Abstract
Background
Early-life stress and exposure to stressful stimuli play a major role in the development of chronic widespread pain in adults. However, how they interact in chronic pain syndromes remains unclear.
Methods
Dams and neonatal litters were submitted to a restriction of nesting material (neonatal limited bedding, NLB) for one week. As adults, these rats were exposed to a painless sound stress protocol. The involvement of sympathoadrenal catecholamines, interleukin 6 (IL-6) and tumor necrosis alpha (TNFα) in nociception, was evaluated through of behavioral and ELISA assays, surgical interventions and intrathecal antisense treatments.
Results
Adult NLB rats exhibited mild muscle hyperalgesia, which was markedly aggravated by sound stress (peaking 15 days after exposure). Adrenal medullectomy did not modify hyperalgesia in NLB rats but prevented its aggravation by sound stress. Sustained administration of epinephrine to NLB rats mimicked sound stress effect. Intrathecal treatment with antisense directed to IL-6-receptor subunit gp130, but not to TNFα type 1 receptor (TNFR1), inhibited hyperalgesia in NLB rats. However, antisense against either gp130 or TNFR1 inhibited sound stress-induced enhancement of hyperalgesia. Compared to control rats, NLB rats exhibit increased plasma levels of IL-6 but decreased levels of TNFα, whereas sound stress increases IL-6 plasma levels in control but not in NLB rats.
Conclusions
Early-life stress induces a persistent elevation of IL-6, hyperalgesia and susceptibility to chronic muscle pain, which is unveiled by exposure to stress in adults. This probably depends on an interaction between adrenal catecholamines and pro-inflammatory cytokines acting at muscle nociceptor level.
Keywords: Neonatal limited bedding, Sound stress, Myalgia, Nociceptors, TNF α, IL-6
INTRODUCTION
Physical or psychological abuse during childhood is not only related to long-lasting vulnerability to stress, anxiety and mood disorders (1), but also to increased risk to develop chronic pain in adulthood (2–5). Such an increased risk seems to be particularly important in syndromes involving musculoskeletal symptoms (6–10). Importantly, stressful life events may further increase chronic musculoskeletal pain (9, 11–14).
Most of the available models of early-life stress in rodents are based on the isolation or separation of pups from the mother (15). While these models are useful as acute or recurrent stressors, they involve some degree of inanition and hypothermia of the pups (16). More importantly, they do not reproduce a key element observed in human chronic early-life stress: the unpredictable and erratic care of the infant despite the presence of the mother (16, 17). To overcome this, models of early-life stress based on the disruption of maternal care behavior induced by a restriction of the nesting/bedding material have been developed (1, 16–19). The neonatal limited bedding (NLB) model of early-life stress consists in reducing the availability of nesting material after parturition, inducing fragmented and aberrant maternal-nurturing behavior (17). These changes in the dam’s behavior lead to a persistent acute stress-like hormonal response in neonatal pups (18), followed by a life-long enhanced neuroendocrine stress response (1). As adults, rats previously submitted to the NLB protocol exhibit increased anxiety (1) and muscle hyperalgesia (20). While the mechanisms of muscle hyperalgesia observed in NLB rats have not been explored, there is evidence that individuals exposed to early-life stress exhibit increased production of pro-inflammatory cytokines such as tumor necrosis alpha (TNFα) and interleukin 6 (IL-6) (21–23). The TNF receptor type 1 (TNFR1) (24) and the subunit of the IL-6 receptor signaling complex gp130 (25, 26) are ubiquitously expressed in nociceptors. Injected locally, these cytokines produce long-lasting muscle mechanical hyperalgesia in rodents (27–29) and nociceptor sensitization (25, 30). Furthermore, increased levels of TNFα and IL-6 are observed in active myofascial trigger points (31) and serum of fibromyalgia patients (32). And, plasma levels of TNFα and IL-6 are raised after exposure to psychological stressors in humans (33). While these reports point towards a link between pro-inflammatory cytokines, acute stress and chronic muscle pain, how they interact in individuals exposed to early-life stress has not been explored.
Here we provide evidence that adult NLB-treated rats exposed to a stressful stimulus, which do not produce pain in normal rats, exhibit a marked increase in muscle pain. Our data indicate that adrenal catecholamines are necessary and sufficient to produce persistent enhancement of muscle pain in NLB rats, probably by acting on muscle nociceptors sensitized by pro-inflammatory cytokines.
MATERIALS AND METHODS
Animals
Primiparous pregnant Sprague-Dawley female rats were obtained from Charles River (Hollister, CA). After delivery, dams were housed with their litter in standard cages on postnatal days 0–1. On postnatal day 2, litters were assigned to limited bedding (NLB) or standard care conditions. Behavioral experiments were performed on adult male rats (weighing 250–350 g; age 50–75 days) from these litters. The animals were housed in the Laboratory Animal Resource Center of UCSF, under a 12-hours light/dark cycle (lights on 7 am–7 pm) and environmentally controlled conditions; ambient room temperature (21–23 °C), with food and water available ad libitum. Care and use in experiments conformed to National Institutes of Health guidelines. Experimental protocols were approved by the UCSF Institutional Animal Care and Use Committee.
Neonatal limited bedding (NLB) stress
Our use of the NLB model has been described previously (20). Briefly, beginning on postnatal day 2, dams and their pups were placed in cages fitted with a stainless steel mesh bottom, raised ~2.5 cm from the floor of the home cage, to allow collection of urine and feces. The nesting/bedding material provided consisted of one sheet of paper towel (~13 × 23 cm). Litters were otherwise undisturbed during postnatal days 2–9.
Sound stress protocol
Exposure to sound stress occurred on days 1, 3 and 4 as described previously (34, 35).
Adrenal medullectomy (AdMdx)
To evaluate the role of catecholamines for the effects of sound stress in NLB rats, their adrenal medullae were excised as previously described (36).
Chronic administration of epinephrine
To evaluate whether increased plasma levels of epinephrine underlie the effects of sound on NLB rats, we administered epinephrine to these rats over a period of 15 days, using micro-osmotic pumps as described previously (35, 36). Epinephrine was given at the rate of 5.4 µg/hr, which mimics plasma levels observed after sound stress (35, 36).
Measurement of muscle mechanical nociceptive threshold
Mechanical nociceptive threshold in the gastrocnemius muscle was quantified using a digital force transducer (Chatillon DFI2; Amtek Inc., Largo, FL) with a custom-made 7 mm-diameter probe (37). Rats were lightly restrained in a cylindrical acrylic holder with lateral slats that allow for easy access to the hind limb and application of the transducer probe to the belly of the gastrocnemius muscle. The nociceptive threshold was defined as the force, in milliNewtons (mN), required to produce a flexion reflex in the hind leg.
Intrathecal injections
Rats were briefly anaesthetized with 2.5% isoflurane in 97.5% O2. Then, a 30-gauge hypodermic needle was inserted into the subarachnoid space, on the midline, between the L4 and L5 vertebrae and the injection performed (20 µl). Proper intrathecal injections were systematically confirmed by checking for a sudden flicking of the tail (38).
Antisense oligodeoxynucleotides
To attenuate the expression of TNFα receptor type-1 (TNFR1) in sensory neurons, the antisense oligodeoxynucleotide (AS ODN) sequence 5’-ACACGGTGTTCTGTTTCTCC-3’ directed against a unique sequence of rat TNFR1 was used. The mismatch ODN (MM ODN) sequence, 5’-ACCCGTTGTTCGGTTGCTCC-3’, with four bases mismatched (denoted by bold face). We have previously shown that this AS ODN against TNFR1, at a dose of 40 µg, decreases TNFR1 protein in primary afferent sensory neurons (39).
To determine the contribution of IL-6, its effect on sensory neurons was disrupted by attenuating the expression of the signal transducing molecule glycoprotein 130 (gp130), a subunit of the IL-6 receptor signaling complex, which is necessary for IL-6 receptor function (40). The dose of ODN (40 µg) was based on prior studies (34, 37). The AS ODN sequence, 5’-TCC TTCCCACCTTCTTCT G-3’, was directed against a unique sequence of rat gp130 mRNA. The corresponding GenBank accession number and ODN position within the cDNA sequence are M92340 and 1834–1852, respectively (41). The MM ODN sequence, 5’-TACTACTCACATTCATCA G-3’, corresponds to the gp130 subunit antisense sequence with six mismatched bases (denoted by bold letters).
Rats were daily injected, intrathecally, with either AS or MM ODN (40 µg/20 µl) against TNFR1 or gp130 mRNA for three consecutive days. The AS- and MM ODN primers were purchased from Invitrogen (San Francisco, CA). Injected intrathecally, antisense oligodeoxynucleotides have been shown to reach the soma of sensory neurons, located in the dorsal root ganglia, and knockdown the expression of several classes of proteins involved in the processing of nociceptive information (for a review see (42).
Measurement of TNFα and IL-6 levels in plasma
Once muscle mechanical nociceptive threshold readings were taken, rats were placed back in their home cage. Then they were briefly anaesthetized with 2.5% isoflurane in 97.5% O2 and blood was collected from a tail vein, using a 25 gauge infusion set that was previously filled with 20 µl (200 U) of heparin (Sagent pharmaceuticals, India). After centrifugation (12,000 rpm for 2 min at 4°C, Eppendorf® centrifuge 5415R), the plasma was separated and stored at −80°C. TNFα and IL-6 concentrations in plasma were determined using an enzyme-linked immunosorbant assay (ELISA) performed with the Quantikine® ELISA Immunoassay kit (R&D Systems, Minneapolis, MN), according to the manufacturer’s instructions. Results from duplicate samples were averaged to obtain the final concentration of TNFα and IL-6 from each sample.
Statistical analysis
Group data are expressed as mean ± SEM of n independent observations. Statistical comparisons were made by one-way ANOVA or two-way repeated measures ANOVA followed by Bonferroni’s multiple comparisons post-hoc test. Statistical significance was set at P < 0.05.
RESULTS
Effects of sound stress on muscle hyperalgesia exhibited by NLB rats
As previously reported (20), rats submitted to the neonatal limited bedding (NLB) protocol exhibited, as adults, decreased muscle mechanical nociceptive threshold (~20% less than control rats) (P < 0.05, Fig. 1).
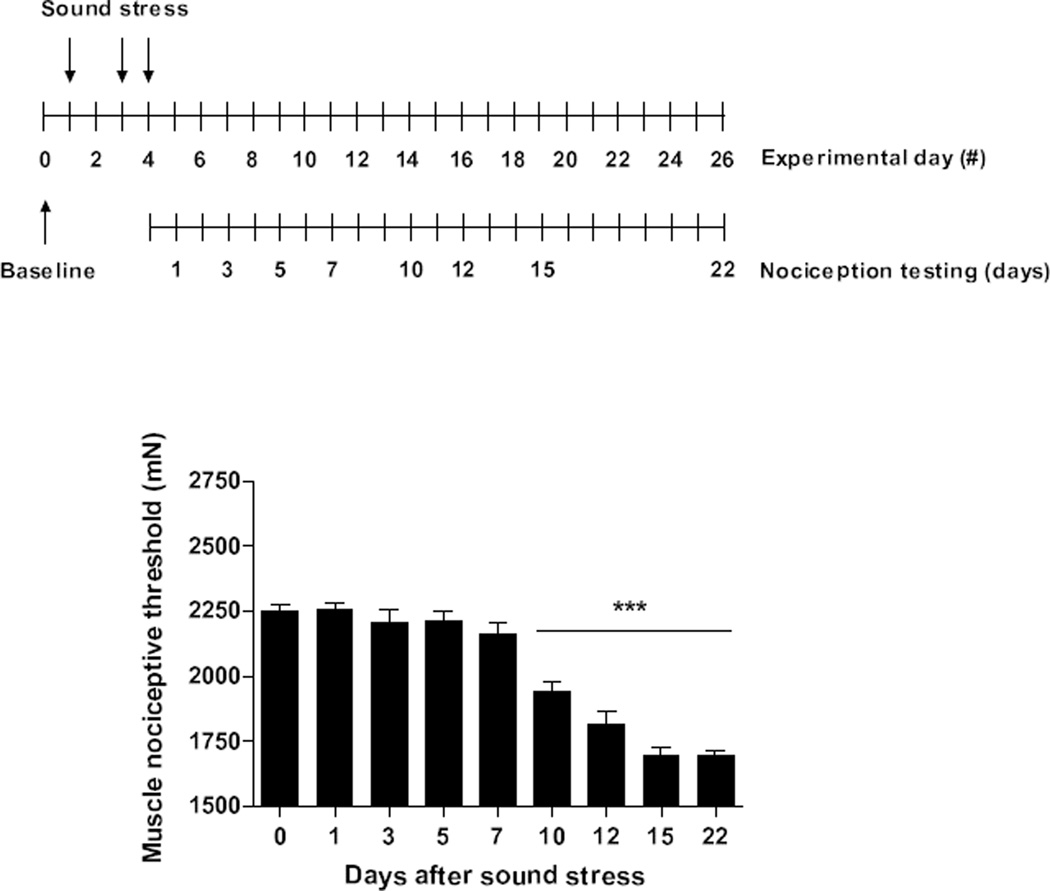
Figure 1. Sound stress enhances muscle hyperalgesia exhibited by NLB rats.
As previously reported (Green et al., 2011), rats submitted to the neonatal limited bedding protocol exhibit as adults (Day 0) decreased in muscle mechanical nociceptive threshold (~20% lower compared to control rats). As shown in the timeline inset, a 4-day cycle of sound stress consists of 30 min of intermittent exposure to sound stress administered on days 1, 3, and 4. Nociceptive threshold measurements were taken on post-stress days 1, 3, 5, 7, 10, 12, 15 and 22. Ten days after exposure to the sound stress protocol, the muscle hyperalgesia is significantly increased, persisting unattenuated for at least 12 days. ***P < 0.001.
Since exposure of normal rats to unpredictable sound stress produces persistent activation of the sympathoadrenal stress axis, but has no effect on muscle nociceptive threshold (20, 35), we used this as the stressful stimulus in adult NLB rats. Mechanical nociceptive thresholds remained unchanged in NLB rats up to 1 week after exposure to sound stress. However, at day 10 after sound stress, a significant diminution in mechanical nociceptive threshold was observed in the gastrocnemius muscle (P < 0.01, Fig. 1). This decrease in nociceptive threshold reached a peak at day 15 after sound stress (P < 0.01, Fig. 1), remaining stable at least for an additional one week (P < 0.01, Fig. 1).
Effects of adrenal medullectomy and epinephrine replacement
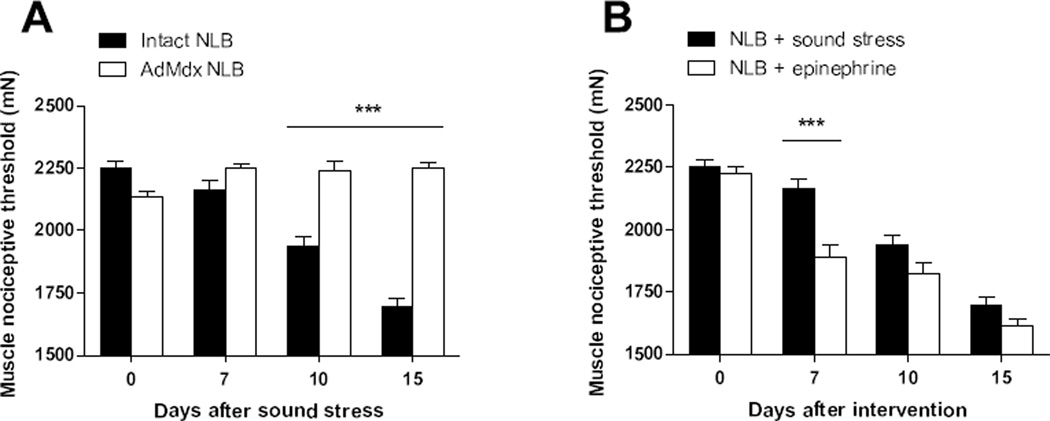
Since raised plasma levels of catecholamines play a central role in the development of latent nociceptive sensitization induced by sound stress (35, 36, 43), we explored whether surgical excision of the adrenal medulla (AdMdx) in NLB rats affects their muscle hyperalgesia or its enhancement by sound stress. While AdMdx did not affect muscle hyperalgesia observed in NLB rats (P > 0.05, Fig. 2A), it prevented its enhancement by sound stress (P < 0.05, Fig. 2A), indicating that adrenal hormones are necessary for stress-induced increase of muscle hyperalgesia in NLB rats. To evaluate the role of adrenal catecholamines in the stress-induced enhancement of muscle hyperalgesia in NLB rats, we explored whether the sustained administration of epinephrine could mimic the effect of sound stress. Compared to their adult baseline nociceptive threshold or to NLB rats after exposure to sound stress, NLB rats implanted with epinephrine containing osmotic pumps exhibited a significant decrease of muscle mechanical nociceptive threshold at day 7 after implanting the pump (P < 0.001, Fig. 2B). By day 15, the nociceptive responses of epinephrine-implanted NLB rats were indistinguishable from those of NLB rats submitted to sound stress (P < 0.001, Fig. 2B), indicating that adrenal catecholamines are sufficient to reproduce the increase of muscle hyperalgesia induced by stress in NLB rats.
Figure 2. Adrenal catecholamines play a role in sound stress-induced enhancement of muscle hyperalgesia exhibited by NLB rats.
(A) While surgical excision of the adrenal medulla (AdMdx, open bars) in NLB rats did not modify the muscle hyperalgesia (Day 0), it prevented its enhancement by sound stress (Days 10–15). (B) Comparison of the effects of sustained administration of epinephrine or sound stress on mechanical nociceptive threshold in NLB rats. NLB rats implanted with epinephrine-releasing pumps (open bars), but not those exposed to sound stress (solid bars), exhibited a significant increase of muscle hyperalgesia by day 7 after implant. Both interventions produced a comparable increase in muscle hyperalgesia between days 10 and 15 after intervention. ***P < 0.001.
Effect of antisense ODN against IL-6 receptor subunit gp130
To evaluate the contribution of IL-6 to the muscle hyperalgesia observed in NLB rats, an AS ODN targeting the gp130 subunit of the IL-6 receptor, or the respective MM ODN, were injected intrathecally for 3 consecutive days.
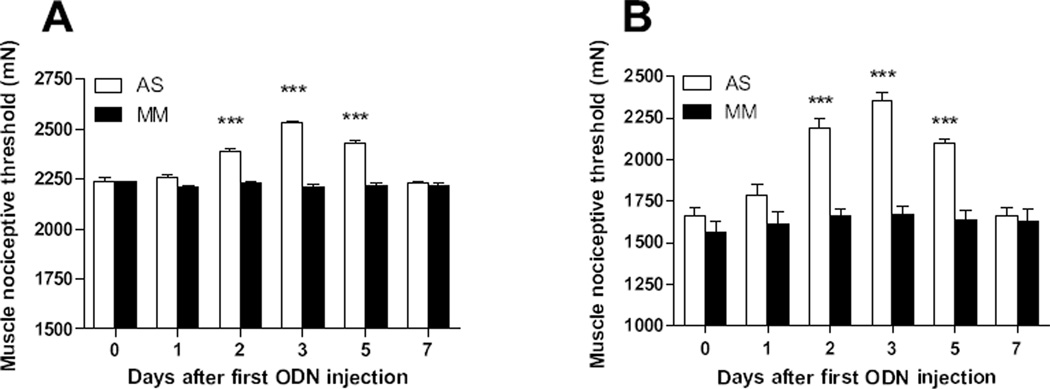
While the injection of MM ODN did not modify the mechanical nociceptive threshold exhibited by NLB rats (solid bars, P > 0.05 [n = 4], Fig. 3), the AS ODN treatment significantly increased muscle nociceptive threshold by day 2 after first AS injection (open bars, P < 0.001 [n = 5], Fig. 3). One day after the last injection, AS-treated rats exhibited a peak in nociceptive threshold value (P < 0.001, Fig. 3); this parameter was still significantly increased up to day 3 after last AS ODN injection (P < 0.001, Fig. 3). By day 5 after last AS ODN injection the nociceptive threshold reached pre-treatment values (P > 0.05, Fig. 3).
Figure 3. IL-6 contributes to baseline muscle hyperalgesia observed in NLB rats and its enhancement by sound stress.
(A) Antisense (AS, open bars), but not mismatch (MM, solid bars) treatment directed to the IL-6 receptor subunit gp130 inhibited baseline muscle hyperalgesia exhibited by NLB rats. (B) This intervention was also able to inhibit the enhancement of muscle hyperalgesia produced by sound stress. ***P < 0.001.
In order to evaluate the contribution of IL-6 in the enhanced muscle hyperalgesia observed after exposure of NLB rats to sound stress, the same AS ODN knockdown strategy directed to the gp130 subunit of the IL-6 receptor was used. The injection of MM ODN did not modified the decrease in mechanical nociceptive threshold produced by sound stress (solid bars, P > 0.05 [n = 7], Fig. 3). However, in the group of AS ODN-treated rats a significant increase in muscle nociceptive threshold was observed at day 2 after first AS injection (open bars, P < 0.001 [n = 6], Fig. 3). By day 3 after the first AS injection, these rats exhibited nociceptive threshold values comparable to prior to sound stress exposure (P < 0.001, Fig. 3). Nociceptive threshold was significantly increased up to day 3 after last administration of AS ODN (P < 0.001, Fig. 3), and 2 days later thresholds returned to pre-treatment values (P > 0.05, Fig. 3), demonstrating reversal of effect of AS ODN.
Effect of antisense ODN against TNFα receptor type-1
We next evaluated the contribution of TNFα in muscle hyperalgesia exhibited by NLB rats. Intrathecal injections of AS ODN targeting the TNFR1 or the respective MM, were performed for 3 consecutive days.
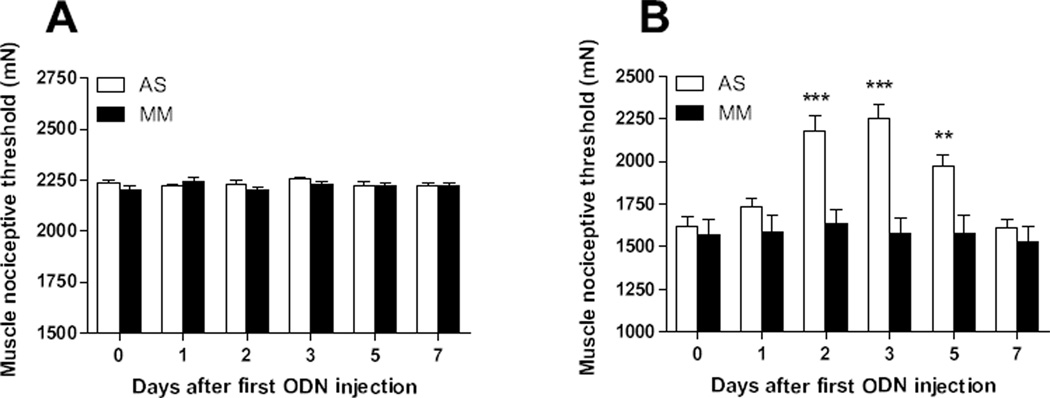
Compared to pre-treatment values, the MM or AS ODN treatments did not modify the decrease in mechanical nociceptive threshold in readings taken up to day 5 after last ODN injection (P > 0.05 [n = 6/group], Fig. 4).
Figure 4. TNFα contributes to the enhancement of muscle hyperalgesia by sound stress in NLB rats.
(A)Intrathecal treatment with either antisense (AS, open bars) or mismatch (MM, solid bars) oligodeoxynucleotides directed to the TNFR1 mRNA did not modified baseline muscle hyperalgesia exhibited by NLB rats. (B) In contrast, antisense (AS, open bars), but not mismatch (MM, solid bars) treatment directed to the TNFR1 mRNA was able to reverse the enhancement of muscle hyperalgesia produced by sound stress. **P < 0.01; ***P < 0.001.
The contribution of TNFα in enhanced muscle hyperalgesia in NLB rats after exposure to sound stress was evaluated by using the same AS ODN knockdown strategy directed to the TNFR1.
While the MM ODN treatment did not modify the decrease in mechanical nociceptive threshold produced by sound stress (solid bars, P > 0.05 [n = 6], Fig. 4), AS ODN-treatment significantly increased muscle nociceptive threshold observed at day 2 after first AS injection (open bars, P < 0.001 [n = 6], Fig. 4) reaching a peak one day after the last AS ODN injection (P < 0.001, Fig. 4). Thereafter, nociceptive thresholds start to decrease returning to pre-treatment values by day 5 after the last AS ODN injection (P > 0.05, Fig. 4), demonstrating reversal of effect of AS ODN.
Plasma levels of TNFα and IL-6
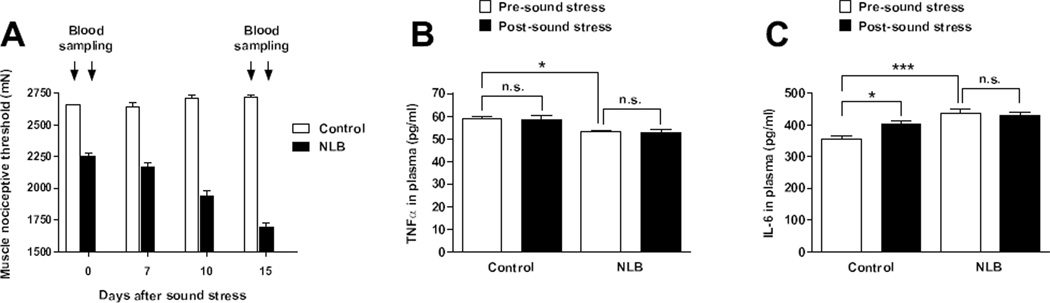
To evaluate the role of TNFα and IL-6 in mechanical hyperalgesia exhibited by NLB rats, we obtained blood samples pre- and post-exposure to sound stress (Fig. 5A). Compared to control rats, NLB rats exhibited lower plasma levels of TNFα (58.8 ± 0.9 [n=6] versus 53.1 ± 0.6 pg/ml [n=6] respectively, P < 0.05) in samples obtained before sound stress (Fig. 5B). The TNFα plasma levels were not modified by sound stress in control rats (58.8 ± 0.9 [n=6] versus 58.3 ± 1.8 pg/ml [n=6] respectively, P > 0.05) or in NLB rats (53.1 ± 0.6 [n=6] versus 52.7 ± 1.3 pg/ml [n=6] respectively, P > 0.05) (Fig. 5B).
Figure 5. Effects of sound stress on TNFα and IL-6 plasma levels.
(A) Blood samples were obtained pre (Day 0) and post (Day 15) exposure to sound stress in control (open bars) and NLB (solid bars) rats, which represent time points of baseline and enhanced muscle hyperalgesia values in NLB rats. (B) While NLB rats exhibited lower plasma levels of TNFα with respect to control rats, at baseline, sound stress did not modify these values in either control or NLB rats. (C) Before exposure to sound stress, NLB rats exhibited higher plasma levels of IL-6 with respect to control rats. Fifteen days after sound stress control rats, but not NLB rats, exhibited increased plasma levels of IL-6. *P < 0.05; ***P < 0.001.
The IL-6 plasma levels in control and NLB rats exhibited marked differences (357.3 ± 10 [n=6] versus 438.7 ± 21 pg/ml [n=6], respectively, P < 0.001) in samples obtained before sound stress (Fig. 5C). The plasma levels of IL-6 were increased by sound stress in control rats (403.2 ± 10.3 [n=6] versus 357.3 ± 10 pg/ml [n=6], respectively, P < 0.05) but not in NLB rats (438.7 ± 12 [n=6] versus 432.4 ± 7.2 pg/ml [n=6], respectively, P > 0.05) (Fig. 5C).
DISCUSSION
In agreement with our previous report, as adults, NLB-treated rats exhibited a moderate muscle hyperalgesia (20). This phenotype is likely due to the abnormal dam/off-spring interaction induced by the restriction of bedding material at the early post-partum period (1, 17). Dams submitted to this protocol exhibit only mild anxiety-like behavior (17) and take care of the litter but this behavior is displayed in an inconsistent/fragmented schedule (16, 17). In contrast to maternal deprivation, this model is more reminiscent of human maternal neglect where the mother is typically present but maternal care is displayed in an inappropriate/unpredictable manner (17). Neonatal limited bedding stress not only produces increased plasma corticosterone at baseline but also a sustained increase of plasma corticosterone after cold-separation stress (18). In these pups, changes consistent with a chronic stress-like state also included greater inter-individual basal and cold stress-induced plasma corticosterone levels, as well as increased adrenal weight and decreased body weight (18, 19). As adults, these rats exhibit selective impairment in cognitive tasks dependent on hippocampal function (44), which is a well-established feature of chronic stress (45, 46).
Growing evidence indicates that early-life stress related to neglect is a risk factor for the development of musculoskeletal pain in adults (10, 47). This risk seems to be associated with a persistent vulnerability to stressful events observed in adult life (12). Consistent with this view, we observed that adult NLB rats exposed to sound stress, which does not produce hyperalgesia in normal rats (35, 43), exhibited persistent aggravation in muscle pain. Remarkably, the development of such chronic muscle pain-like behavior in NLB rats occurred in the absence of any lesion or previous injury. This latter element is the hallmark of many musculoskeletal chronic pain syndromes, including fibromyalgia (6, 7) and posttraumatic stress disorder (48), which are clinical entities where early-life adversity is an important risk factor (6, 7, 10).
Muscle hyperalgesia in NLB rats
It has been reported that adults with a history of early-life adversity have increased circulating levels of pro-inflammatory cytokines such as IL-6 (49) and TNFα (50). Since local injection of these cytokines produces muscle hyperalgesia (27–29), we evaluated whether they could contribute to muscle hyperalgesia exhibited by NLB rats. Our data showing increased IL-6 plasma levels and inhibition of muscle hyperalgesia by disruption of the IL-6 signaling in nociceptors in NLB rats support this idea. In contrast, TNFα plasma levels were lower in NLB rats and antisense treatment against TNFR1 failed to modify muscle nociceptive threshold in these animals, suggesting that TNFα is not involved in hyperalgesia observed in NLB rats. Taken together these results indicate that muscle hyperalgesia observed in NLB rats has a particular pro-inflammatory cytokine signature, which involves IL-6 but not TNFα. The mechanism underlying increased levels of IL-6 was not investigated, but there is evidence that maternal separation produces a persistent dysfunction of intestinal barrier, bacterial translocation and activation of immune response, including increases in circulating levels of IL-6 (22, 51). Since most sensory neurons express the IL-6 receptor/signal transducer gp130 (52) and it is required for long lasting nociceptor sensitization to mechanical stimuli and mechanical hyperalgesia (25), such as that observed here, muscle hyperalgesia observed in NLB rats is likely due to IL-6 effects. Indeed, NLB rats exhibit changes in electrophysiological parameters, indicative of ongoing muscle nociceptor sensitization (20).
Enhanced muscle hyperalgesia in NLB rats by sound stress
Early-life adversity produces a persistent vulnerability to later stressful events (1) and increased levels of circulating pro-inflammatory cytokines (53, 54). Since exposure to subsequent stressful stimuli produces a further increase in plasma levels of such cytokines (53, 54), we asked whether this could contribute to the enhancement of muscle hyperalgesia produced by sound stress in NLB rats. Unexpectedly, while IL-6 plasma levels were increased in control rats after sound stress, neither TNFα nor IL-6 plasma levels were modified by sound stress in NLB rats. However, disruption of either the TNFα or IL-6 signaling reversibly inhibited the sound stress-induced enhancement of muscle hyperalgesia in NLB rats. This suggests that the exacerbation of muscle hyperalgesia by sound stress involves changes in nociceptor expression/signaling by TNFR1 or IL-6 signaling complex, or the induction of a factor interacting synergistically with these cytokine signaling systems. This is consistent with our previous observation that antisense knockdown of TNFα receptor 1 or IL-6 signaling subunit gp130 completely blocks the hyperalgesia induced by lipopolysaccharide (LPS) in rats previously exposed to sound stress, but not that produced by LPS in control rats (34). While a contribution of spinal and supraspinal structures in pain behavior observed here cannot be ruled out (55–57), the intrathecal treatment to knockdown cytokine receptor TNFR1 or subunit gp130 of IL-6 signaling complex was sufficient to block the enhancement of muscle hyperalgesia induced by sound stress. This indicates that such exacerbation is due to a cytokine action at a very early stage of nociceptive processing, presumably at the level of muscle nociceptor.
Contribution of catecholamines
An important observation of this study is that adrenal catecholamines are necessary and sufficient to produce chronic enhancement of muscle pain in NLB rats. Since maternal deprivation does not produce an increase in plasma catecholamine levels in male rats (58), it is not surprising that adrenal medullectomy did not affect baseline muscle hyperalgesia observed in NLB rats. However, adrenal medullectomy prevented sound stress-induced enhancement of muscle pain in NLB rats. Conversely, sustained administration of epinephrine mimicked sound stress-induced enhanced muscle hyperalgesia, with a time course difference probably due to a faster achievement of epinephrine plasma levels in implanted rats. Of note, rats with excised adrenal medulla receiving sustained epinephrine infusion did not exhibited basal muscle hyperalgesia but enhanced mechanical hyperalgesia to intramuscular epinephrine (35). These data indicate that, as in normal rats (35, 43), the exposure to unpredictable sound stress increases catecholamine plasma levels which are necessary for the enhancement of muscle pain observed in NLB rats. Of note, our sound stress protocol does not produce muscle pain in normal rats (35, 43), suggesting that a vulnerability to this stressful stimulus is needed before it can induce muscle hyperalgesia.
Since increased catecholamine levels are observed shortly after activation of the sympathoadrenal axis by sound stress (43), they cannot explain the delayed exacerbation of hyperalgesia observed here and other mechanisms may underlie this phenomenon. As previously indicated, rats exposed to early-life stress exhibit persistent dysfunction of the gastrointestinal barrier, enhanced bacterial translocation to systemic circulation, activation of immune response and raised levels of pro-inflammatory cytokines (22, 51). On the other hand, models of stress-induced bowel dysfunction exhibit concomitant changes in catecholamine plasma levels (59). In addition, catecholamines have direct effects on enteric bacteria and increase their growth capacity and ability to cause an infection (for a review see (60)), which might contribute to enhanced pro-inflammatory responses. Our group has shown that vagotomy produces a sympathoadrenal-dependent enhancement of mechanical hyperalgesia (61), which also induces visceral and muscle mechanical hyperalgesia (62). Interestingly, it has been shown that vagotomy also induces marked bacterial translocation, bacterial invasion to abdominal organs and damage of the intestinal wall (63). Remarkably, these changes were observed at least a week after the intervention and vagotomy-induced mechanical hyperalgesia lasted for weeks thereafter (61–63). Taken together these observations indicate that early-life stress produces a long term dysfunction of gut barrier, which might be aggravated in a catecholamine-dependent manner by the exposure to further stressful stimuli. This might account for the delayed enhancement of muscle hyperalgesia induced by sound stress observed here. This hypothesis is consistent with the remarkable co-morbidity exhibited by patients suffering widespread pain and chronic gastrointestinal symptoms (64).
Altogether, these data indicate that the aggravation of muscle pain in NLB rats observed after exposure to sound stress requires raised levels of plasma catecholamines.
The mechanism underlying the contribution of catecholamines in enhanced muscle hyperalgesia observed in NLB rats was not explored. In vitro studies have shown that exposure of muscle nociceptors to epinephrine causes increased spontaneous firing and enhanced responsiveness to mechanical stimulation (65) and sensitizes cultured dorsal root ganglion neurons to mechanical stimulation in a beta-adrenergic receptor-dependent manner (66). However, since sound stress increases plasma catecholamines without producing muscle hyperalgesia (35, 43), the enhanced hyperalgesia observed in NLB rats is probably related to an action of catecholamines on muscle nociceptors previously sensitized by the activation of either IL-6 or TNFR1 signaling pathways.
In addition to a direct effect on muscle nociceptors, catecholamines can also affect plasma levels of pro-inflammatory cytokines: systemic administration of epinephrine (67, 68) or the exposure to a “psychological” stressor such as open field (69) increase plasma levels of IL-6, which fits with our results in control rats. However, reports about the effect of adrenergic agonists on systemic LPS-induced TNFα and IL-6 production have been contradictory (70–72). Thus, while patients affected by a chronic widespread pain syndrome exhibit sympathoadrenal dysfunction (73), increased release of TNFα/IL-6 (74, 75) and aggravation of their symptoms after stress (9, 11–14), their muscle pain symptoms cannot be explained solely by changes on TNFα/IL-6 plasma levels.
In conclusion, the exposure to unpredictable stress produces persistent aggravation of muscle pain in rats submitted to a model of early-life stress. Such enhanced muscle pain proved to be related to a concerted action of catecholamines and IL-6 or TNFR1 signaling pathways on muscle nociceptors. These data underline the role of early-life adversity in the acquisition of a persistent susceptibility to stressful stimuli and its contribution to chronic widespread muscle pain.
Acknowledgements
This work was support by a grant from the National Institutes of Health and the Rosalind Russell Medical Research Center for Arthritis at UCSF, honoring the violinist, Itzhak Perlman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Baram TZ, Davis EP, Obenaus A, Sandman CA, Small SL, Solodkin A, et al. Fragmentation and unpredictability of early-life experience in mental disorders. The American journal of psychiatry. 2012;169:907–915. doi: 10.1176/appi.ajp.2012.11091347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis DA, Luecken LJ, Zautra AJ. Are reports of childhood abuse related to the experience of chronic pain in adulthood? A meta-analytic review of the literature. The Clinical journal of pain. 2005;21:398–405. doi: 10.1097/01.ajp.0000149795.08746.31. [DOI] [PubMed] [Google Scholar]
- 3.Raphael KG, Widom CS. Post-traumatic stress disorder moderates the relation between documented childhood victimization and pain 30 years later. Pain. 2011;152:163–169. doi: 10.1016/j.pain.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sachs-Ericsson N, Kendall-Tackett K, Hernandez A. Childhood abuse, chronic pain, and depression in the National Comorbidity Survey. Child abuse & neglect. 2007;31:531–547. doi: 10.1016/j.chiabu.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Tietjen GE. Is there a link between abuse in childhood and pain disorders? Expert review of neurotherapeutics. 2010;10:1625–1627. doi: 10.1586/ern.10.152. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg RT, Pachas WN, Keith D. Relationship between traumatic events in childhood and chronic pain. Disability and rehabilitation. 1999;21:23–30. doi: 10.1080/096382899298061. [DOI] [PubMed] [Google Scholar]
- 7.Imbierowicz K, Egle UT. Childhood adversities in patients with fibromyalgia and somatoform pain disorder. Eur J Pain. 2003;7:113–119. doi: 10.1016/S1090-3801(02)00072-1. [DOI] [PubMed] [Google Scholar]
- 8.Kopec JA, Sayre EC. Stressful experiences in childhood and chronic back pain in the general population. The Clinical journal of pain. 2005;21:478–483. doi: 10.1097/01.ajp.0000139909.97211.e1. [DOI] [PubMed] [Google Scholar]
- 9.Korszun A, Papadopoulos E, Demitrack M, Engleberg C, Crofford L. The relationship between temporomandibular disorders and stress-associated syndromes. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 1998;86:416–420. doi: 10.1016/s1079-2104(98)90366-3. [DOI] [PubMed] [Google Scholar]
- 10.Low LA, Schweinhardt P. Early life adversity as a risk factor for fibromyalgia in later life. Pain research and treatment. 2012;2012:140832. doi: 10.1155/2012/140832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruflat AK, Baiter JE, McGuire D, Fethke NB, Maluf KS. Stress management as an adjunct to physical therapy for chronic neck pain. Physical therapy. 2012;92:1348–1359. doi: 10.2522/ptj.20110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis MC, Zautra AJ, Reich JW. Vulnerability to stress among women in chronic pain from fibromyalgia and osteoarthritis. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2001;23:215–226. doi: 10.1207/S15324796ABM2303_9. [DOI] [PubMed] [Google Scholar]
- 13.Giske L, Bautz-Holter E, Sandvik L, Roe C. Relationship between pain and neuropathic symptoms in chronic musculoskeletal pain. Pain Med. 2009;10:910–917. doi: 10.1111/j.1526-4637.2009.00622.x. [DOI] [PubMed] [Google Scholar]
- 14.Glaros AG, Williams K, Lausten L. The role of parafunctions, emotions and stress in predicting facial pain. J Am Dent Assoc. 2005;136:451–458. doi: 10.14219/jada.archive.2005.0200. [DOI] [PubMed] [Google Scholar]
- 15.Nishi M, Horii-Hayashi N, Sasagawa T, Matsunaga W. Effects of early life stress on brain activity: Implications from maternal separation model in rodents. General and comparative endocrinology. 2013;181C:306–309. doi: 10.1016/j.ygcen.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 16.Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 2008;154:1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilles EE, Schultz L, Baram TZ. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatric neurology. 1996;15:114–119. doi: 10.1016/0887-8994(96)00153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avishai-Eliner S, Gilles EE, Eghbal-Ahmadi M, Bar-El Y, Baram TZ. Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. Journal of neuroendocrinology. 2001;13:799–807. doi: 10.1046/j.1365-2826.2001.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green PG, Chen X, Alvarez P, Ferrari LF, Levine JD. Early-life stress produces muscle hyperalgesia and nociceptor sensitization in the adult rat. Pain. 2011;152:2549–2556. doi: 10.1016/j.pain.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loizzo A, Loizzo S, Lopez L, d'Amore A, Renzi P, Spampinato S, et al. Naloxone prevents cell-mediated immune alterations in adult mice following repeated mild stress in the neonatal period. British journal of pharmacology. 2002;135:1219–1226. doi: 10.1038/sj.bjp.0704577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, et al. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biological psychiatry. 2009;65:263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 23.O'Malley D, Liston M, Hyland NP, Dinan TG, Cryan JF. Colonic soluble mediators from the maternal separation model of irritable bowel syndrome activate submucosal neurons via an interleukin-6-dependent mechanism. American journal of physiology Gastrointestinal and liver physiology. 2011;300:G241–G252. doi: 10.1152/ajpgi.00385.2010. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Ji A, Weihe E, Schafer MK. Cell-specific expression and lipopolysaccharide-induced regulation of tumor necrosis factor alpha (TNFalpha) and TNF receptors in rat dorsal root ganglion. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:9623–9631. doi: 10.1523/JNEUROSCI.2392-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quarta S, Vogl C, Constantin CE, Uceyler N, Sommer C, Kress M. Genetic evidence for an essential role of neuronally expressed IL-6 signal transducer gp130 in the induction and maintenance of experimentally induced mechanical hypersensitivity in vivo and in vitro. Molecular pain. 2011;7:73. doi: 10.1186/1744-8069-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Summer GJ, Romero-Sandoval EA, Bogen O, Dina OA, Khasar SG, Levine JD. Proinflammatory cytokines mediating burn-injury pain. Pain. 2008;135:98–107. doi: 10.1016/j.pain.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008;152:521–525. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manjavachi MN, Motta EM, Marotta DM, Leite DF, Calixto JB. Mechanisms involved in IL-6-induced muscular mechanical hyperalgesia in mice. Pain. 2010;151:345–355. doi: 10.1016/j.pain.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 29.Schafers M, Sorkin LS, Sommer C. Intramuscular injection of tumor necrosis factor-alpha induces muscle hyperalgesia in rats. Pain. 2003;104:579–588. doi: 10.1016/S0304-3959(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 30.Hakim AW, Dong XD, Svensson P, Kumar U, Cairns BE. TNFalpha mechanically sensitizes masseter muscle afferent fibers of male rats. Journal of neurophysiology. 2009;102:1551–1559. doi: 10.1152/jn.00326.2009. [DOI] [PubMed] [Google Scholar]
- 31.Shah JP, Danoff JV, Desai MJ, Parikh S, Nakamura LY, Phillips TM, et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Archives of physical medicine and rehabilitation. 2008;89:16–23. doi: 10.1016/j.apmr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 32.Uceyler N, Hauser W, Sommer C. Systematic review with meta-analysis: cytokines in fibromyalgia syndrome. BMC musculoskeletal disorders. 2011;12:245. doi: 10.1186/1471-2474-12-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, et al. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- 34.Dina OA, Levine JD, Green PG. Enhanced cytokine-induced mechanical hyperalgesia in skeletal muscle produced by a novel mechanism in rats exposed to unpredictable sound stress. Eur J Pain. 2011;15:796–800. doi: 10.1016/j.ejpain.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khasar SG, Dina OA, Green PG, Levine JD. Sound stress-induced long-term enhancement of mechanical hyperalgesia in rats is maintained by sympathoadrenal catecholamines. The journal of pain : official journal of the American Pain Society. 2009;10:1073–1077. doi: 10.1016/j.jpain.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khasar SG, Green PG, Levine JD. Repeated sound stress enhances inflammatory pain in the rat. Pain. 2005;116:79–86. doi: 10.1016/j.pain.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez P, Levine JD, Green PG. Eccentric exercise induces chronic alterations in musculoskeletal nociception in the rat. The European journal of neuroscience. 2010;32:819–825. doi: 10.1111/j.1460-9568.2010.07359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. Journal of pharmacological and toxicological methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 39.Parada CA, Yeh JJ, Joseph EK, Levine JD. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. The European journal of neuroscience. 2003;17:1847–1852. doi: 10.1046/j.1460-9568.2003.02626.x. [DOI] [PubMed] [Google Scholar]
- 40.Muller-Newen G. The cytokine receptor gp130: faithfully promiscuous. Science's STKE: signal transduction knowledge environment. 2003;2003:PE40. doi: 10.1126/stke.2003.201.pe40. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Nesbitt JE, Fuentes NL, Fuller GM. Molecular cloning and characterization of the rat liver IL-6 signal transducing molecule, gp130. Genomics. 1992;14:666–672. doi: 10.1016/s0888-7543(05)80166-1. [DOI] [PubMed] [Google Scholar]
- 42.Stone LS, Vulchanova L. The pain of antisense: in vivo application of antisense oligonucleotides for functional genomics in pain and analgesia. Advanced drug delivery reviews. 2003;55:1081–1112. doi: 10.1016/s0169-409x(03)00105-4. [DOI] [PubMed] [Google Scholar]
- 43.Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, et al. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brunson KL, Kramar E, Lin B, Chen Y, Colgin LL, Yanagihara TK, et al. Mechanisms of late-onset cognitive decline after early-life stress. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alfarez DN, Joels M, Krugers HJ. Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. The European journal of neuroscience. 2003;17:1928–1934. doi: 10.1046/j.1460-9568.2003.02622.x. [DOI] [PubMed] [Google Scholar]
- 46.Pavlides C, Nivon LG, McEwen BS. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus. 2002;12:245–257. doi: 10.1002/hipo.1116. [DOI] [PubMed] [Google Scholar]
- 47.Nicolson NA, Davis MC, Kruszewski D, Zautra AJ. Childhood maltreatment and diurnal cortisol patterns in women with chronic pain. Psychosomatic medicine. 2010;72:471–480. doi: 10.1097/PSY.0b013e3181d9a104. [DOI] [PubMed] [Google Scholar]
- 48.Gibson CA. Review of posttraumatic stress disorder and chronic pain: the path to integrated care. Journal of rehabilitation research and development. 2012;49:753–776. doi: 10.1682/jrrd.2011.09.0158. [DOI] [PubMed] [Google Scholar]
- 49.Gouin JP, Glaser R, Malarkey WB, Beversdorf D, Kiecolt-Glaser JK. Childhood abuse and inflammatory responses to daily stressors. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2012;44:287–292. doi: 10.1007/s12160-012-9386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosomatic medicine. 2011;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Mahony SM, Hyland NP, Dinan TG, Cryan JF. Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology. 2011;214:71–88. doi: 10.1007/s00213-010-2010-9. [DOI] [PubMed] [Google Scholar]
- 52.Gardiner NJ, Cafferty WB, Slack SE, Thompson SW. Expression of gp130 and leukaemia inhibitory factor receptor subunits in adult rat sensory neurones: regulation by nerve injury. Journal of neurochemistry. 2002;83:100–109. doi: 10.1046/j.1471-4159.2002.01101.x. [DOI] [PubMed] [Google Scholar]
- 53.Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:2617–2623. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. The American journal of psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 55.Jia D, Gao GD, Liu Y, He SM, Zhang XN, Zhang YF, et al. TNF-alpha involves in altered prefrontal synaptic transmission in mice with persistent inflammatory pain. Neuroscience letters. 2007;415:1–5. doi: 10.1016/j.neulet.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 56.Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei F, Guo W, Zou S, Ren K, Dubner R. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:10482–10495. doi: 10.1523/JNEUROSCI.3593-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Renard GM, Suarez MM, Levin GM, Rivarola MA. Sex differences in rats: effects of chronic stress on sympathetic system and anxiety. Physiology & behavior. 2005;85:363–369. doi: 10.1016/j.physbeh.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Ibeakanma C, Ochoa-Cortes F, Miranda-Morales M, McDonald T, Spreadbury I, Cenac N, et al. Brain-gut interactions increase peripheral nociceptive signaling in mice with postinfectious irritable bowel syndrome. Gastroenterology. 2011;141:2098–2108. e2095. doi: 10.1053/j.gastro.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 60.Lyte M, Vulchanova L, Brown DR. Stress at the intestinal surface: catecholamines and mucosa-bacteria interactions. Cell and tissue research. 2011;343:23–32. doi: 10.1007/s00441-010-1050-0. [DOI] [PubMed] [Google Scholar]
- 61.Khasar SG, Miao FJ, Janig W, Levine JD. Vagotomy-induced enhancement of mechanical hyperalgesia in the rat is sympathoadrenal-mediated. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:3043–3049. doi: 10.1523/JNEUROSCI.18-08-03043.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Furuta S, Shimizu T, Narita M, Matsumoto K, Kuzumaki N, Horie S, et al. Subdiaphragmatic vagotomy promotes nociceptive sensitivity of deep tissue in rats. Neuroscience. 2009;164:1252–1262. doi: 10.1016/j.neuroscience.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 63.Doganay M, Kama NA, Yazgan A, Aksoy M, Ergul G, Tekeli A. The effects of vagotomy on bacterial translocation: an experimental study. The Journal of surgical research. 1997;71:166–171. doi: 10.1006/jsre.1997.5157. [DOI] [PubMed] [Google Scholar]
- 64.Wallace DJ, Hallegua DS. Fibromyalgia: the gastrointestinal link. Current pain and headache reports. 2004;8:364–368. doi: 10.1007/s11916-996-0009-z. [DOI] [PubMed] [Google Scholar]
- 65.Kieschke J, Mense S, Prabhakar NR. Influence of adrenaline and hypoxia on rat muscle receptors in vitro. Progress in brain research. 1988;74:91–97. doi: 10.1016/s0079-6123(08)63003-4. [DOI] [PubMed] [Google Scholar]
- 66.Khasar SG, McCarter G, Levine JD. Epinephrine produces a beta-adrenergic receptor-mediated mechanical hyperalgesia and in vitro sensitization of rat nociceptors. Journal of neurophysiology. 1999;81:1104–1112. doi: 10.1152/jn.1999.81.3.1104. [DOI] [PubMed] [Google Scholar]
- 67.DeRijk RH, Boelen A, Tilders FJ, Berkenbosch F. Induction of plasma interleukin-6 by circulating adrenaline in the rat. Psychoneuroendocrinology. 1994;19:155–163. doi: 10.1016/0306-4530(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 68.van Gool J, van Vugt H, Helle M, Aarden LA. The relation among stress, adrenalin, interleukin 6 and acute phase proteins in the rat. Clinical immunology and immunopathology. 1990;57:200–210. doi: 10.1016/0090-1229(90)90034-n. [DOI] [PubMed] [Google Scholar]
- 69.LeMay LG, Vander AJ, Kluger MJ. The effects of psychological stress on plasma interleukin-6 activity in rats. Physiology & behavior. 1990;47:957–961. doi: 10.1016/0031-9384(90)90024-x. [DOI] [PubMed] [Google Scholar]
- 70.Izeboud CA, Monshouwer M, van Miert AS, Witkamp RF. The beta-adrenoceptor agonist clenbuterol is a potent inhibitor of the LPS-induced production of TNF-alpha and IL-6 in vitro and in vivo. Inflammation research : official journal of the European Histamine Research Society [et al] 1999;48:497–502. doi: 10.1007/s000110050493. [DOI] [PubMed] [Google Scholar]
- 71.Sekut L, Champion BR, Page K, Menius JA, Jr, Connolly KM. Anti-inflammatory activity of salmeterol: down-regulation of cytokine production. Clinical and experimental immunology. 1995;99:461–466. doi: 10.1111/j.1365-2249.1995.tb05573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Szabo C, Hasko G, Zingarelli B, Nemeth ZH, Salzman AL, Kvetan V, et al. Isoproterenol regulates tumour necrosis factor, interleukin-10, interleukin-6 and nitric oxide production and protects against the development of vascular hyporeactivity in endotoxaemia. Immunology. 1997;90:95–100. doi: 10.1046/j.1365-2567.1997.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sarzi-Puttini P, Atzeni F, Diana A, Doria A, Furlan R. Increased neural sympathetic activation in fibromyalgia syndrome. Annals of the New York Academy of Sciences. 2006;1069:109–117. doi: 10.1196/annals.1351.009. [DOI] [PubMed] [Google Scholar]
- 74.Bote ME, Garcia JJ, Hinchado MD, Ortega E. Inflammatory/Stress feedback dysregulation in women with fibromyalgia. Neuroimmunomodulation. 2012;19:343–351. doi: 10.1159/000341664. [DOI] [PubMed] [Google Scholar]
- 75.Malhotra D, Saxena AK, Dar SA, Kumar V, Nasare N, Tripathi AK, et al. Evaluation of Cytokine Levels in Fibromyalgia Syndrome Patients and its Relationship to the Severity of Chronic Pain. Musculoskelet Pain. 2012;20:164–169. [Google Scholar]