Abstract
This focused review serves to explore relevant issues in regard to blood pressure regulation and by doing so, provides the initial stimulus paper for the Thematic Review series “Blood Pressure Regulation” to be published in the European Journal of Applied Physiology over the coming months. In this introduction, we highlight how variable normal blood pressure can be and challenge the reader to take another look at some key concepts related to blood pressure regulation. We point out that there is frequently an underappreciated balance between peripheral vasodilation and systemic blood pressure regulation and ask the question: Are changes in blood pressure, in effect, reasonable and integrated adaptations to the physiological challenge at hand? We conclude with the idea that blood pressure regulatory systems are both flexible and redundant; ensuring a wide variety of activities associated with life can be accompanied by a perfusion pressure that can serve multiple masters.
INTRODUCTION
This focused review serves to explore relevant issues in regard to blood pressure regulation and by doing so, provides the initial stimulus paper for the Thematic Review series “Blood Pressure Regulation” to be published in the European Journal of Applied Physiology over the coming months. The title, “Every Adaptation is an Integration” is designed to highlight how blood pressure changes in response to acute physiological “stressors” and ask if the changes in blood pressure are, in fact, reasonable and integrated adaptations to the physiological challenge at hand. It is hoped that this approach will provide thought exercises that highlight key concepts and questions about blood pressure regulation in humans.
Is blood pressure a regulated variable?
The classic view is that blood pressure is a highly regulated variable. The simple equation, Blood Pressure = Cardiac Output × Total Peripheral Resistance is frequently used to illustrate this point. The idea is that blood pressure is sensed by baroreceptors (mechanoreceptors), located at strategic locations in the body like the aortic arch and carotid sinus, which deform with changes in blood pressure. These mechanical deformations evoke afferent nerve signals that are proportional to blood pressure. These signals are then integrated in the brain stem cardiovascular centers and appropriate adjustments in heart rate and efferent sympathetic traffic are made. The adjustments fundamentally regulate cardiac output and total peripheral resistance [For detailed review, please see (Hart et al, 2012)].
The simple scheme outlined above overlooks a couple of important points. First, the ability of changes in heart rate to modulate cardiac output is critically dependent on how full the heart is and other factors that affect stroke volume. In this way, things that reduce venous return – like the upright posture – will influence the ability of heart rate to change cardiac output.
Dehydration, heat stress, and blood loss can have similar effects. For example during heat stress cutaneous vasodilation causes both a reduction in systemic vascular resistance and venous return, contributing to a fall in systemic blood pressure and orthostatic intolerance [For detailed review, please see (Crandall 2008; Charkoudian 2003)]. Thus, the upright posture on a hot day can be especially challenging. This basic example highlights how various regulatory priorities (e.g. blood pressure vs. thermoregulation) can be in competition. For more detailed discussion, please see Review III in the Thematic Review series.
Second, longer-term changes in blood volume can also have significant effects on blood pressure control. Following the classic, renal-centric view of blood pressure regulation, if blood volume and blood pressure are low, sodium and water retention are enhanced and if blood volume is high there can be natriuresis and diuresis [For detailed review, please see (Cowley, 1992), see also Lohmeirer & Iliescu 2004]. These mechanisms are thought to be inappropriately activated in hypertension so that blood volume and blood pressure are maintained at higher levels. In this context, many anti-hypertensive drugs target either blood volume (diuretics) or some element of the renin-angiotensin-aldosterone axis.
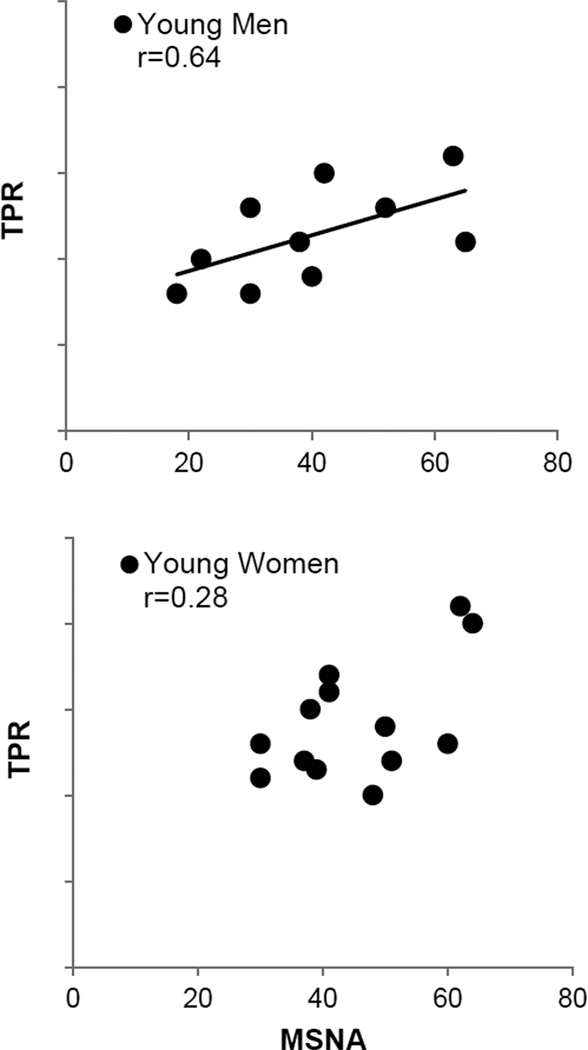
Lastly, in addition to changes in cardiac output, the ability of efferent sympathetic outflow to evoke vasoconstriction can also vary. There are marked inter-individual differences in baseline levels of efferent sympathetic outflow and important age- and sex-related differences in sympathetic vasoconstrictor responsiveness (Fagius & Wallin, 1993; Hart et al, 2011; Sundlof & Wallin, 1977). For example older men typically have higher baseline levels of muscle sympathetic nerve activity (MSNA) than young men, but they also vasoconstrict less to a given level of α-adrenergic stimulation [For detailed review, please see (Seals & Dinenno, 2004); see also Review VI in the Thematic Review series]. In regard to blood pressure control, in younger men there is a tight relationship between baseline MSNA and total peripheral resistance that is not seen in younger women and findings suggest, in women, α-adrenergic vasoconstriction is offset by concurrent β-adrenergic vasodilation (Hart et al 2011; Figure 1). Additionally, vasodilation in the active muscles can limit the ability of sympathetic nerves to evoke vasoconstriction during exercise through a process known as functional sympatholysis (Remensnyder et al, 1962; Tschakovsky et al, 2002). Although, perhaps a better example of sympatholysis occurs during sepsis when there is marked and essentially “uncontrolled” peripheral vasodilation that is resistant to adrenergic vasoconstriction (Holmes & Walley, 2009). Taken together, the ability of an increase in sympathetic nerve activity to evoke vasoconstriction is modulated by a number of factors and while blood pressure is generally seen as “regulated variable”, changes in heart rate and efferent sympathetic nerve activity do not always cause predictable changes in cardiac output and total peripheral resistance.
Figure 1.
This figure demonstrates that there are important sex-related differences in the ability of efferent sympathetic outflow to evoke vasoconstriction and changes in muscle sympathetic nerve activity (MSNA) do not always cause predictable changes in total peripheral resistance (TPR). For example, in younger men there is a tight relationship between baseline MSNA and TPR that is not present in younger women (Hart et al, 2011). Figures such as this demonstrate that blood pressure regulation is much more complicated than generally appreciated. Figure adapted from Hart et al, 2011.
How regulated is blood pressure?
The above overview demonstrates that blood pressure regulation is in fact more complicated than generally appreciated. Blood pressure might also rise or fall, depending on physiological circumstances and various homeostatic challenges encountered. This raises the question, what is the range of blood pressure seen in a normal healthy person over the course of their daily activities?
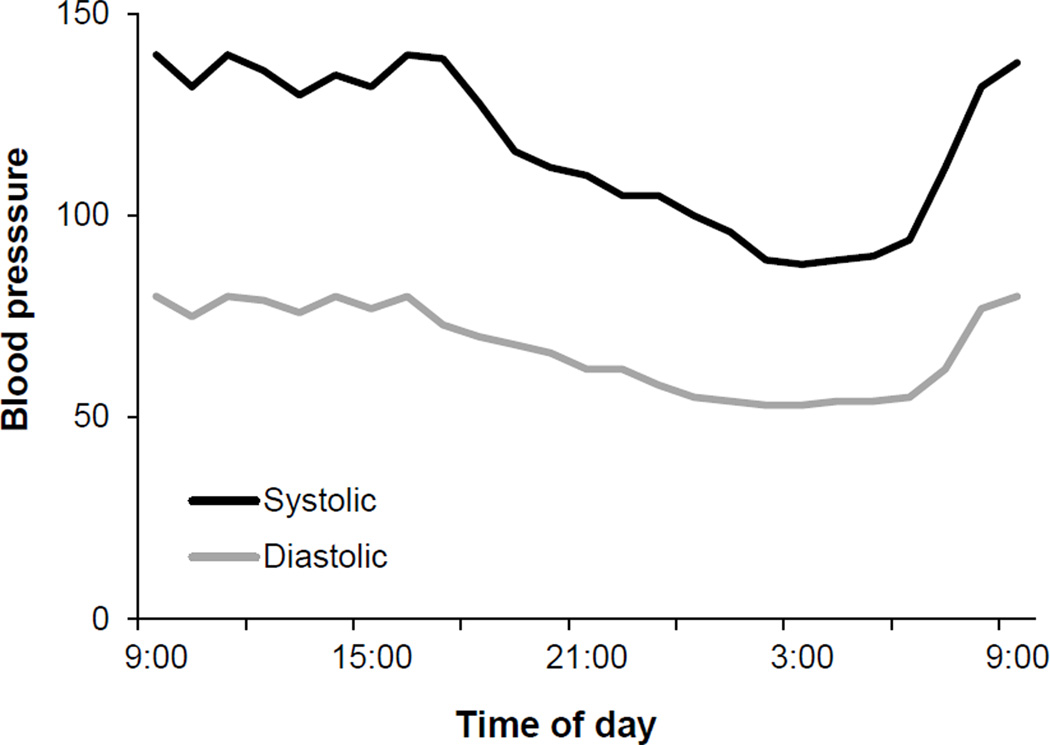
The textbook idea that blood pressure is “normal” if it is approximately 120/80 mmHg, is misleading. Blood pressure can vary throughout the day during activities and can dip at night in normal healthy normotensive humans. Figure 2 is a blood pressure record showing a typical response over 24-hrs in a single individual. This shows that mean pressure can vary by about 30% during daily living [(Millar-Craig et al, 1978); see also Review VII in the Thematic Review series]. What follows are a few examples to think about:
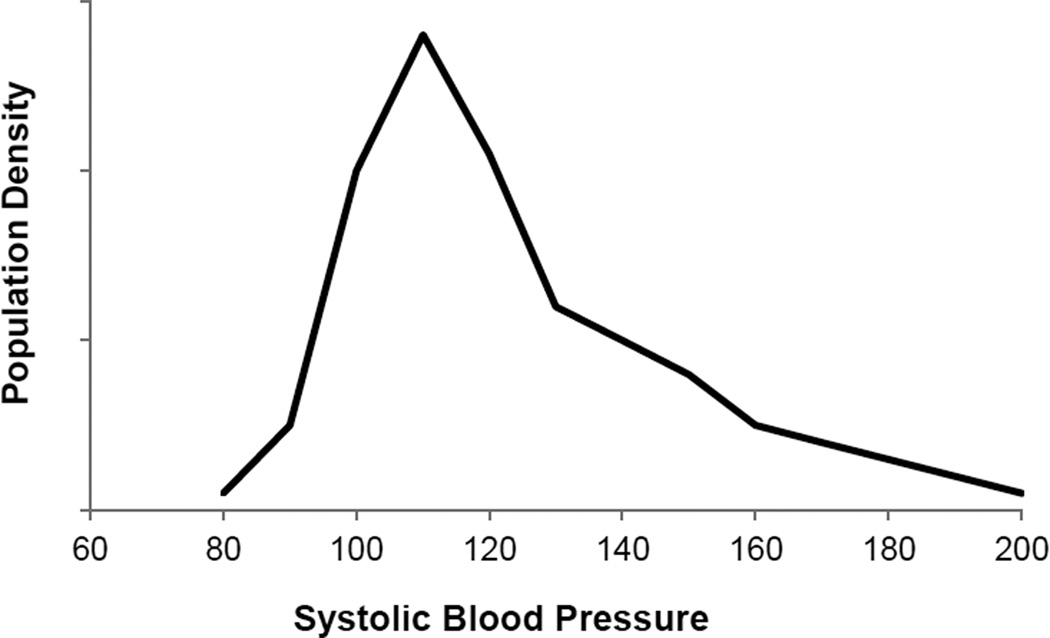
Resting blood pressure: Excluding people with the clinical definition of hypertension (>140/90 for the purposes of this paper), there is wide variability in casual resting blood pressure in humans. Figure 3 shows a typical population distribution of systolic blood pressures.
Post-exercise hypotension: Acute periods of aerobic exercise are associated with modest increases in mean arterial pressure. However, following exercise, there is a period of “post-exercise hypotension” that can last for hours (Halliwill et al, 2013). If blood pressure is a “regulated” variable, why doesn’t this post-exercise hypotension evoke counter-regulatory responses that return blood pressure to pre-exercise “normal” values? For more detailed discussion, please see Review X in the Thematic Review series.
Dynamic exercise: During dynamic exercise there is an increase in systolic pressure and in young healthy subjects a fall in diastolic pressure as active skeletal muscles vasodilate [For detailed review, please see (Clausen, 1977)]. The effect of this is a small change in mean arterial pressure in normotensive young subjects. In general changes in blood pressure during exercise continue to be “regulated” by the baroreflex – albeit the blood pressure operating point is “reset” to accommodate the exercise-induced increase in blood pressure [For detailed reviews, please see (Raven et al, 1997; Rowell, 1992), see also (Melcher & Donald, 1981)].
Static exercise: During static exercise, like an ischemic handgrip, there can be marked increases in arterial blood pressure with even small muscle mass exercise. These increases in pressure can be sustained during periods of post-exercise circulatory occlusion. Under these conditions, the initial rise in blood pressure is thought to be due to “central command” whereas the slow rise pressure as contractions continue is likely the result of a combination of increased central command – as the effort associated with the contractions increases due to fatigue —and the so-called “muscle chemoreflex,” which is driven by metabolites in the contracting muscles stimulating blood pressure-raising afferents (Mark et al, 1985).
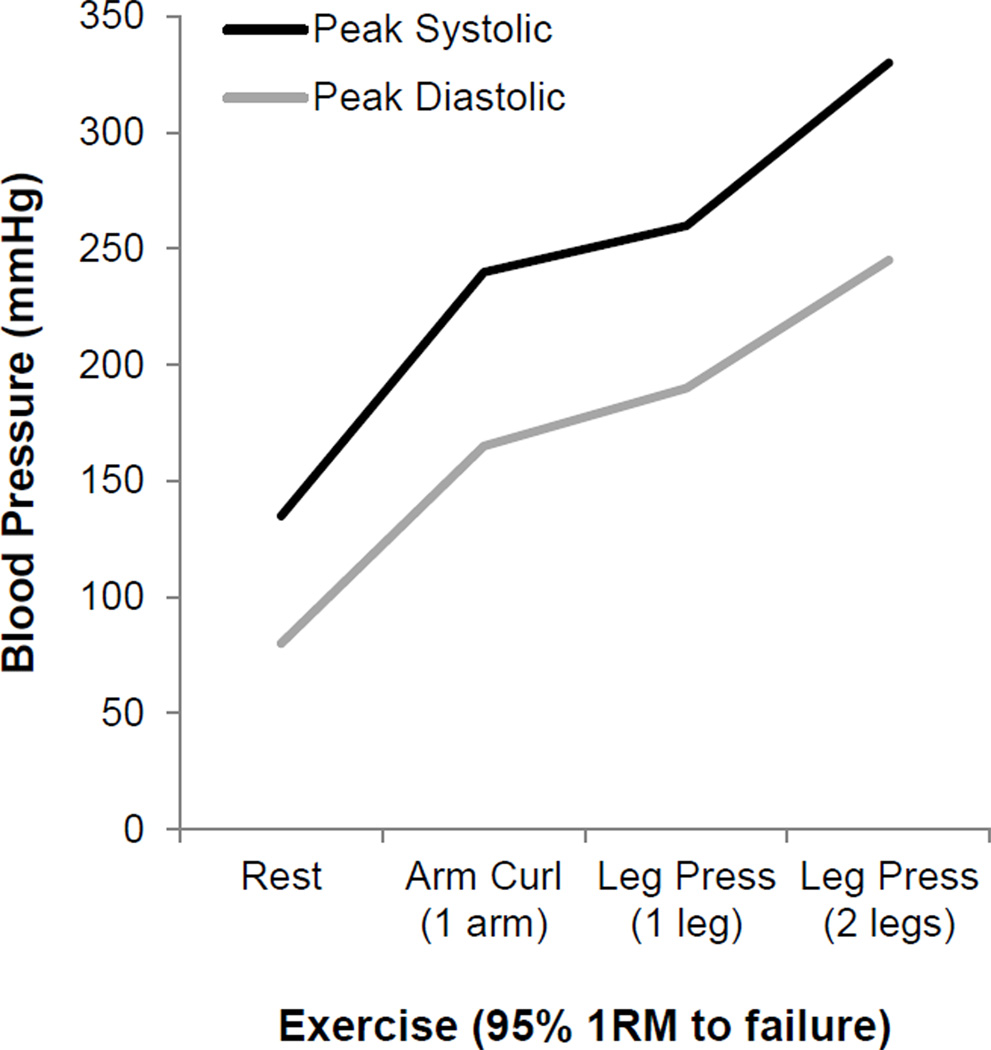
Heavy weight lifting: Perhaps the most extreme increases in blood pressure that can be seen in normal healthy humans are those associated with heavy weight lifting as shown in Figure 4. In their remarkable study, MacDougall and colleagues made direct measurements of arterial pressure during leg press exercise in humans and observed blood pressure values as high as 480/350 mmHg or nearly 4 times above normal (MacDougall et al, 1985).
Figure 2.
This 24-hour blood pressure record shows the variability in blood pressure that occurs throughout the day in healthy, normotensive individuals. These observations offer clear evidence that blood pressure is not an absolutely stable value. With these data in mind, if blood pressure is “regulated,” how are these changes possible and why doesn’t blood pressure immediately return to normal - especially during the dips observed at night? Figure adapted from Millar-Craig et al., 1978.
Figure 3.
This figure clearly depicts marked inter-individual differences in resting levels of blood pressure, even in healthy, normotensive individuals. This observation would suggest the textbook idea that blood pressure is “normal” if it is 120/80 mmHg is misleading. Figure adapted from Wright et al, 2011.
Figure 4.
This figure offers clear evidence that blood pressure can vary dramatically during weight lifting and MacDougall and colleagues (1985) have shown pressures can rise nearly 4 times above resting levels during leg press exercise in humans (MacDougall et al, 1985). These data suggest blood pressure is not as rigidly regulated as one might have thought and raises the question: “What is a pathological level of blood pressure?” Figure adapted from MacDougall et al, 1985.
The point of the above examples is that blood pressure is not rigidly regulated or an absolutely stable value; it varies throughout the day and it can vary more dramatically under some circumstances like heavy weight lifting.
Blood pressure “regulation” as an integrated adaptation to physiological challenges?
It is possible both the dipping in blood pressure seen at night and the vast increases in blood pressure seen during heavy weight lifting can be explained by a common, overarching theme. One idea is that the brain and heart need oxygen under all circumstances, while other tissues need varying amounts of oxygen depending on the situation. In this conceptual framework, blood pressure “can” be lower during sleep when myocardial oxygen demand and cerebral metabolic rate are both low, and gravitational forces on perfusion pressure are removed. These simple observations raise the question: Is oxygen delivery as important, or perhaps even more important, than the support of an “arbitrary” arterial pressure?
The best example of a “tissue driven” change in blood pressure comes from the brain. Normally brain blood flow is thought to be autoregulated so that flow remains relatively constant during the swings in blood pressure seen during normal daily life [For detailed review, please see (Lassen, 1974)]. However, an increase in intracranial pressure after a serious head injury can compress arterioles within the brain and reduce brain blood flow. Under these circumstances, reflex increases in sympathetic nerve activity, systemic vasoconstriction, and resultant elevation of systemic blood pressure (i.e. the Cushing response) are combined to ensure brain perfusion is preserved [For detailed review, please see (Paton et al, 2009), see also (Ursino et al, 2009)]. Ultimately, this response can be maladaptive and lead to further increases in intracranial pressure as the brain swells; however, the key is that blood pressure is altered in order to maintain perfusion to the brain at all costs. A related idea is that subtle, chronic “underperfusion” of the brain may contribute to the genesis of hypertension in humans [For detailed review, please see (Paton et al, 2009)]. At the other end of the pathological spectrum, it is interesting to note that vasopressin released during hemorrhage or other reductions in blood pressure evokes vasoconstriction in many vascular beds but can dilate the cerebral circulation (Vanhoutte et al, 1984). Once again, peripheral vascular tone is altered and perfusion of the brain is protected. For more detailed discussion, please see Review IX in the Thematic Review series.
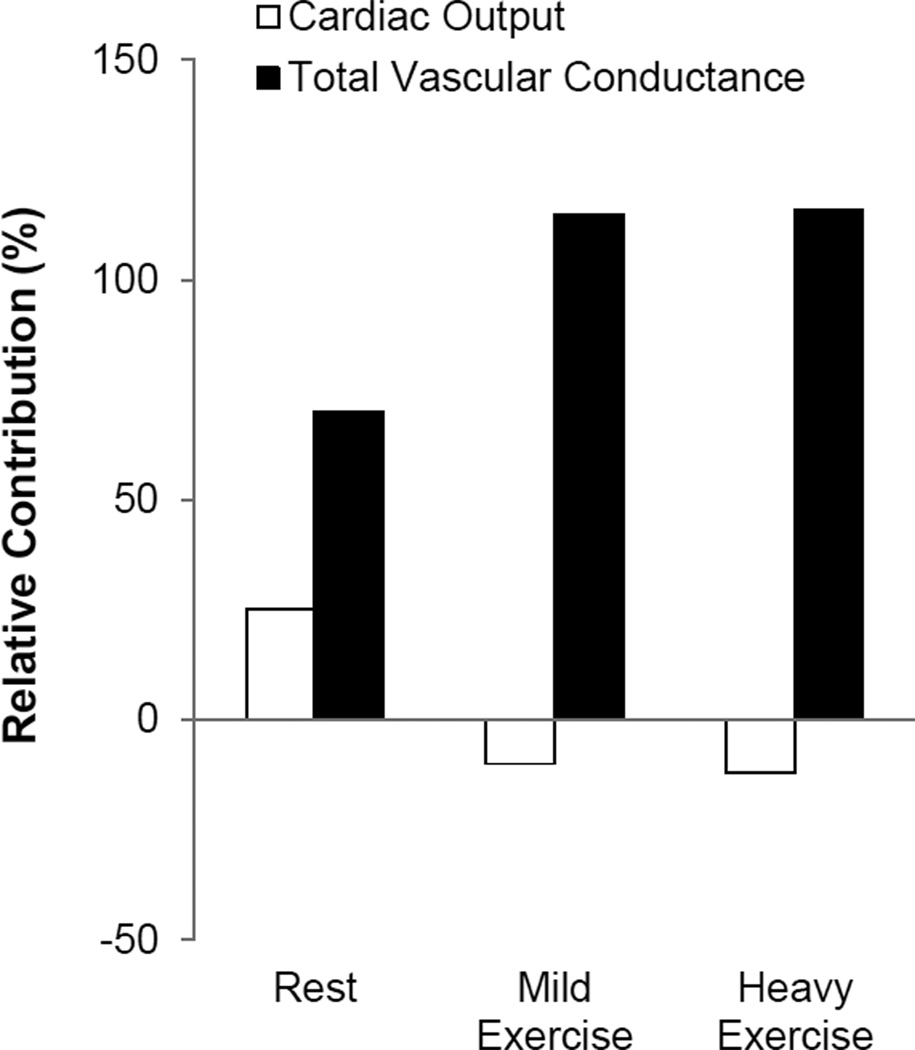
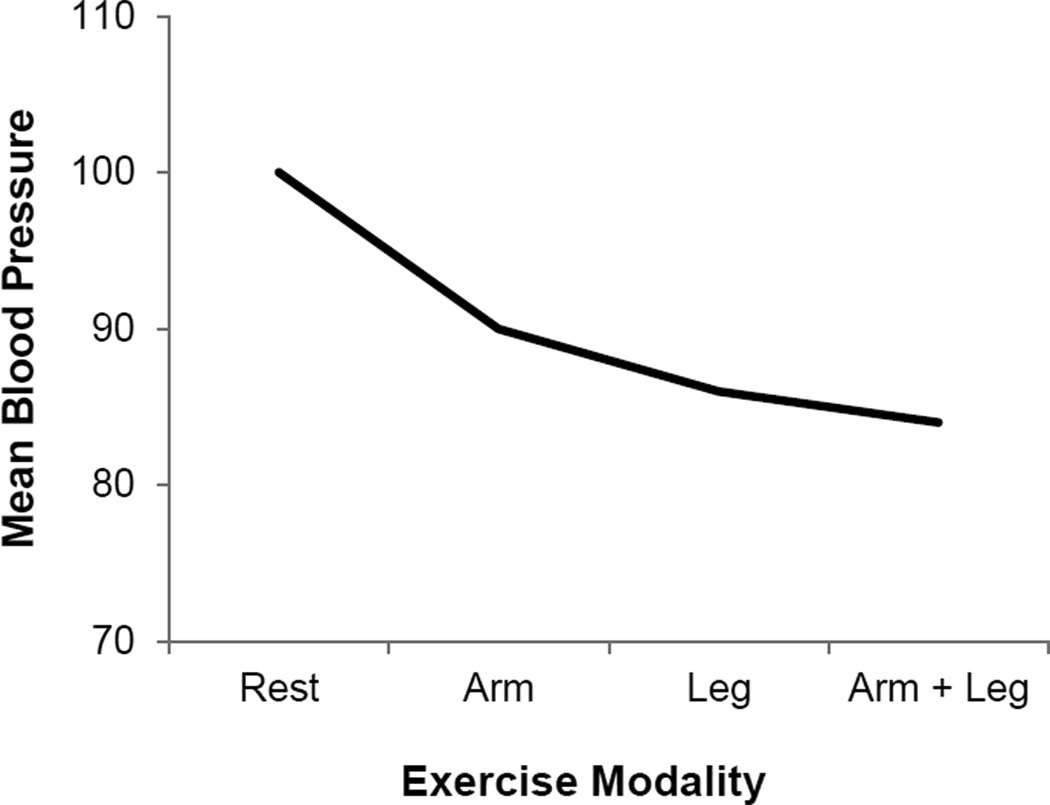
At rest, reflex changes in arterial pressure are the result of both changes in cardiac output and vascular conductance (i.e. sympathetically-mediated vasoconstriction). However during exercise, the vast majority of cardiac output is directed toward the skeletal muscle. Thus, the relative contribution of cardiac output to blood pressure regulation is reduced and the importance of vascular resistance increases (Ogoh et al, 2003; Figure 5). One can argue that adequate perfusion of skeletal muscle becomes an important regulated variable under these conditions as the skeletal muscle, heart, and brain compete for blood flow. Consistent with this concept, highly trained athletes are able to tolerate low levels of arterial pressures during intense whole-body exercise – ensuring skeletal muscle perfusion is preserved (Figure 6) [For detailed review, please see (Clausen, 1977); see also (Calbet et al, 2004; Volianitis & Secher, 2002) and Review II in the Thematic Review series].
Figure 5.
At rest, blood pressure is determined by changes in both cardiac output and vascular conductance. However, during exercise an increase in peripheral vasodilation is observed that ensures oxygen delivery matches metabolic demand. Because of this, the relative contribution of cardiac output to blood pressure regulation is reduced and the importance of sympathetically-mediated vasoconstriction increases. These data demonstrate that, while blood pressure may be “regulated,” changes in cardiac output and total vascular conductance do not always result in similar changes in blood pressure. Figure adapted from Ogoh et al, 2003.
Figure 6.
The heart and brain require oxygen under all circumstances, while other tissues need varying amounts depending on the situation. During whole-body exercise, highly trained athletes are able to tolerate lower arterial blood pressures to ensure high levels of skeletal muscle perfusion. This observation supports the interpretation that oxygen delivery may become more important than the support of an “arbitrary” arterial pressure during whole-body exercise. Figure designed with data from Calbet et al, 2004.
An extreme example of the competition (or loss of competition) and integration between blood pressure regulation and peripheral vasodilation comes from patients with pure autonomic failure. In these patients sympathetic outflow is lost and the ability to increase efferent sympathetic outflow to constrict peripheral blood vessels is absent. Thus, the upright posture is a challenge for such patients and when patients with this syndrome exercise, the metabolite-driven peripheral vasodilation causes a substantial and unopposed reduction in blood pressure (Marshall et al, 1961). In an attempt to compensate for lost sympathetically-mediated vasoconstriction, maneuvers such as abdominal compression, muscle tensing, or lower-limb elastic stockings are used to increase venous return and reduce or delay hypotension [For detailed review, please see (Wieling et al, 2004)].
How much regulation is enough?
As highlighted above, highly trained athletes are able to tolerate low arterial pressures during intense whole-body exercise, by contrast blood pressure increases drastically in order to preserve cerebral perfusion after a serious head injury, and even under normal resting conditions, baseline blood pressure is highly variable in healthy adults. This raises the question, “How much regulation is enough?” We have no obvious answer to this question but close with a classic example of what happens when various blood pressure regulating systems are interrupted.
Conceptually, denervation of the arterial baroreceptors should produce maintained hypertension. In support of this concept, denervation of arterial baroreceptors in dogs can increase resting mean arterial blood pressure by 25 mmHg (Melcher & Donald, 1981). However, Cowley and colleagues observed “no significant difference” between mean 24-hour arterial pressures in intact verses baroreceptor-denervated dogs, but pressor responses to acute stressors were enhanced (Cowley & DeClue, 1976; Cowley et al, 1973). Consistent with this concept, data from humans without a carotid baroreflex (either as a result of bilateral glomectomy for glomus tumors or because of neck irradiation) report more variable blood pressure values with a larger-than-normal rise in arterial pressure in response to physical and mental stress, but not obvious hypertension (Smit et al, 2002; Timmers et al 2003).
What about exercise? In a classic series of studies, Donald and colleagues removed, isolated, and/or denervated various sensory afferents in the cardiovascular system of dogs [For detailed review, please see (Joyner, 2006)]. They demonstrated that with complete barodenervation the rise in blood pressure during exercise could be dramatic (Walgenbach & Donald, 1983). However, they also showed that blood pressure responses to exercise were remarkably normal when only one carotid sinus nerve was intact (Shepherd, personal communication). This means that the blood pressure responses were normal in spite of the loss of input from vagal cardiopulmonary afferents, aortic receptors and one of two carotid baroreceptors. So, at one level blood pressure is highly variable and at another level redundant control mechanisms keep it within the broad range of normal under most circumstances.
Summary
In this paper we have highlighted just how variable normal blood pressure can be. It can vary dramatically both within and between so-called “normotensive” individuals. Additionally, values that might be seen as pathologic or dangerously high or low under some circumstances are in fact normal responses to various behavioral states and stressors. We have also pointed out that there is an underappreciated balance between the “demand” for blood flow by peripheral tissues and systemic blood pressure regulation. Finally, blood pressure regulatory systems are classically seen as redundant so that values in the normal range can be maintained under many circumstances. Altogether, perhaps a better way to look at “blood pressure regulation” is to say that blood pressure regulatory systems are both flexible and redundant so that that the wide variety of activities associated with life can be accompanied by a perfusion pressure that can serve several masters. We encourage you to explore these ideas in more detail within the European Journal of Applied Physiology’s Thematic Review series “Blood Pressure Regulation.”
Literature Cited
- Calbet JA, Jensen-Urstad M, van Hall G, Holmberg HC, Rosdahl H, Saltin B. Maximal muscular vascular conductances during whole body upright exercise in humans. J Physiol. 2004;558:319–331. doi: 10.1113/jphysiol.2003.059287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc. 2003;78:603–612. doi: 10.4065/78.5.603. [DOI] [PubMed] [Google Scholar]
- Clausen JP. Effect of physical training on cardiovascular adjustments to exercise in man. Physiol Rev. 1977;57:779–815. doi: 10.1152/physrev.1977.57.4.779. [DOI] [PubMed] [Google Scholar]
- Cowley AW, DeClue JW. Quantification of baroreceptor influence on arterial pressure changes seen in primary angiotension-induced hypertension in dogs. Circ Res. 1976;39:779–787. doi: 10.1161/01.res.39.6.779. [DOI] [PubMed] [Google Scholar]
- Cowley AW, Jr, Liard JF, Guyton AC. Role of baroreceptor reflex in daily control of arterial blood pressure and other variables in dogs. Circ Res. 1973;32:564–576. doi: 10.1161/01.res.32.5.564. [DOI] [PubMed] [Google Scholar]
- Crandall CG. Heat stress and baroreflex regulation of blood pressure. Med Sci Sports Exerc. 2008;40:2063–2070. doi: 10.1249/MSS.0b013e318180bc98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagius J, Wallin BG. Long-term variability and reproducibility of resting human muscle nerve sympathetic activity at rest, as reassessed after a decade. Clin Auton Res. 1992;3:201–205. doi: 10.1007/BF01826234. [DOI] [PubMed] [Google Scholar]
- Halliwill JR, Buck TM, Lacewell AN, Romero SA. Postexercise hypotension and sustained postexercise vasodilatation: what happens after we exercise? Exp Physiol. 2013;98:7–18. doi: 10.1113/expphysiol.2011.058065. [DOI] [PubMed] [Google Scholar]
- Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J, Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the β-adrenergic receptors. J Physiol. 2011;589:5285–5297. doi: 10.1113/jphysiol.2011.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EC, Joyner MJ, Wallin BG, Charkoudian N. Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J Physiol. 2012;590:2069–2079. doi: 10.1113/jphysiol.2011.224642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes CL, Walley KR. Vasoactive drugs for vasodilatory shock in ICU. Curr Opin Crit Care. 2009;15:398–402. doi: 10.1097/MCC.0b013e32832e96ef. [DOI] [PubMed] [Google Scholar]
- Joyner MJ. Baroreceptor function during exercise: resetting the record. Exp Physiol. 2006;91:27–36. doi: 10.1113/expphysiol.2005.032102. [DOI] [PubMed] [Google Scholar]
- Lassen NA. Control of cerebral circulation in health and disease. Circ Res. 1974;34:749–760. doi: 10.1161/01.res.34.6.749. [DOI] [PubMed] [Google Scholar]
- MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol. 1985;58:785–790. doi: 10.1152/jappl.1985.58.3.785. [DOI] [PubMed] [Google Scholar]
- Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57:461–469. doi: 10.1161/01.res.57.3.461. [DOI] [PubMed] [Google Scholar]
- Marshall RJ, Schirger A, Shepherd JT. Blood pressure during supine exercise in idiopathic orthostatic hypotension. Circulation. 1961;24:76–81. doi: 10.1161/01.cir.24.1.76. [DOI] [PubMed] [Google Scholar]
- Melcher A, Donald DE. Maintained ability of carotid baroreflex to regulate arterial pressure during exercise. Am J Physiol. 1981;24:H838–H849. doi: 10.1152/ajpheart.1981.241.6.H838. [DOI] [PubMed] [Google Scholar]
- Millar-Craig MW, Bishop CN, Raftery EB. Circadian variation of blood-pressure. Lancet. 1978;1:795–797. doi: 10.1016/s0140-6736(78)92998-7. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Fadel PJ, Nissen P, Jans O, Selmer C, Secher NH, Raven PB. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol. 2003;550:317–324. doi: 10.1113/jphysiol.2003.041517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JF, Dickinson CJ, Mitchell G. Harvey Cushing and the regulation of blood pressure in giraffe, rat and man: introducing “Cushing’s mechanism”. Exp Physiol. 2009;94:11–17. doi: 10.1113/expphysiol.2008.043455. [DOI] [PubMed] [Google Scholar]
- Raven PB, Potts JT, Shi X. Baroreflex regulation of blood pressure during dynamic exercise. Exerc Sport Sci Rev. 1997;25:365–389. [PubMed] [Google Scholar]
- Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res. 1962;11:370–380. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Reflex control of the circulation during exercise. Int J Sports Med. 1992;13:S26–S27. doi: 10.1055/s-2007-1024583. [DOI] [PubMed] [Google Scholar]
- Seals DR, Dinenno FA. Collateral damage: cardiovascular consequences of chronic sympathetic activation with human aging. Am J Physiol Heart Circ Physiol. 2004;287:H1895–H1905. doi: 10.1152/ajpheart.00486.2004. [DOI] [PubMed] [Google Scholar]
- Smit AA, Timmers HJ, Wieling W, Wagenaar M, Marres HA, Lenders JW, van Montfrans GA, Karemaker JM. Long-term effects of carotid sinus denervation on arterial blood pressure in humans. Circulation. 2002;105:1329–1335. doi: 10.1161/hc1102.105744. [DOI] [PubMed] [Google Scholar]
- Sundlöf G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol. 1977;272:383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers HJ, Karemaker JM, Wieling W, Marres HA, Lenders JW. Baroreflex control of muscle sympathetic nerve activity after carotid body tumor resection. Hypertension. 2003;42:143–149. doi: 10.1161/01.HYP.0000080495.07301.31. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol. 2002;541:623–635. doi: 10.1113/jphysiol.2001.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursino M, Giannessi M, Frapparelli M, Magosso E. Effect of cushing response on systemic arterial pressure. IEEE Eng Med Biol Mag. 2009;28:63–71. doi: 10.1109/MEMB.2009.934622. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Katusic ZS, Shepherd JT. Vasopressin induces endothelium-dependent relaxations of cerebral and coronary, but not of systemic arteries. J Hypertens. 1984;2:S421–S422. [PubMed] [Google Scholar]
- Volianitis S, Secher NH. Arm blood flow and metabolism during arm and combined arm and leg exercise in humans. J Physiol. 2002;544:977–984. doi: 10.1113/jphysiol.2002.023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walgenbach SC, Donald DE. Inhibition by carotid baroreflex of exercise-induced increases in arterial pressure. Circ Res. 1983;52:253–262. doi: 10.1161/01.res.52.3.253. [DOI] [PubMed] [Google Scholar]
- Wieling W, Colman N, Krediet CT, Freeman R. Nonpharmacological treatment of reflex syncope. Clin Auton Res. 2004;14(Suppl 1):62–70. doi: 10.1007/s10286-004-1009-x. [DOI] [PubMed] [Google Scholar]
- Wright JD, Hughes JP, Ostchega Y, Yoon SS, Nwankwo T. Mean systolic and diastolic blood pressure in adults aged 18 and over in the United States, 2001–2008. Natl Health Stat Report. 2011;35:1–22. 24. [PubMed] [Google Scholar]