0%–33%) | 309 (42.0%) | 31 (50.8%) | |
| 2 (>33%–66%) | 252 (34.3%) | 18 (29.5%) | |
| 3 (>66%) | 174 (23.7%) | 12 (19.7%) | |
| Location (predominant) | 0.02 | ||
| Zone 3 (central) | 307 (41.8%) | 15 (24.6%) | |
| Zone 1(periportal) | 11 (1.5%) | 0 (0.0%) | |
| Azonal | 195 (26.6%) | 26 (42.6%) | |
| Panacinar | 221 (30.1%) | 20 (32.8%) | |
| Microvesicular steatosis: present | 74 (10.1%) | 9 (14.8%) | 0.27 |
| Fibrosis: | |||
| Stage: | |||
| None (0) | 200 (27.4%) | 5 (8.2%) | 0.001 |
| Mild/moderate ( zone 3), portal/periportal (1A, 1B, 1C) | 209 (28.3%) | 13 (21.3%) | |
| Zone 3 & periportal (2) | 141 (19.3%) | 16 (26.2%) | |
| Bridging (3) | 122 (16.7%) | 19 (31.0%) | |
| Cirrhosis (4) | 61 (8.3%) | 8 (13.1%) | |
| Advanced fibrosis: | 0.002 | ||
| None (0), mild (1), moderate (2) | 548 (75.0%) | 34 (55.7%) | |
| Bridging (3) or cirrhosis (4) | 183 (25.0%) | 27 (44.3%) | |
| Inflammation: | |||
| Lobular inflammation (score) (no. foci per 200X field) | 0.007 | ||
| 0 to <2 foci (0–1) | 392 (53.3%) | 22 (36.1%) | |
| 2 to 4 foci (2) | 267 (36.3%) | 26 (42.6%) | |
| >4 foci (3) | 76 (10.3%) | 13 (21.3%) | |
| Microgranulomas: present | 603 (82.0%) | 52 (82.3%) | 0.60 |
| Large lipogranulomas: present | 279 (38.0%) | 33 (54.1%) | 0.02 |
| Portal Inflammation (score) | 0.23 | ||
| None (0) | 111 (15.1%) | 5 (8.2%) | |
| Mild (1) | 466 (63.4%) | 39 (63.9%) | |
| More than mild (2) | 158 (21.5%) | 17 (27.9%) | |
| Liver cell injury: | |||
| Ballooning (score): | 0.004 | ||
| None (0) | 251 (34.2%) | 15 (24.6%) | |
| Few (1) | 199 (27.1%) | 9 (14.8%) | |
| Many (2) | 285 (38.8%) | 37 (60.7%) | |
| Acidophil bodies: many | 215 (29.3%) | 26 (42.6%) | 0.04 |
| Pigmented macrophages: many | 644 (87.6%) | 54 (88.5%) | 1.00 |
| Megamitochondria: many | 106 (14.4%) | 15 (24.6%) | 0.04 |
| Other findings | |||
| Mallory Denk bodies: many | 194 (26.4%) | 32 (52.5%) | < 0.001 |
| Glycogenated nuclei: many | 392 (53.3%) | 29 (47.5%) | 0.42 |
| NAFLD Activity Score (NAS) | 0.23 | ||
| 0 – 4 | 384 (52.2%) | 27 (44.3%) | |
| 5 – 8 | 351 (47.8%) | 34 (55.7%) | |
| Mean ± SD | 4.38 ± 1.67 | 4.82 ± 1.69 | 0.05 |
| Diagnostic classification: | |||
| Steatohepatitis (diagnosis): | 0.12 | ||
| Not steatohepatitis (0) | 166 (22.6%) | 10 (16.4%) | |
| Possible/borderline: | |||
| Zone 3 pattern (1A) | 148 (20.1%) | 7 (11.5%) | |
| Zone 1, periportal (1B) | 9 (1.2%) | 0 (0.0%) | |
| Definite steatohepatitis (2) | 412 (56.1%) | 44 (72.1%) | |
| Definite NASH (yes) | 412 (56.1%) | 44 (72.1%) | 0.02 |
Determination of histological features from centrally reviewed biopsies using the NASH CRN Scoring System9
P values determined from Fisher’s exact test or Cuzick non-parametric test for trend across ordered categories, except for NAS, a t-test was used
Figure 1. Prevalence of definite NASH between non-elderly and elderly patients with NAFLD.
Compared to non-elderly (green dotted bar) patients with NAFLD, elderly patients (red bar) had a higher prevalence of NASH (56% versus 74%, P=0.02).
Figure 2. Distribution of fibrosis stage between non-elderly and elderly patients with NAFLD.
The distribution of fibrosis between non-elderly (green dotted bar) versus elderly patients (red bar) for various stage of fibrosis was as follows: stage 0: 27.4% vs. 8.2%, stage 1–28.3% vs. 21.3%, stage 2: 19.3% vs. 26.2%, stage 3: 16.7% vs. 31%, and stage 4: 8.3% vs. 13.1%, respectively.
Furthermore, elderly patients had other features consistent with progressive liver disease including a higher degree of lobular inflammation and a higher prevalence of acidophil bodies, megamitochondria, Mallory-Denk bodies, as well as more prominent ballooning (Table 2). As expected, elderly patients had a higher prevelence of lipogranulomas.
Histological comparison between elderly and non-elderly patients with definite NASH
In order to examine whether the advanced histologic features in elderly patients with NAFLD were due to the increased prevalence of NASH or whether these features were seen across the spectrum of NAFLD irrespective of presence or absence of NASH, we compared the detailed liver histologic features between elderly versus non-elderly patients who had biopsy-proven NASH. There were 44 patients with biopsy proven NASH in the elderly patients group and 412 patients with biopsy-proven NASH in the non-elderly patients group (Table 3). Compared to non-elderly patients with NASH, elderly patients with NASH had higher rates of advanced fibrosis (35% versus 52%, P=0.03), as well as other features suggestive of severe liver disease including ballooning degeneration, acidophil bodies, megamitochondria, and Mallory-Denk bodies (P≤0.05 for each) (Table 3). In contrast, compared to non-elderly patients with NASH, elderly patients had lesser degrees of steatosis (67% versus 48% >33% steatosis, P=0.01).
Table 3.
Histological features of patients with definite NASH comparing elderly to non-elderly patients
| Histological Feature* | Age Group
|
P† | |
|---|---|---|---|
| Non-elderly (N=412) No. (%) | Elderly (N=44) No. (%) | ||
| Steatosis: | |||
| Grade | 0.01 | ||
| 0–1 (0%–33%) | 136 (33.0%) | 23 (52.3%) | |
| 2–3 (>33%) | 276 (67.0%) | 21 (47.7%) | |
| Location (predominant) | 0.07 | ||
| Zone 3 (central) | 152 (36.9%) | 9 (20.5%) | |
| Zone 1(periportal) | 2 (0.5%) | 0 (0.0%) | |
| Azonal | 119 (28.9%) | 20 (45.5%) | |
| Panacinar | 139 (33.7%) | 15 (34.1%) | |
| Microvesicular steatosis | 0.82 | ||
| Not present | 353 (85.7%) | 37 (84.1%) | |
| Present | 59 (14.3%) | 7 (15.9%) | |
| Fibrosis: | |||
| Stage: | 0.03 | ||
| None (0) | 32 (7.8%) | 1 (2.3%) | |
| Mild/moderate ( zone 3), portal/periportal (1A, 1B, 1C) | 133 (32.4%) | 7 (15.9%) | |
| Zone 3 & periportal (2) | 101 (24.6%) | 13 (29.6%) | |
| Bridging (3) | 107 (26.1%) | 15 (34.1%) | |
| Cirrhosis (4) | 37 (9.0%) | 8 (18.2%) | |
| Advanced fibrosis: | 0.03 | ||
| None (0), mild (1), moderate (2) | 266 (64.9%) | 21 (47.7%) | |
| Bridging (3) or cirrhosis (4) | 144 (35.1%) | 23 (52.3%) | |
| Inflammation: | |||
| Lobular inflammation (score) (no. foci per 200X field) | 0.08 | ||
| 0 to <2 foci (0–1) | 162 (39.3%) | 13 (29.6%) | |
| 2 to 4 foci (2) | 183 (44.4%) | 18 (40.9%) | |
| >4 foci (3) | 67 (16.3%) | 13 (29.6%) | |
| Microgranulomas: present | 357 (86.7%) | 40 (90.9%) | 0.64 |
| Large lipogranulomas: present | 172 (41.8%) | 29 (65.9%) | 0.002 |
| Portal Inflammation (score) | 0.69 | ||
| None (0) | 43 (10.4%) | 3 (6.8%) | |
| Mild (1) | 260 (63.1%) | 27 (61.4%) | |
| More than mild (2) | 109 (26.5%) | 14 (31.8%) | |
| Liver cell injury: | |||
| Ballooning (score)‡: many (2) | 276 (67.0%) | 37 (84.1%) | 0.03 |
| Acidophil bodies: many | 160 (38.8%) | 25 (56.8%) | 0.02 |
| Pigmented macrophages: many | 372 (90.3%) | 41 (93.2%) | 0.79 |
| Megamitochondria: many | 76 (18.5%) | 14 (31.8%) | 0.05 |
| Other findings: | |||
| Mallory Denk bodies: present | 186 (45.2%) | 32 (72.7%) | < 0.001 |
| Glycogenated nuclei: many | 171 (41.5%) | 23 (52.3%) | 0.20 |
| NAFLD Activity Score (NAS) | 0.72 | ||
| 0 – 4 | 111 (26.9%) | 13 (29.5%) | |
| 5 – 8 | 301 (73.1%) | 31 (70.5%) | |
| Mean ± SD | 5.36 ± 1.25 | 5.43 ± 1.37 | 0.73 |
Determination of histological features from centrally reviewed biopsies using the NASH CRN Scoring System9
P values determined from Fisher’s exact test or Cuzick non-parametric test for trend across ordered categories, except for NAS, a t-test was used
1 non-elderly patient and 0 elderly patients had no ballooning
Characteristics of elderly patients with definite NASH
We then investigated the characteristics of presence of NASH in elderly patients by comparing how it differs from those without NASH in this age group (supplementary table 1). Elderly patients with NASH had significantly higher average values for AST (70 ± 48 vs. 38 ± 12 U/L; P < 0.001), ALT (75 ± 49 vs. 49 ± 21 U/L; P=0.006) and GGT (88 ± 82 vs. 49 ± 44 U/L; P=0.02). In addition, the average platelet count was lower (204 ± 59 vs. 254 ± 71 x1000/mm3; P=0.02) (supplementary table 1). The mean APRI score was significantly higher in elderly patients with NASH compared to elderly patients without NASH (0.8 ± 0.7 vs. 0.4 ± 0.3; P < 0.001). There was no significant difference in steatosis and degree of lobular inflammation between those with and without NASH. However, as would be expected, NASH patients were more likely to have ballooning degeneration. In addition, Mallory-Denk bodies were present in 72% of NASH patients, while these were absent in those who did not have NASH (P <0.001) (supplementary table 1). The NAFLD activity score as indicated by the percentage of patients with NAS ≥ 5 was higher in elderly patients with NASH compared to elderly patients without NASH (70% vs. 18%; P <0.001). Elderly patients with NASH were more likely to have advanced fibrosis compared to elderly patients who did not have NASH (52% vs. 24%, P = 0.05) (supplementary table 1).
Independent predictors of NASH among elderly patients determined from multivariable-adjusted logistic regression analyses were: younger age among this cohort with age ≥ 65 (OR= 0.65, 95% CI: 0.46–0.91, P= 0.01); higher AST value (OR=1.12, 95% CI: 1.03–1.22, P=0.007) and lower platelet count (OR= 0.98, 95% CI: 0.96–1.00, P=0.02) (Table 4).
Table 4.
Multivariable logistic regression analysis of demographic, clinical and histological characteristics in elderly patients with NAFLD: Characteristics independently associated with definite NASH and advanced fibrosis
| Characteristics | Unadjusted†
|
Adjusted‡
|
||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Definite NASH* | ||||
| Demographics | ||||
| Female (vs male) | 1.62 (0.45, 5.80) | 0.46 | 1.15 (0.18, 7.50) | 0.79 |
| Age (years) | 0.86 (0.72,1.04) | 0.11 | 0.65 (0.46, 0.91) | 0.01 |
| White (vs non-white) | 0.69 (0.16, 2.88) | 0.61 | 0.30 (0.04, 2.27) | 0.25 |
| Hispanic (vs nonHispanic) | 2.53 (0.28, 22.71) | 0.41 | n/s | |
| Ever smoked regular (vs not) | 0.89 (0.29, 2.73) | 0.84 | -- | |
| Clinical | ||||
| Cardiovascular disease (yes vs no) | 1.04 (0.24, 4.48) | 0.91 | -- | |
| Type 2 diabetes (yes vs no) | 1.75 (0.43, 7.19) | 0.44 | n/s | |
| BMI (kg/m2) | 1.04 (0.93, 1.18) | 0.47 | n/s | |
| Laboratory markers | ||||
| AST (U/L) | 1.08 (1.02, 1.13) | 0.01 | 1.12 (1.03, 1.22) | 0.007 |
| ALT (U/L) | 1.02 (1.00, 1.05) | 0.06 | n/s | |
| GGT (U/L) | 1.01 (1.00, 1.03) | 0.10 | n/s | |
| Platelets (1000/mm3) | 0.99 (0.98, 1.00) | 0.02 | 0.98 (0.96, 1.00) | 0.02 |
| Hypertriglyceridemia (yes vs no) | 1.09 (0.35, 3.38) | 0.89 | n/s | |
| Low HDL (yes vs no) | 1.41 (0.46, 4.37) | 0.55 | n/s | |
| High blood pressure (yes vs no) | 0.60 (0.18, 2.01) | 0.41 | n/s | |
| HOMA-IR (mg/dL × lU/mL/405) | 1.03 (0.89, 1.18) | 0.70 | n/s | |
| Ferritin (ng/mL) | 1.00 (1.00, 1.00) | 0.48 | n/s | |
| Advanced fibrosis (yes vs no) | 3.56 (1.00, 12.64) | 0.05 | n/s | |
| Advanced Fibrosis* | ||||
| Demographics | ||||
| Female (vs males) | 1.08 (0.32, 3.59) | 0.90 | 0.44 (0.06, 2.79) | 0.38 |
| Age (years) | 1.13 (0.5, 1.36) | 0.17 | 1.34 (0.97, 1.87) | 0.08 |
| Ever smoked regular (vs not) | 1.41 (0.51, 3.88) | 0.51 | n/s | |
| White (vs non-white) | 0.82 (0.24, 2.83) | 0.76 | 2.92 (0.40, 21.19) | 0.29 |
| Clinical | ||||
| Cardiovascular disease (yes vs not) | 4.35 (1.03, 18.4) | 0.05 | n/s | |
| Type 2 diabetes (yes vs no) | 1.14 (0.35, 3.66) | 0.83 | n/s | |
| BMI (kg/m2) | 1.18 (1.03, 135) | 0.02 | n/s | |
| Laboratory markers | ||||
| AST (U/L) | 1.00 (0.99, 1.01) | 0.64 | 1.08 (1.02, 1.14) | 0.007 |
| ALT (U/L) | 0.99 (0.98, 1.00) | 0.12 | 0.91 (0.86, 0.97) | 0.002 |
| GGT (U/L) | 1.01 (1.00, 1.02) | 0.04 | n/s | |
| Platelets (1000/mm3) | 0.98 (0.97, 1.00) | 0.01 | n/s | |
| Hypertriglyceridemia (yes vs no) | 1.50 (0.54, 4.18) | 0.44 | n/s | |
| Low HDL (yes vs no) | 3.21 (1.08, 9.59) | 0.04 | 8.35 (1.50, 46.5) | 0.02 |
| HOMA-IR (mg/dL × lU/mL/405) | 1.15 (1.00, 1.32) | 0.05 | n/s | |
| High blood pressure (yes vs no) | 2.54 (0.85, 7.58) | 0.10 | n/s | |
| Ferritin (ng/mL) | 1.00 (1.00, 1.00) | 0.58 | n/s | |
| Definite NASH (yes vs no) | 3.56 (1.00, 12.64) | 0.05 | 10.37 (1.22, 87.94) | 0.03 |
Total number=59; no. with definite NASH = 42, not definite NASH =17; no. with advanced fibrosis = 25, not advanced =34
Unadjusted odds ratios and p-values determined from logistic regression of either definite NASH or advanced fibrosis on each characteristic.
The adjusted model determined from backward stepwise regression of either definite NASH or advanced fibrosis on the candidate set forcing age, gender and race into the model. If the characteristic was not in the final model, n/s was used to indicate to indicate this. Smoking and CVD were excluded from the candidate set for definite NASH due to non-collinearity (noted with --); ethnicity was excluded from the candidate set for the advanced fibrosis model due to no non-Hispanic males with fibrosis.
Characteristics of elderly patients with advanced fibrosis
Characteristics of elderly patients with advanced fibrosis compared to those with stage 0–2 fibrosis are shown in supplementary table 2. Patients with advanced fibrosis were more likely to have metabolic syndrome, higher average BMI and increased fasting serum insulin, HOMA-IR, INR, and AST/ALT ratio. In addition, patients with advanced fibrosis had lower mean platelet count, total cholesterol, and LDL cholesterol levels. Patients with advanced fibrosis had significantly less steatosis but more portal inflammation, ballooning, Mallory-Denk bodies, and higher prevalence of NASH.
In multivariable-adjusted logistic regression analysis, the independent predictors of advanced fibrosis included a higher AST level (OR=1.08, 95% CI: 1.02–1.14, P=0.007), a lower ALT value (OR=0.91,95% CI: 0.86–0.97, P=0.002), and increased odds of having a low HDL cholesterol (OR=8.35, 95% CI: 1.50–46.50, P=0.02) (Table 4).
Discussion
This is a secondary analysis of prospectively collected clinical, biochemical and histologic data of a large number (n = 796) of adult patients with biopsy-proven NAFLD that allowed the detailed characterization of NAFLD in elderly versus non-elderly patients. The main findings of this study are that elderly patients with NAFLD have significantly higher rates of NASH and advanced fibrosis than non-elderly patients with NAFLD. Furthermore, among those NAFLD patients who already have NASH on liver biopsy, elderly patients are more likely to have advanced fibrosis as well as other features suggestive of severe liver disease including presence of ballooning degeneration, acidophil bodies, and Mallory-Denk bodies compared to non-elderly patients with NASH. These findings suggest that the severity of liver histology is shifted to a more aggressive phenotype in elderly patients across the spectrum of NAFLD including those with or without NASH. This is further supported by the surprising finding that the prevalence of advanced fibrosis was 24% in elderly patients who did not have evidence of NASH on biopsy, and the advanced fibrosis prevalence rate increased to 52% in elderly patients who had evidence of NASH on liver histology (p-value <0.05). Further studies with larger sample size are needed to confirm this phenomenon of presence of advanced fibrosis in non-NASH elderly patients with NAFLD.
A higher AST, a lower platelet count, and a lower (not higher) ALT and a low HDL cholesterol are independent predictors of NASH and advanced fibrosis; these commonly available factors can be utilized to aid clinical decision-making regarding when to consider a liver biopsy in elderly patients with NAFLD. These data are in agreement with previously published studies by the NASH-CRN and other cohorts but highlight that ALT may be lower in elderly patients and low HDL was the most significant predictor of advanced disease in elderly patients26,36, 40.
NAFLD is reported to be more prevalent in men than in women, and its prevalence may increase with age 16, 37–39. Other studies have also suggested that the prevalence of NAFLD may be influenced by menopause, and it may be more prevalent in women after menopause 39–41. Our results are consistent with previous studies that elderly patients with NAFLD are more likely to be women. Frith et al17 have shown that fibrosis and cirrhosis are higher in elderly patients with NAFLD. Using this well-characterized clinico-pathologic cohort, we confirm their findings by demonstrating that elderly patients had laboratory findings that are suggestive of more advanced liver disease including, higher AST/ALT ratio, higher APRI score and lower platelet count42, 43. Furthermore, we showed that elderly patients have more definite NASH, advanced fibrosis and cirrhosis compared to non-elderly patients. Given that this a cross-sectional study, one can argue that higher prevalence of advanced liver disease found in elderly patients can be due to the fact that they have more metabolic risk factors44. However; in our cohort; the elderly patients didn’t have more risk factors such as diabetes or insulin resistance42. Indeed elderly patients had lower BMI and waist circumference.
The novelty of the study is the detailed histological description of NAFLD and NASH by a panel of expert pathologists, and availability of paired clinical, demographic, and biochemical dataset that allowed the comparison between elderly and non-elderly patients with biopsy-proven NAFLD.
Our findings in the context of the previous studies may suggest that early in the natural history of NAFLD, the steatosis starts in zone 3 and with progressive aging (as well as with disease progression because they are collinear with each other), steatosis spreads to other zones and the pattern of steatosis distribution becomes pan-acinar with more cellular injury, and then perhaps due to progressive fibrosis and regeneration/remodelling, the pattern is further modified, and steatosis distribution becomes azonal as patients develop more advanced fibrosis. In addition steatosis paradoxically decreases in elderly patients despite having more severe disease. Firth et al and Permutt et al have previously shown that steatosis grade on histology and liver fat content estimated by MRI, respectively, are significantly lower in patients with cirrhosis compared to those with less degree of fibrosis10, 45, 46. One plausible explanation of this paradoxical reduction in steatosis may be related to reduced ability of the stiffened fibrotic liver to store and accumulate fat in the hepatocytes. The collagen deposition in the liver tissue replaces fat in the liver and restricts further accumulation of fat in hepatocytes. Prospective studies are needed to confirm this hypothesis. Moreover, the mechanisms underlying these alterations in steatosis distribution by age need to be studied further.
Strengths and limitations
The strengths of the study include prospective design of the study, and availability of well-characterized liver histology data. The study utilized the well-accepted and previously validated NASH-CRN Histologic Scoring System9, 47. Liver biopsy assessment was performed by a panel of expert liver pathologists during central review by consensus of the members of the pathology committee. This study included comparisons between elderly and non-elderly patients with NAFLD as well as NASH. Although our cohort is large, the number of elderly patients was relatively small but provided sufficient power to detect clinically significant differences. This also illustrates the challenges in the recruitment and enrollment of elderly patients in cohort studies especially with the requirement of liver biopsy. It took several years for this large multicenter study to recruit patients with biopsy-proven NAFLD. Age correlates with duration of disease and the association with more advanced disease may not be attributable to age alone, but also to duration of disease. However, it is not possible to control for duration of disease. Despite this limitation, it is elderly patients that have higher rates of NASH and advanced fibrosis whether it is due to aging or due to duration of disease. Longitudinal studies with serial liver biopsies will be required to investigate the natural progression of the disease in younger and older adults and to examine the evolution of fat distribution.
Conclusion
Elderly patients with NAFLD are more likely to have features of advanced fibrosis as well as aggressive NASH. NAFLD cannot be considered a benign disease in elderly patients. Elderly patients are at increased risk of NASH and advanced fibrosis but are underrepresented in cohort studies. Advanced fibrosis can also occur in elderly patients with NAFLD without specific histologic features of NASH. This observation may reflect the previous observation that key features of NASH such as steatosis, ballooning and Mallory-Denk bodies may be lost as the disease progresses towards cirrhosis. Thus liver biopsy evaluation can be helpful in this age group to guide the implementation of treatment recommendations such as weight reduction and increased physical activity. Due to the aging of American society, further research is needed in NAFLD in elderly patients. It is important to identify elderly NAFLD patients who are at risk of progressive liver disease, especially because newer treatment modalities are emerging 48–55. Furthermore, clinical trials should be conducted to test the efficacy and safety of the available treatment modalities, such as vitamin E, in this sub-population and every effort should be made to avoid excluding patients older than 65 years in future trials and cohort studies.
Supplementary Material
Acknowledgments
Funding Support: The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, U01DK061713). Several clinical centers use support from General Clinical Research Centers or Clinical and Translational Science Awards in conduct of NASH CRN Studies (grants UL1RR024989, M01RR000750, UL1RR02413101, M01RR000827, UL1RR02501401, M01RR000065, M01RR020359). This work is supported in part by the American Gastroenterological Association (AGA) Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award; Funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Association of Specialty Professors, and the American Gastroenterological Association and K23DK090303 to Rohit Loomba, MD, MHSc. This work was supported in part by the intramural research program of the NIH, National Cancer Institute.
Role of Sponsor: The study was sponsored by the NIDDK, NIH. As per the policy of the network the manuscript was reviewed by the NIDDK prior to publication. Authors take full responsibility of the data analyses and credibility of findings.
Appendix Members of the Nonalcoholic Steatohepatitis Clinical Research Network
Members of the Nonalcoholic Steatohepatitis Clinical Research Network Adult Clinical Centers:
Case Western Reserve University Clinical Centers
Metro Health Medical Center, Cleveland, OH: Arthur J. Mc Cullough, MD; Patricia Brandt; Diane Bringman, RN (2004–2008); Srinivasan Dasarathy, MD; Jaividhya Dasarathy MD; Carol Hawkins, RN; Yao-Chang Liu, MD (2004–2009); Nicholette Rogers, PhD, PAC(2004–2008)
Cleveland Clinic Foundation, Cleveland, OH: Arthur J. Mc Cullough, MD; Srinivasan Dasarathy, MD; Mangesh Pagadala, MD; Ruth Sargent, LPN; Lisa Yerian, MD; Claudia Zein, MD
California Pacific Medical Center, San Francisco, CA: Raphael Merriman, MD; Anthony Nguyen
Columbia University, New York, NY: Joel E. Lavine, MD, PhD
Duke University Medical Center, Durham, NC: Manal F. Abdelmalek, MD; Stephanie Buie; Anna Mae Diehl, MD; Marcia Gottfried, MD (2004–2008); Cynthia Guy, MD; Meryt Hanna (2010);Christopher Kigongo; Paul Killenberg, MD (2004–2008); Samantha Kwan, MS (2006–2009); Yi-Ping Pan; Dawn Piercy, FNP; Melissa Smith (2007–2010); Savita Srivastava, MD
Indiana University School of Medicine, Indianapolis, IN: Naga Chalasani, MD; Oscar W. Cummings, MD; Marwan Ghabril, MD; Ann Klipsch, RN; Linda Ragozzino, RN; Girish Subbarao, MD; Sweta Tandra, MD; Raj Vuppalanchi, MD
Saint Louis University, St Louis, MO: Brent A. Neuschwander-Tetri, MD; Joan Siegner, RN; Susan Stewart, RN; Debra King, RN; Judy Thompson, RN
University of California San Diego, San Diego, CA: Cynthia Behling, MD, PhD; Jennifer Collins;Janis Durelle; Tarek Hassanein, MD (2004–2009); Joel E. Lavine, MD, PhD (2002–2010); Rohit Loomba, MD; Anya Morgan (2009–2010); Thu Nguyen; Heather Patton, MD; Claude Sirlin, MD
University of California San Francisco, San Francisco, CA: Bradley Aouizerat, PhD; Kiran Bambha, MD (2006–2010); Marissa Bass; Nathan M. Bass, MD, PhD; Linda D. Ferrell, MD; Bo Gu (2009–2010); Bilal Hameed, MD; Mark Pabst; Monique Rosenthal (2005–2010); Tessa Steel (2006–2008)
University of Washington Medical Center, Seattle, WA: Matthew Yeh, MD, PhD
Virginia Commonwealth University, Richmond, VA: Sherry Boyett, RN, BSN; Melissa J. Contos, MD; Michael Fuchs, MD; Amy Jones; Velimir AC Luketic, MD; Puneet Puri, MD; Bimalijit Sandhu, MD (2007–2009); Arun J. Sanyal, MD; Carol Sargeant, RN, BSN, MPH; Kimberly Noble; Melanie White, RN, BSN (2006–2009)
Virginia Mason Medical Center, Seattle, WA: Sarah Ackermann; Kris V. Kowdley, MD; Jane Park; Tracey Pierce; Jody Mooney, MS; James Nelson, PhD; Cheryl Shaw, MPH; Alice Stead; Chia Wang, MD
Washington University, St. Louis, MO: Elizabeth M. Brunt, MD
Resource Centers
National Cancer Institute, Bethesda, MD: David E. Kleiner, MD, PhD
National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD: Edward C. Doo, MD; Jay H. Hoofnagle, MD; Patricia R. Robuck, PhD, MPH; Averell Sherker, MD
Johns Hopkins University, Bloomberg School of Public Health (Data Coordinating Center), Baltimore, MD: Patricia Belt, BS; Frederick L. Brancati, MD, MHS (2003–2009); Jeanne M. Clark, MD, MPH; Ryan Colvin, MPH (2004–2010); Michele Donithan, MHS; Mika Green, MA; Rosemary Hollick (2003–2005); Milana Isaacson, BS; Wana K. Jin, BS; Alison Lydecker, MPH (2006–2008), Pamela Mann, MPH (2008–2009); Kevin P. May, MS; Laura Miriel, BS; Alice Sternberg, ScM; James Tonascia, PhD; Aynur Ünalp-Arida, MD, PhD; Mark Van Natta, MHS; Ivana Vaughn, MPH; Laura Wilson, ScM; Katherine Yates, ScM
Footnotes
Conflict of interest: No conflicts of interest exist
Authors Contributions: Mazen Noureddin: Drafting of the manuscript, interpretation of data, critical revision of the manuscript, approved final submission
Katherine P Yates: Analysis and interpretation of data, statistical analysis, critical revision of the manuscript, approved final submission
Ivana A Vaughn: Analysis and interpretation of data, statistical analysis, critical revision of the manuscript, approved final submission
Brent A. Neuschwander-Tetri: Critical revision of the manuscript, approved final submission
Arun J. Sanyal: Critical revision of the manuscript, approved final submission
Arthur McCullough: Critical revision of the manuscript, approved final submission
Raphael Merriman: Critical revision of the manuscript, approved final submission
Bilal Hameed: Critical revision of the manuscript, approved final submission
Edward Doo: Critical revision of the manuscript, approved final submission
David E. Kleiner: Critical revision of the manuscript, approved final submission
Cynthia Behling: Critical revision of the manuscript, approved final submission
Rohit Loomba: Study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, obtained funding, study supervision, approved final submission
References
- 1.Kim WR, Brown RS, Jr, Terrault NA, et al. Burden of liver disease in the United States: summary of a workshop. Hepatology. 2002;36:227–42. doi: 10.1053/jhep.2002.34734. [DOI] [PubMed] [Google Scholar]
- 2.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. The American journal of gastroenterology. 2003;98:960–7. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 3.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–31. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Ratziu V, Bellentani S, Cortez-Pinto H, et al. A position statement on NAFLD/NASH based on the EASL 2009 special conference. Journal of hepatology. 2010;53:372–84. doi: 10.1016/j.jhep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 6.Chen CH, Huang MH, Yang JC, et al. Prevalence and etiology of elevated serum alanine aminotransferase level in an adult population in Taiwan. Journal of gastroenterology and hepatology. 2007;22:1482–9. doi: 10.1111/j.1440-1746.2006.04615.x. [DOI] [PubMed] [Google Scholar]
- 7.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–21. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. The American journal of gastroenterology. 2003;98:2042–7. doi: 10.1111/j.1572-0241.2003.07659.x. [DOI] [PubMed] [Google Scholar]
- 9.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 10.Brunt EM, Kleiner DE, Wilson LA, et al. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): a histologic marker of advanced NAFLD-Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49:809–20. doi: 10.1002/hep.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter-Kent C, Brunt EM, Yerian LM, et al. Relations of steatosis type, grade, and zonality to histological features in pediatric nonalcoholic fatty liver disease. Journal of pediatric gastroenterology and nutrition. 2011;52:190–7. doi: 10.1097/MPG.0b013e3181fb47d3. [DOI] [PubMed] [Google Scholar]
- 12.Alkhouri N, De Vito R, Alisi A, et al. Development and Validation of a New Histological Score for Pediatric Nonalcoholic Fatty Liver Disease (NAFLD) Journal of hepatology. 2012 doi: 10.1016/j.jhep.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 13.Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–9. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 14.Lonardo A, Lombardini S, Scaglioni F, et al. Fatty liver, carotid disease and gallstones: a study of age-related associations. World journal of gastroenterology: WJG. 2006;12:5826–33. doi: 10.3748/wjg.v12.i36.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki A, Angulo P, Lymp J, et al. Chronological development of elevated aminotransferases in a nonalcoholic population. Hepatology. 2005;41:64–71. doi: 10.1002/hep.20543. [DOI] [PubMed] [Google Scholar]
- 16.Kagansky N, Levy S, Keter D, et al. Non-alcoholic fatty liver disease--a common and benign finding in octogenarian patients. Liver international: official journal of the International Association for the Study of the Liver. 2004;24:588–94. doi: 10.1111/j.1478-3231.2004.0969.x. [DOI] [PubMed] [Google Scholar]
- 17.Frith J, Day CP, Henderson E, et al. Non-alcoholic fatty liver disease in older people. Gerontology. 2009;55:607–13. doi: 10.1159/000235677. [DOI] [PubMed] [Google Scholar]
- 18.Arias E, Curtin LR, Wei R, et al. U.S. decennial life tables for 1999–2001, United States life tables National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System. 2008;57:1–36. [PubMed] [Google Scholar]
- 19.Arias E, Rostron BL, Tejada-Vera B. United States life tables, 2005. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2010;58:1–132. [PubMed] [Google Scholar]
- 20.Arias E. United States life tables, 2006. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2010;58:1–40. [PubMed] [Google Scholar]
- 21.Trends in aging--United States and worldwide. MMWR Morbidity and mortality weekly report. 2003;52:101–4. 106. [PubMed] [Google Scholar]
- 22.Dong MH, Bettencourt R, Barrett-Connor E, et al. Alanine aminotransferase decreases with age: the Rancho Bernardo Study. PLoS One. 2010;5:e14254. doi: 10.1371/journal.pone.0014254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. The New England journal of medicine. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chalasani NP, Sanyal AJ, Kowdley KV, et al. Pioglitazone versus vitamin E versus placebo for the treatment of non-diabetic patients with non-alcoholic steatohepatitis: PIVENS trial design. Contemporary clinical trials. 2009;30:88–96. doi: 10.1016/j.cct.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Annals of internal medicine. 2007;147:W163–94. doi: 10.7326/0003-4819-147-8-200710160-00010-w1. [DOI] [PubMed] [Google Scholar]
- 26.Neuschwander-Tetri BA, Clark JM, Bass NM, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52:913–24. doi: 10.1002/hep.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.US census Bureau; 2012. http://www.census.gov/prod/1/pop/profile/95/24_ps.pdf. [Google Scholar]
- 28.National Institute of Aging, National Institutes of Health; 2012. http://www.nia.nih.gov/about. [Google Scholar]
- 29.Hosmer D, Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons, Inc; 2000. [Google Scholar]
- 30.Chaurasia A, Harel O. Using AIC in Multiple Linear Regression framework with Multiply Imputed Data. Health services & outcomes research methodology. 2012;12:219–233. doi: 10.1007/s10742-012-0088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paul P, Pennell ML, Lemeshow S. Standardizing the power of the Hosmer-Lemeshow goodness of fit test in large data sets. Statistics in medicine. 2012 doi: 10.1002/sim.5525. [DOI] [PubMed] [Google Scholar]
- 32.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974:716–723. [Google Scholar]
- 33.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. Springer-Verlag; 2002. [Google Scholar]
- 34.SAS Institute, Inc. SAS software, version 9.3 of the SAS system for Windows. Cary, NC: 2002–2010. [Google Scholar]
- 35.StataCorp. Stata statistical software: release 12. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- 36.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–54. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 37.Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clinics in liver disease. 2009;13:511–31. doi: 10.1016/j.cld.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Okanoue T, Umemura A, Yasui K, et al. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in Japan. Journal of gastroenterology and hepatology. 2011;26 (Suppl 1):153–62. doi: 10.1111/j.1440-1746.2010.06547.x. [DOI] [PubMed] [Google Scholar]
- 39.Gutierrez-Grobe Y, Ponciano-Rodriguez G, Ramos MH, et al. Prevalence of non alcoholic fatty liver disease in premenopausal, posmenopausal and polycystic ovary syndrome women. The role of estrogens Annals of hepatology. 2010;9:402–9. [PubMed] [Google Scholar]
- 40.Hashimoto E, Tokushige K. Prevalence, gender, ethnic variations, and prognosis of NASH. Journal of gastroenterology. 2011;46 (Suppl 1):63–9. doi: 10.1007/s00535-010-0311-8. [DOI] [PubMed] [Google Scholar]
- 41.Fan JG, Farrell GC. Epidemiology of non-alcoholic fatty liver disease in China. Journal of hepatology. 2009;50:204–10. doi: 10.1016/j.jhep.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 42.Loaeza-del-Castillo A, Paz-Pineda F, Oviedo-Cardenas E, et al. AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Annals of hepatology. 2008;7:350–7. [PubMed] [Google Scholar]
- 43.Harrison SA, Oliver D, Arnold HL, et al. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–7. doi: 10.1136/gut.2007.146019. [DOI] [PubMed] [Google Scholar]
- 44.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA: the journal of the American Medical Association. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 45.Permutt Z, Le TA, Peterson MR, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Alimentary pharmacology & therapeutics. 2012 doi: 10.1111/j.1365-2036.2012.05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adams LA, Sanderson S, Lindor KD, et al. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–8. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Zein CO, Yerian LM, Gogate P, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610–9. doi: 10.1002/hep.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Promrat K, Lutchman G, Uwaifo GI, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39:188–96. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- 51.Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, et al. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38:1008–17. doi: 10.1053/jhep.2003.50420. [DOI] [PubMed] [Google Scholar]
- 52.Nair S, Diehl AM, Wiseman M, et al. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Alimentary pharmacology & therapeutics. 2004;20:23–8. doi: 10.1111/j.1365-2036.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- 53.Loomba R, Lutchman G, Kleiner DE, et al. Clinical trial: Pilot study of metformin for the treatment of nonalcoholic steatohepatitis. Aliment Pharmacol Ther. 2008 doi: 10.1111/j.1365-2036.2008.03869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caldwell SH, Hespenheide EE, Redick JA, et al. A pilot study of a thiazolidinedione, troglitazone, in nonalcoholic steatohepatitis. The American journal of gastroenterology. 2001;96:519–25. doi: 10.1111/j.1572-0241.2001.03553.x. [DOI] [PubMed] [Google Scholar]
- 55.Van Wagner LB, Koppe SW, Brunt EM, et al. Pentoxifylline for the treatment of non-alcoholic steatohepatitis: a randomized controlled trial. Annals of hepatology. 2011;10:277–86. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.