Abstract
Rationale
The dynorphin (DYN)/kappa opioid receptor (KOR) system is involved in the dysphoric properties of drugs of abuse. Given that adolescents show reduced sensitivity to aversive effects of many drugs, alterations in the DYN/KOR system may contribute to the prevalence of drug use during adolescence.
Objectives
The present study was designed to assess dysphoric properties of a selective kappa agonist, U62,066, in adolescent and adult rats using both conditioned taste aversion (CTA) and conditioned place aversion (CPA) paradigms.
Methods
For CTA, water-restricted rats were administered U62,066 following 30 min access to a saccharin solution, with subsequent saccharin consumption used to index aversion. For CPA, animals were allowed access to both compartments of a two-compartment chamber for a 15-minute pre- and post-conditioning test. For conditioning, subjects were administered U62,066 prior to confinement to one side of the chamber and saline prior to confinement to the other side for a total of four pairings.
Results
Overall, adolescents displayed reduced sensitivity to the kappa agonist relative to adults. Adults demonstrated taste aversions to the 0.2 and 0.3 mg/kg doses of U62,066 whereas adolescents did not display aversions to any tested doses. Adults demonstrated a place aversion to the 0.1 and 0.2 mg/kg dose of U62,066 when paired with the preferred side of the conditioning chamber. Adolescents did not display aversions to any of the doses tested.
Conclusions
Reduced sensitivity to DYN/KOR system activation during adolescence may be a contributing factor to the age-typical insensitivity to aversive properties of drugs commonly abused by adolescents.
Keywords: Adolescence, Age differences, Rat, Kappa opioid system, Dynorphin, U62, 066, Spiradoline, Conditioned taste aversion, Conditioned place aversion
Individuals make the transition from youth to maturity during adolescence, a developmental period characterized by numerous neurobehavioral changes that are highly conserved across mammalian species (see Spear 2010, for references and review). In addition to the hormonal and physiological transformations associated with pubertal development, adolescents also exhibit a number of age-typical behavioral characteristics that include elevated levels of social interactions with peers (Hartup and Stevens 1997; Primus and Kellogg 1989; Varlinskaya and Spear 2008), novelty-seeking (Adriani et al. 1998), risk-taking (Laviola et al. 2003; Douglas et al. 2003; Steinberg 2008; Vetter-O’Hagen and Spear 2012) and impulsivity (Adriani and Laviola 2003). These behaviors are commonly observed in both humans and animal models of adolescence. In rats, adolescence is conservatively defined as the age range between postnatal day (P)28 and P42 during which adolescent-typical behavioral alterations are evident in males and females (Spear 2000).
Adolescence is the period during which substance abuse is commonly initiated: Among high school seniors, over 40% have smoked cigarettes or marijuana, 25% have used illicit drugs, and 70% have consumed ethanol (Johnston et al. 2012). Elevated drug use during adolescence may, in part, reflect age differences in sensitivity to the rewarding and aversive effects of drugs of abuse. Given the ethical constraints that limit or preclude such research in humans, animal models of adolescence provide a useful way to examine these effects experimentally. Dysphoria and other adverse effects of drugs can be assessed using conditioned aversion procedures. With conditioned taste aversion (CTA), ingestion of a novel flavor (conditioned stimulus; CS) is paired with the effects of a specific drug (unconditioned stimulus; US), whereas with conditioned place aversion (CPA), an environmental context serves as the CS paired with the US. Interestingly, evidence suggests that adolescents may be less sensitive than adults to aversive properties of several drugs of abuse. For example, adolescent rats demonstrate attenuated CTAs to ethanol (Anderson et al. 2010; Schramm-Sapyta et al. 2010; Vetter-O’Hagen et al. 2009), cocaine (Schramm-Sapyta et al. 2006), nicotine (Shram et al. 2006; Wilmouth and Spear 2004), THC (Schramm-Sapyta et al. 2007), morphine (Hurwitz et al. 2012) and amphetamine (Infurna and Spear 1979). Conversely, adolescents appear more sensitive to the positive rewarding properties of a number of drugs of abuse, including nicotine (Torres et al. 2008; Vastola et al. 2002), methamphetamine (Zakharova et al. 2009a) and cocaine (Badanich et al. 2006; Zakharova et al. 2009a; Zakharova et al. 2009b).
Although the specific underlying mechanisms contributing to the adolescent-typical insensitivity to aversive properties of drugs are still unknown, evidence supports a role for the dynorphin (DYN)/kappa opioid receptor (KOR) system in negative effects of many drugs of abuse (see Shippenberg et al. 2007; Wee and Koob 2010, for references and review). Potential age-related alterations in this endogenous opioid system could contribute to the adolescent-typical insensitivity that has been reported to the aversive effects of a number of drugs. If this were the case, similar age differences in sensitivity to aversive effects of KOR activation would be expected. Although there has been little investigation of possible ontogenetic differences in this opioid system between adolescence and adulthood, one recent developmental study reported age differences in KOR function following chronic nicotine exposure (Tejeda et al. 2012), results that support differential activation of the KOR system across age under some circumstances.
The present study was designed to assess aversions induced by pharmacological activation of kappa opioid receptors in adolescent and adult male rats using both CTA (Experiment 1) and CPA (Experiment 2) paradigms. Males were selected because previous research focusing on age differences in aversive drug properties has focused almost exclusively on male subjects (though see Vetter-O’Hagen et al. 2009; Zakharova et al. 2009b). We hypothesized that adolescents would be less sensitive to the dysphoric effects induced by the kappa agonist, U62,066 (Wadenberg 2003), with higher doses of the drug needed for CTAs and CPAs in adolescents than in adults.
Experiment 1 – Conditioned Taste Aversion
Subjects
A total of 60 male Sprague Dawley rats bred in our animal facility at Binghamton University were used as subjects. Animals were weaned on postnatal day (P)21 and pair-housed with a same-sex littermate. Subjects were maintained in a temperature- and humidity-controlled vivarium on a 12:12 hr light/dark cycle (lights on at 0700) and were treated in accordance with guidelines for animal care established by the National Research Council (Committee for the Update of the Guide for the Care and Use of Laboratory Animals, 2011) under protocols approved by the Binghamton University Institutional Animal Care and Use Committee. Food was available in the home cage at all times, with water restricted as described below. All testing occurred during the light cycle between the hours of 0900 and 1200 given that the majority of studies assessing aversive effects of kappa agonists using CTA and CPA procedures have tested animals during the light phase of their light/dark cycle (i.e., Bals-Kubik et al. 1993; Logrip et al. 2009; McLaughlin et al. 2006; Nizhnikov et al. 2012; Sante et al. 2000; Tejeda et al. 2012).
Procedure
On P30/P72, all animals were 50% water deprived by providing each pair of subjects with a bottle containing half the amount of water they had ingested during the previous 24 hours. On the following day, the first conditioning session, subjects were separated in their home cage by a mesh divider to allow for measurement of individual consumption. Approximately 10 minutes later, each animal was then given access to a bottle of 0.1% saccharin for 30 minutes, immediately followed by an injection of the selective kappa agonist U62,066 (0, 0.1, 0.2, or 0.3 mg/kg, s.c.). Subjects remained separated from each other in the home cage for 2 hours post-injection to allow any drug effects to dissipate prior to reuniting animals with their cagemates. Following the conditioning session, animals were given ad libitum access to food and water for the next 24 hours, after which they were again water-deprived prior to the next conditioning session. This cycle of 50% water deprivation and conditioning followed by 24 hr of unlimited water access (and no conditioning) was repeated for a total of five conditioning/test sessions (with the final session not followed by an injection).
Experimental Design and Data Analyses
The design of this experiment was a 2 (age) × 4 (U62,066 dose) factorial, with 7–8 animals placed into each of the 8 experimental conditions. To avoid the possible confounding of litter with drug effects (Holson and Pearce 1992; Zorrilla 1997), animals were assigned semi-randomly to experimental groups, such that no more than one subject from a given litter was assigned to a particular drug dose. Baseline saccharin intake (ml) data were subjected to a one-way analysis of variance (ANOVA). Saccharin intake data (ml) across the four test days were analyzed via a 2 (age) × 4 (U62,066 dose) × 4 (day) repeated measures ANOVA. Significant effects were further explored using Fisher’s LSD tests.
Results
Analysis of baseline intake data revealed no significant differences between adolescents and adults (see Figure 1A). Analysis of saccharin intake data across the four test days revealed an age × dose interaction [F(3,52) = 3.6, p < .02], with adults injected with the 0.2 and 0.3 mg/kg dose of U62,066 drinking less saccharin solution than saline-injected controls. Adolescents did not demonstrate aversions to any doses of U62,066. A day × age interaction also emerged [F(3,156) = 3.0, p < .04); however, no relevant group differences were revealed. Accordingly, data are shown collapsed across test day (see Figure 1B).
Fig. 1. Conditioned taste aversion.
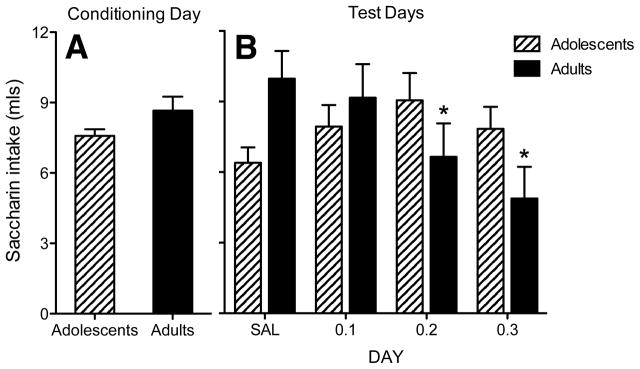
(A) Adolescents (striped bars) and adults (solid bars) consumed similar amounts of saccharin on the conditioning day (B) On the test days, adult rats administered the 0.2 and 0.3 mg/kg doses of U62,066 consumed less saccharin (indicated by asterisks) relative to saline controls, whereas no differences were seen in adolescents (data shown are collapsed across four test days)
Experiment 2 – Conditioned Place Aversion
Subjects
One hundred seventeen male Sprague Dawley rats bred in our facility were used as subjects. Food and water were available in the home cage at all times. All testing occurred during the light cycle between the hours of 0900 and 1200.
Apparatus
Testing occurred in three-compartment chambers that were size-adjusted for animals of each age. Each chamber consisted of two outer compartments (adults: 30.5 × 25 × 32 cm; adolescents: 22.8 × 18.8 × 24 cm) that flanked a smaller central compartment (adults: 11 × 25 × 32 cm; adolescents: 8.3 × 18.8 × 24 cm). In the adult-sized chambers, one outer compartment had solid black walls (Side A) and the other had vertical black and white stripes (Side B). In the adolescent-sized chambers, one outer compartment had solid black walls with white polka dots (Side A) whereas the other contained vertical black and white stripes (Side B). The central compartments were white, and all chambers had metal bar floors. This chamber configuration was based on preliminary work that varied chamber characteristics to produce equivalent baseline side preferences at each age; however, the majority of experimental subjects of both ages demonstrated a preference for Side A in the current study. Pre- and post-conditioning sessions were recorded by dome cameras (model CD33W, SuperCircuits, Austin, TX), and time spent in each compartment was scored later by an experienced observer with no knowledge of the experimental condition of any animal.
Procedure
One day prior to the start of conditioning, on P30 (adolescents) or P70 (adults), subjects were allowed to freely explore the entire chamber for a 15-min (pre-conditioning session). On each of the following four days, subjects were injected with either vehicle or a dose of U62,066 (0, 0.1, 0.2, or 0.3 mg/kg, s.c.) and restricted to one side of the conditioning chamber, for a total of two pairings of vehicle with one side of the chamber (designated the “paired side”) and 2 pairings of U62,066 with the other side (the “non-paired side”). For approximately half of the subjects, U62,066 was paired with Side A; the remaining subjects received the drug paired with Side B. Inclusion of saline-injected controls resulted in two groups of rats that received saline paired with both sides of the conditioning apparatus, with these rats matched for “paired side” based on the cohort of rats that were tested on the same day at the same time. Each conditioning session lasted 40 min, with drug order and side of chamber used as the “paired side” counterbalanced across subjects. After each conditioning trial, each pair of animals was returned to their home cage but were separated from each other by a wire mesh divider for 80 min to allow drug effects to dissipate prior to being reunited. When combined with the time spent in the chamber, this amount of time (2 hr) was equivalent to the amount of time animals were separated post-drug in Experiment 1. Twenty-four hours after the last conditioning session (i.e., on P35/P75), animals were given a 15-min post-conditioning test during which they had access to the entire chamber.
Experimental Design and Data Analyses
Pre-conditioning data were inspected to determine initial side preference for each individual subject and whether U62,066 was paired with the initially preferred side or the non-preferred side of the conditioning chamber. Pre- and post-conditioning data were expressed as percent of total time spent on the drug-paired side of the conditioning chamber: [(time on paired side)/(time on paired side + unpaired side)] x 100. Prior to analysis, three subjects with post-conditioning test values more than 2 standard deviations away from the group mean were identified as outliers and eliminated (2 adult saline, 1 adolescent 0.3 mg/kg). Data were analyzed via a 2 (age) × 4 (dose) × 2 (paired side: preferred or non-preferred) × 2 (test: pre- or post-conditioning) repeated measures ANOVA, with 6–9 subjects placed in each group. Place aversions were signified by significant decreases in post-conditioning values relative to pre-conditioning values. Time spent (seconds) in the central compartment during the pre- and post-conditioning tests was analyzed via 2 × 4 × 2 factorial ANOVAs. All significant effects were further explored with Fisher’s LSD tests.
Results
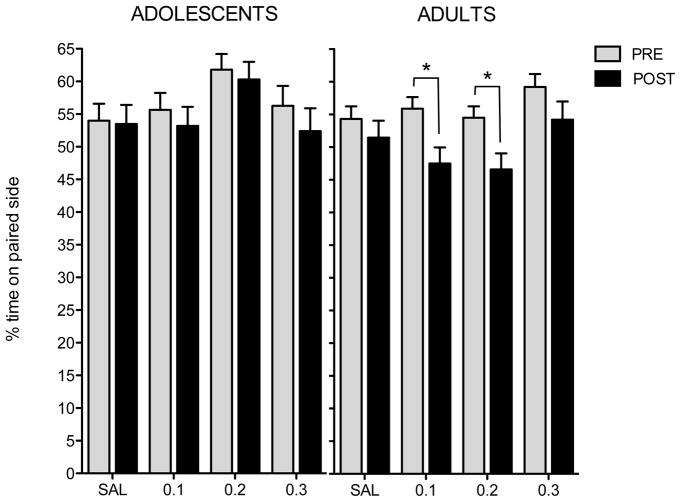
Analysis of the pre- and post-conditioning data revealed significant effects of paired side, test, and multiple interactions that were tempered by a significant 4-way interaction of all factors [F(3,98) = 3.2, p < .03]. Among animals that received pairings of U62,066 with the initially preferred chamber compartment (56% of adults and 49% of adolescents), adults demonstrated aversions to the 0.1 and 0.2 mg/kg doses whereas adolescents showed no aversions (see Figure 2). Regardless of age, no aversions to any dose were seen among animals for which U62,066 was paired with the initially non-preferred compartment (data not shown).
Fig. 2. Conditioned place aversion: Percent of time spent on the drug-paired side.
No differences between pre- and post-conditioning values were seen among adolescents (left panel), whereas adults (right panel) demonstrated reduced post-test (black bars) relative to pre-test (gray bars) values following conditioning with the 0.1 and 0.2 mg/kg doses of U62,066 (indicated by asterisks)
Analysis of time spent in the center compartment during the pre-conditioning revealed only a main effect of age [F(1,98) = 28.3, p < .001], with adolescents spending more time in the center (Mean and standard error: 242.2 ± 12.6) than adults (159.4 ± 10.4). Similarly, analysis of the post-conditioning data indicated that adolescents again spent more time in the center (282.6 ± 11.4) than adults (228.2 ± 12.1)[main effect of age: F(1,98) = 12.1, p < .001].
Discussion
KOR agonists have been found to consistently induce aversions in adult animals using both place and taste conditioning procedures following either peripheral (Bals-Kubik et al. 1989; Mucha and Herz 1985; Shippenberg and Herz 1986; Iwamoto 1986; Morales et al. 2007; Zhang et al. 2005) or central administration (Sante et al. 2000; Bals-Kubik et al. 1989). Results from the present studies are among the first to suggest differences in sensitivity to KOR agonists in adolescents relative to adults, with generally similar patterns of reduced sensitivity to KOR activation in adolescents relative to adults emerging with both the CTA and CPA paradigms. Adolescents failed to demonstrate taste or place aversions to any of the U62,066 doses tested, whereas adults consumed less saccharin solution following pairings with the 0.2 and 0.3 mg/kg doses (Experiment 1) and spent less time on the drug-paired side following conditioning with the 0.1 and 0.2 mg/kg doses on the initially preferred side (Experiment 2).
The attenuated aversion to the KOR agonist seen among adolescents is congruent with numerous reports of reduced sensitivity to aversion induced by drugs of abuse in adolescents relative to adults (e.g., Anderson et al. 2010; Hurwitz et al. 2012; Infurna and Spear 1979; Schramm-Sapyta et al. 2006; 2007; Wilmouth and Spear 2004). In Experiment 1 (CTA), adults consumed less saccharin solution following pairings with the 0.2 and 0.3 mg/kg doses of U62,066, whereas adolescents did not demonstrate an aversion to any of the doses tested. It is important to note that adolescents demonstrate conditioned taste aversion to U62,066 under some conditions. As a follow-up study, our laboratory recently assessed conditioned taste aversions to higher doses of U62,066 (0.3, 0.4, and 0.5 mg/kg) in adolescent and adult rats using a novel CTA procedure designed to eliminate the need for water deprivation (Anderson et al., in press). Adolescents in that study demonstrated a conditioned taste aversion to all doses tested, an effect that was blocked by stressor exposure. Thus, the results of Experiment 1 likely reflect differential sensitivity to KOR activation across age and not an aversion learning impairment.
Interestingly, saline-injected adults consumed more saccharin across test days relative to adolescents although no age differences emerged during the initial baseline intake. Given that baseline saccharin intake was not adjusted for body weight differences, it is surprisingly that adults did not consume more than adolescents. Baseline adult consumption may reflect an enhanced neophobic response relative to adolescents. Indeed, a previous CTA study from our laboratory found this same pattern of higher CS consumption after the initial conditioning day among saline-injected adults (Anderson et al., 2010; Experiment 2). On the other hand, for adolescents, the novelty of the saccharin solution on the conditioning day may have enhanced the incentive value of the solution, with that value diminishing following repeated exposures and thus potentially explaining the apparent reduction in consumption following pairings with saline injections. Although the potential influence of age differences in saccharin consumption among saline-injected controls should be considered when interpreting our CTA results, it is important to note that similar differences have been reported in other CTA studies comparing adolescents and adults (e.g., Hurwitz et al. 2012; Anderson et al. in press). In these studies, relatively low consumption among adolescents did not preclude expression of CTA among adolescents.
Though Experiment 2 was intended to examine CPA using an unbiased, counterbalanced procedure (see Cunningham et al., 2003), a preference for Side A emerged in a large number of adolescent and adult animals, although some animals demonstrated initial preference for Side B. Thus, the design of our study afforded an opportunity to assess the aversive effects of U62,066 in both a preferred and non-preferred context. When the drug was paired with the initially preferred chamber compartment, adults spent less time on the paired side (relative to the pre-conditioning values) following conditioning with the 0.1 and 0.2 mg/kg doses of U62,066—an effect that was not seen among adults when the drug was paired with their initially non-preferred side. Adolescents did not demonstrate place aversion following any of the tested doses. Given that conditioned place preferences are seen more easily (and arguably sometimes spuriously) when a drug is paired with a non-preferred context (e.g., Cunningham et al., 2003; Roma and Riley, 2005; Tzschentke, 1998; 2007), it is not surprising that place aversion emerged when the drug was paired with the preferred context. Indeed, previous assessments of place aversion induced by KOR agonists have involved pairings of the drug with the preferred side (McLaughlin et al. 2006; Michaels and Holtzman 2008; Tejeda et al. 2012).
Although similar results were revealed by the CTA and CPA paradigms, these models differ in several ways that must be considered. The CTA procedure involved water-deprivation, a condition that likely resulted in heightened stress among subjects in the CTA study. Similarly, U62,066 was administered a total of four times for the CTA study, whereas the CPA procedure involved only two drug pairings. Given evidence that administration of a kappa agonist mimics stressor exposure, the subjects in the CTA study were likely more stressed and may have experienced more salient dysphoria. Additionally, a feature of the place conditioning apparatus, the neutral center compartment, may have influenced the sensitivity of our CPA procedure. A previous study comparing CPP and CPA using both a two- and three-compartment conditioning chamber found that morphine produced similar conditioned place preference in both types of apparatus (an effect also described by Bardo et al. 1995), whereas the CPA induced by the kappa agonist, U50,488, was more pronounced when assessed in a two-compartment apparatus (Morales et al. 2007). Furthermore, counterbalanced designs typically assess time spent on one of two chamber sides (e.g., Cunningham et al. 2003). Inclusion of the central compartment may complicate interpretation of our CPA data, especially given that adolescents spent more time in the center than adults. This finding may reflect a preference for the relative novelty of this neutral area, in line with enhanced novelty preference/seeking behavior commonly reported during adolescence (Douglas et al. 2003; Kelley et al. 2004).
Overall, the results of this study provide evidence that adolescents are less sensitive to KOR agonist-induced aversion than adults. These findings extend prior ontogenetic studies of KOR agonists conducted in younger animals. A number of these studies reported reduced sensitivity to KOR agonists among preweanling animals relative to adults. For example, administration of various KOR agonists attenuated psychostimulant-induced locomotor activity in adults but not preweanling rats (McDougall et al. 1997; Collins et al. 2000). Likewise, U50,488 attenuated cocaine-induced dopamine release in adults but not preweanling rats (Cortez et al. 2010). Although at least one study has shown that KOR agonists can also produce conditioned odor aversions in infant rat pups (Barr et al. 1994), in other work, an ontogenetic switch has been reported in the effects of KOR agonists, with DYN shown to be a powerful appetitive reinforcer in neonatal rats (Petrov et al. 2006), in contrast to the aversive properties of KOR agonists consistently demonstrated in adult rats. Likewise, U62,066 was found to elicit conditioned taste preferences in P4 infant rats, an effect that dissipated by P12 (Nizhnikov et al. 2012). It should also be noted that when assessing CTA induced by higher doses of U62,066 using a different CTA paradigm (Anderson et al., in press), we found no age differences between adolescents and adults—results that may suggest age differences in sensitivity to this KOR agonist may be expressed selectively at low doses. Alternatively, differences between the CTA procedures used may have influenced the pattern of sensitivity.
There are two possible explanations for the reduced sensitivity to the KOR agonist seen in adolescent animals. First, the DYN/KOR system in adolescents may not be fully developed and therefore less likely to be activated by kappa agonists. Supporting this possibility, a recent study from our laboratory found that adolescents were insensitive to KOR antagonist-induced alterations in ethanol intake seen in adult subjects (Morales et al., submitted). Alternatively, adolescents may exhibit an enhanced kappa tone relative to adults under these test circumstances. In this case, administration of an agonist may not produce a change in adolescents if the kappa system is already activated (by stress, for example), thereby reducing their sensitivity to further kappa stimulation. A recent study in our laboratory, however, found similar effects of prior stress on KOR sensitivity in animals of both ages (Anderson et al., in press)
Although further studies are needed to illuminate the contribution of the DYN/KOR system to adolescent-typical insensitivities to the aversive properties of drugs and other stimuli (e.g., see Doremus-Fitzwater et al. 2010; Spear 2011, for review), findings of differential sensitivity to KOR activation in adolescents and adults provide evidence supporting a potential role of this system in adolescent drug abuse.
Footnotes
The authors have no conflicts of interest to disclose.
The work presented in this manuscript was funded by grants AA017823 to LPS and AA012453 to EIV.
References
- Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112:1152–66. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. Elevated levels of impulsivity and reduced place conditioning with d-amphetamine: two behavioral features of adolescence in mice. Behav Neurosci. 2003;117:695–703. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Agoglia AE, Morales M, Varlinskaya EI, Spear LP. Stress, kappa manipulations, and aversive effects of ethanol in adolescent and adult male rats. Neuroscience. doi: 10.1016/j.neuroscience.2012.12.028. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Varlinskaya EI, Spear LP. Ethanol-induced conditioned taste aversion in male sprague-dawley rats: impact of age and stress. Alcohol Clin Exp Res. 2010;34:2106–15. doi: 10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Herz A, Shippenberg TS. Evidence that the aversive effects of opioid antagonists and kappa-agonists are centrally mediated. Psychopharmacology (Berl) 1989;98:203–6. doi: 10.1007/BF00444692. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Barr GA, Wang S, Carden S. Aversive properties of the kappa opioid agonist U50,488 in the week-old rat pup. Psychopharmacology (Berl) 1994;113:422–8. doi: 10.1007/BF02245218. [DOI] [PubMed] [Google Scholar]
- Collins RL, Zavala AR, Nazarian A, McDougall SA. kappa-Opioid receptors in the substantia nigra pars reticulata mediate the U-50,488-induced locomotor activity of preweanling rats. Brain Res Dev Brain Res. 2000;119:97–103. doi: 10.1016/s0165-3806(99)00153-4. [DOI] [PubMed] [Google Scholar]
- Cortez AM, Charntikov S, Der-Ghazarian T, Horn LR, Crawford CA, McDougall SA. Age-dependent effects of kappa-opioid receptor stimulation on cocaine-induced stereotyped behaviors and dopamine overflow in the caudate-putamen: an in vivo microdialysis study. Neuroscience. 2010;169:203–13. doi: 10.1016/j.neuroscience.2010.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 2003;170:409–22. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Effects of pretest manipulation on elevated plus-maze behavior in adolescent and adult male and female Sprague-Dawley rats. Pharmacol Biochem Behav. 2009;92:413–23. doi: 10.1016/j.pbb.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Novel-object place conditioning in adolescent and adult male and female rats: effects of social isolation. Physiology & Behavior. 2003;80:317–325. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Hartup WW, Stevens N. Friendships and adaptation in the life course. Psychological Bulletin. 1997;121:355–370. [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–8. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Hurwitz ZE, Merluzzi AP, Riley AL. Age-dependent differences in morphine-induced taste aversions. Dev Psychobiol. 2012 doi: 10.1002/dev.21046. [DOI] [PubMed] [Google Scholar]
- Infurna RN, Spear LP. Developmental changes in amphetamine-induced taste aversions. Pharmacol Biochem Behav. 1979;11:31–5. doi: 10.1016/0091-3057(79)90293-4. [DOI] [PubMed] [Google Scholar]
- Iwamoto EI. Place-conditioning properties of mu, kappa, and sigma opioid agonists. Alcohol and Drug Research. 1986;6:327–339. [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2011. Ann Arbor: Institute for Social Research, The University of Michigan; 2012. [Google Scholar]
- Kelley AE, Schochet T, Landry CF. Risk taking and novelty seeking in adolescence: introduction to part I. Ann N Y Acad Sci. 2004;1021:27–32. doi: 10.1196/annals.1308.003. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Blockade of ethanol reward by the kappa opioid receptor agonist U50,488H. Alcohol. 2009;43:359–65. doi: 10.1016/j.alcohol.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006;31:787–94. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SA, Garmsen GM, Meier TL, Crawford CA. Kappa opioid mediated locomotor activity in the preweanling rat: role of pre- and postsynaptic dopamine receptors. Psychopharmacology (Berl) 1997;133:62–8. doi: 10.1007/s002130050372. [DOI] [PubMed] [Google Scholar]
- Michaels CC, Holtzman SG. Early postnatal stress alters place conditioning to both mu- and kappa-opioid agonists. J Pharmacol Exp Ther. 2008;325:313–8. doi: 10.1124/jpet.107.129908. [DOI] [PubMed] [Google Scholar]
- Morales L, Perez-Garcia C, Herradon G, Alguacil LF. Place conditioning in a two- or three-conditioning compartment apparatus: a comparative study with morphine and U-50,488. Addict Biol. 2007;12:482–4. doi: 10.1111/j.1369-1600.2007.00071.x. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Herz A. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology (Berl) 1985;86:274–80. doi: 10.1007/BF00432213. [DOI] [PubMed] [Google Scholar]
- Nizhnikov ME, Pautassi RM, Varlinskaya EI, Rahmani P, Spear NE. Ontogenetic differences in ethanol’s motivational properties during infancy. Alcohol. 2012;46:225–34. doi: 10.1016/j.alcohol.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov ES, Nizhnikov ME, Kozlov AP, Varlinskaya EI, Kramskaya TA, Spear NE. Repetitive exposures to a surrogate nipple providing nutritive and non-nutritive fluids: effects on suckling behavior of the newborn rat. Appetite. 2004;43:185–94. doi: 10.1016/j.appet.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Dev Psychobiol. 1989;22:633–43. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- Roma PG, Riley AL. Apparatus bias and the use of light and texture in place conditioning. Pharmacol Biochem Behav. 2005;82:163–9. doi: 10.1016/j.pbb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Sante AB, Nobre MJ, Brandao ML. Place aversion induced by blockade of mu or activation of kappa opioid receptors in the dorsal periaqueductal gray matter. Behav Pharmacol. 2000;11:583–9. doi: 10.1097/00008877-200011000-00005. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Cha YM, Chaudhry S, Wilson WA, Swartzwelder HS, Kuhn CM. Differential anxiogenic, aversive, and locomotor effects of THC in adolescent and adult rats. Psychopharmacology (Berl) 2007;191:867–77. doi: 10.1007/s00213-006-0676-9. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, DiFeliceantonio AG, Foscue E, Glowacz S, Haseeb N, Wang N, Zhou C, Kuhn CM. Aversive effects of ethanol in adolescent versus adult rats: potential causes and implication for future drinking. Alcohol Clin Exp Res. 2010;34:2061–9. doi: 10.1111/j.1530-0277.2010.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Morris RW, Kuhn CM. Adolescent rats are protected from the conditioned aversive properties of cocaine and lithium chloride. Pharmacol Biochem Behav. 2006;84:344–52. doi: 10.1016/j.pbb.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A. Differential effects of mu and kappa opioid systems on motivational processes. NIDA Res Monogr. 1986;75:563–66. [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–21. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Le AD. Adolescent male Wistar rats are more responsive than adult rats to the conditioned rewarding effects of intravenously administered nicotine in the place conditioning procedure. Behav Brain Res. 2006;206:240–4. doi: 10.1016/j.bbr.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The Behavioral Neuroscience of Adolescence. W. W. Norton; 2010. [Google Scholar]
- Spear LP. Rewards, aversions and affect in adolescence: Emerging convergences across laboratory animal and human data. Dev Cogn Neurosci. 2011;1:390–403. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A Social Neuroscience Perspective on Adolescent Risk-Taking. Dev Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda HA, Natividad LA, Orfila JE, Torres OV, O’Dell LE. Dysregulation of kappa-opioid receptor systems by chronic nicotine modulate the nicotine withdrawal syndrome in an age-dependent manner. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Tejeda HA, Natividad LA, O’Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol Biochem Behav. 2008;90:658–63. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–72. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague-Dawley rats: impact of social deprivation and test context familiarity. Behav Brain Res. 2008;188:398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107–14. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Vetter-O’Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 2009;44:547–54. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP. Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Dev Psychobiol. 2012;54:523–35. doi: 10.1002/dev.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadenberg ML. A review of the properties of spiradoline: a potent and selective kappa-opioid receptor agonist. CNS Drug Rev. 2003;9:187–98. doi: 10.1111/j.1527-3458.2003.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 2010;210:121–35. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Adolescent and adult rats’ aversion to flavors previously paired with nicotine. Ann N Y Acad Sci. 2004;1021:462–4. doi: 10.1196/annals.1308.065. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav Brain Res. 2009a;198:45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol Biochem Behav. 2009b;92:131–4. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effects of the plant-derived hallucinogen salvinorin A on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: agonist actions at kappa opioid receptors. Psychopharmacology (Berl) 2005;179:551–8. doi: 10.1007/s00213-004-2087-0. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Developmental Psychobiology. 1997;30:141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]