Abstract
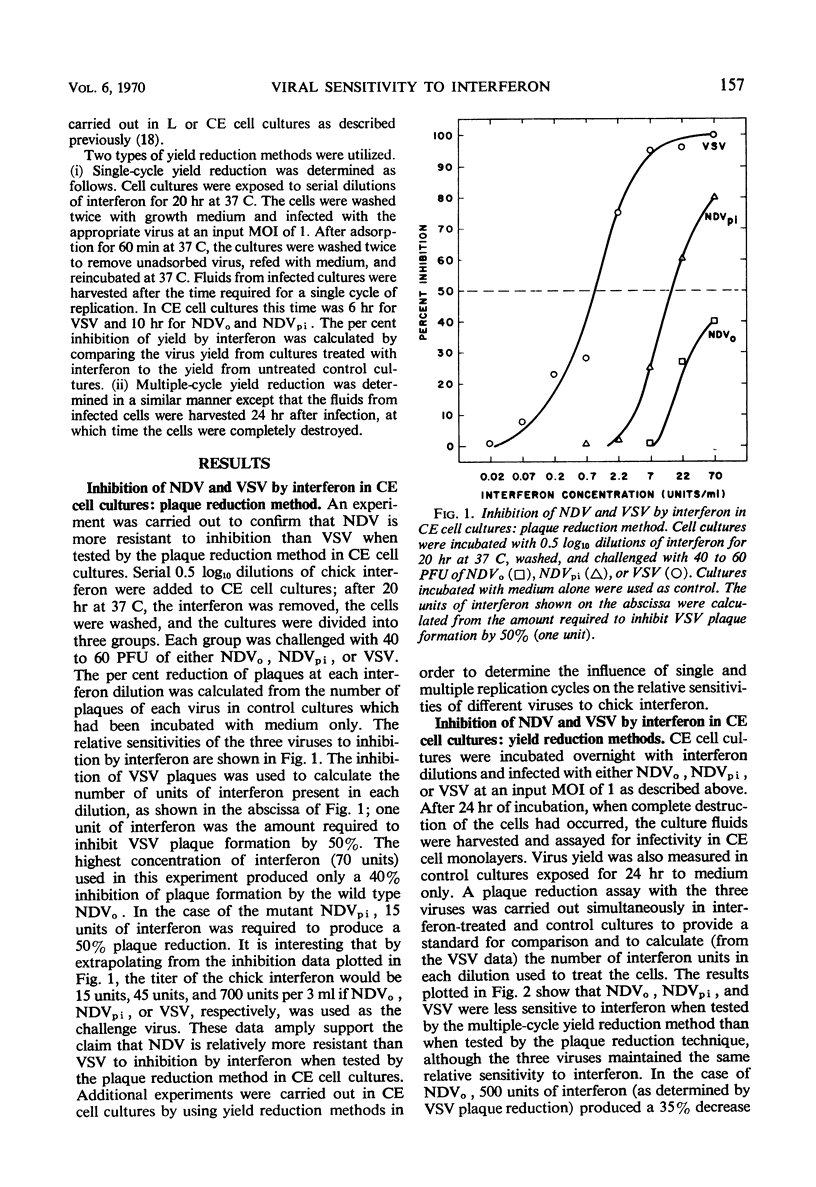
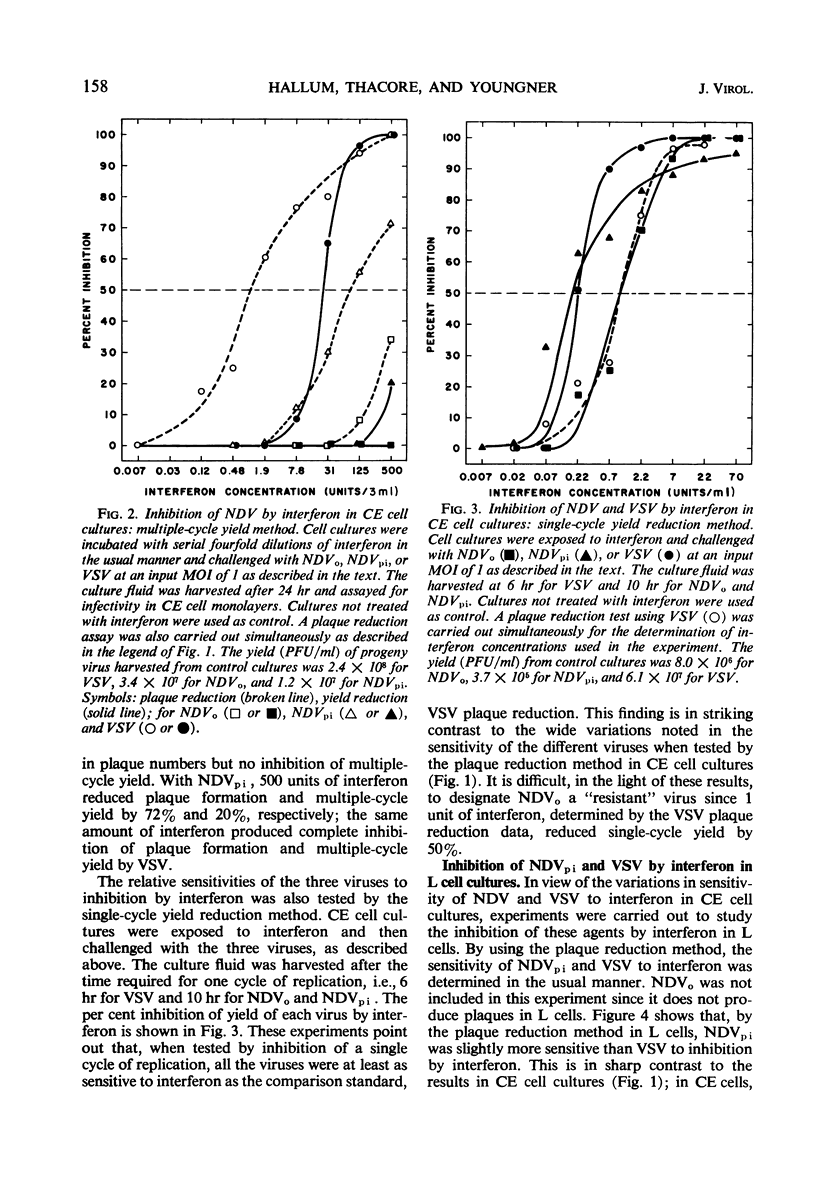
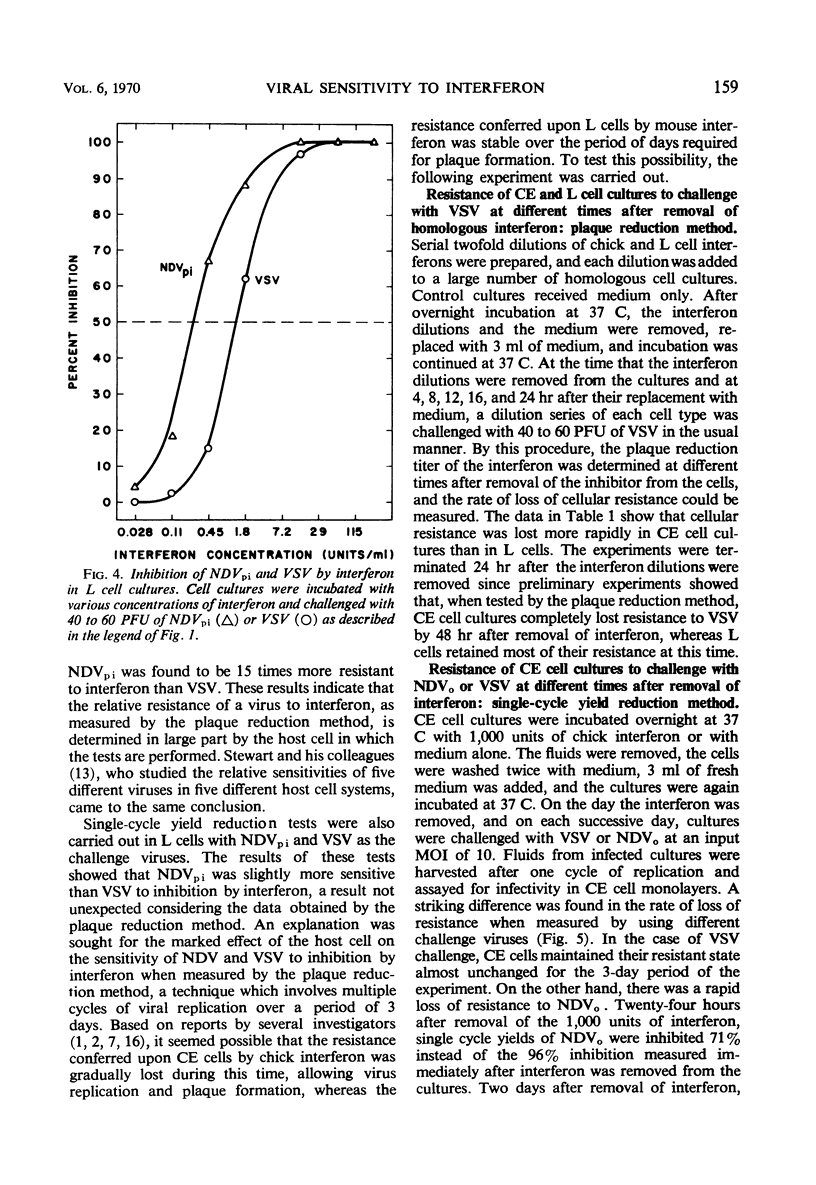
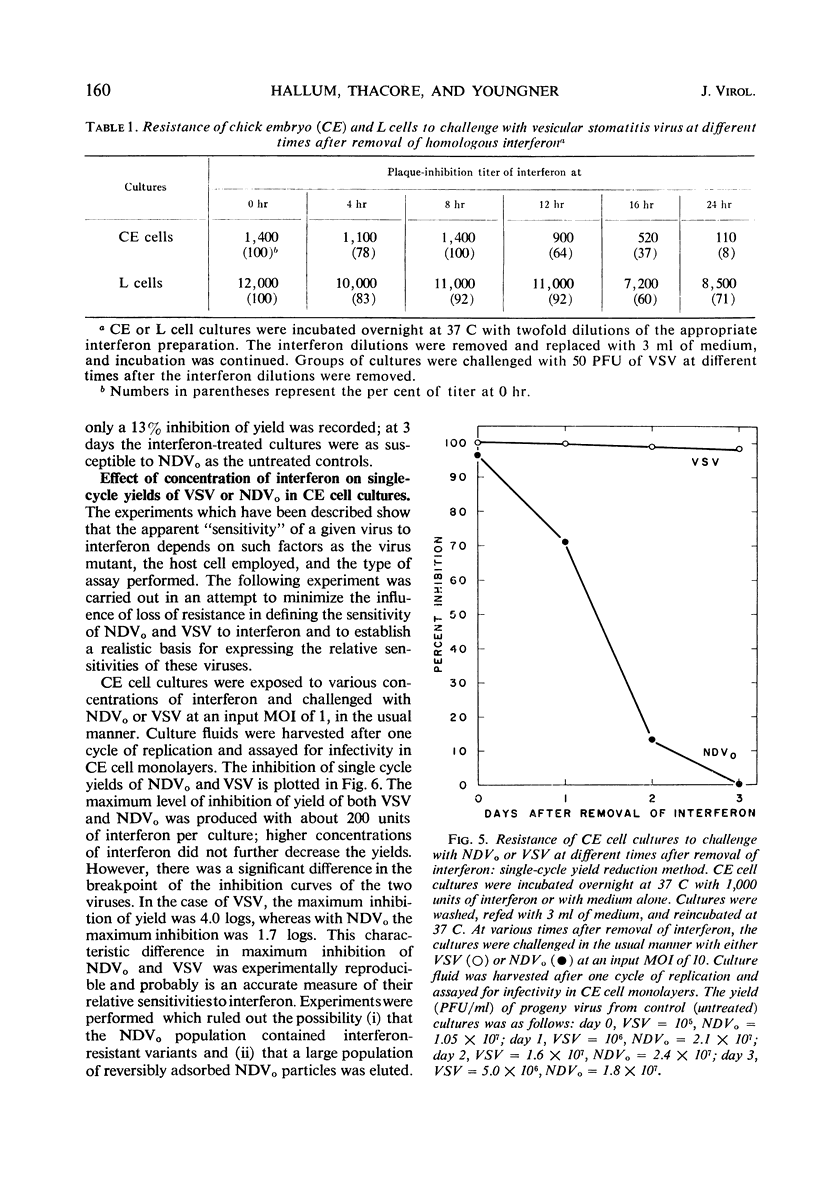
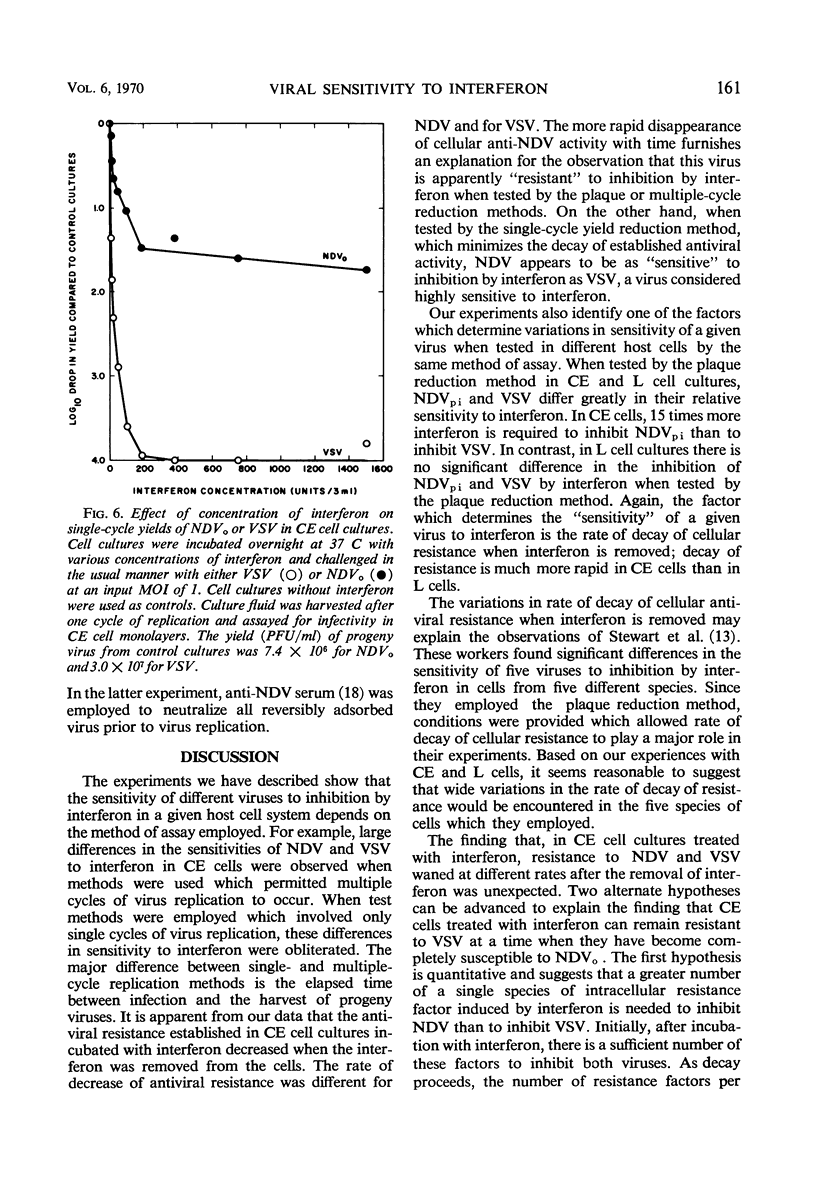
When the sensitivities to interferon of Newcastle disease virus (NDV) and vesicular stomatitis virus (VSV) were compared by the plaque reduction method in chick embryo cell cultures, NDV was found to be 45-fold more resistant than VSV. This difference was exaggerated when a multiple-cycle yield inhibition method was employed. In marked contrast, when the same viruses were tested by a single-cycle yield inhibition method, the difference in sensitivity to interferon of the two viruses was virtually eliminated. Further investigation showed that, in chick embryo cells exposed to interferon, the resistance to NDV decayed more rapidly than resistance to VSV. This finding explained the divergent results obtained with the two viruses when single- or multiple-cycle replication techniques were employed. Experiments carried out with L cells showed that cellular antiviral resistance decayed much more slowly in these cells than in chick embryo cells. Consequently, when measured by the plaque reduction method in L cells, no difference was observed in the sensitivity to interferon of VSV and NDVpi, a mutant of NDV which replicates efficiently in L cells. A procedure is suggested for determining the relative sensitivities to interferon of different viruses under conditions which minimize the role of decay of antiviral resistance in the host cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron S., Buckler C. E., Levy H. B., Friedman R. M. Some factors affecting the interferon-induced antiviral state. Proc Soc Exp Biol Med. 1967 Aug-Sep;125(4):1320–1326. doi: 10.3181/00379727-125-32347. [DOI] [PubMed] [Google Scholar]
- Buckler C. E., Wong K. T., Baron S. Induction of the interferon system by various inducers. Proc Soc Exp Biol Med. 1968 Apr;127(4):1258–1262. doi: 10.3181/00379727-127-32924. [DOI] [PubMed] [Google Scholar]
- Campbell J. B., Colter J. S. Sensitivity to, and production of interferon by three variants of Mengo virus. Can J Microbiol. 1967 Aug;13(8):931–937. doi: 10.1139/m67-125. [DOI] [PubMed] [Google Scholar]
- Hallum J. V., Younger J. S. Quantitative aspects of inhibition of virus replication by interferon in chick embryo cell cultures. J Bacteriol. 1966 Oct;92(4):1047–1050. doi: 10.1128/jb.92.4.1047-1050.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallum J. V., Youngner J. S., Stinebring W. R. Interferon activity associated with high molecular weight proteins in the circulation of mice injected with endotoxin or bacteria. Virology. 1965 Nov;27(3):429–431. doi: 10.1016/0042-6822(65)90124-8. [DOI] [PubMed] [Google Scholar]
- Ke Y. H., Ho M., Merigan T. C. Heterogeneity of rabbit serum interferons. Nature. 1966 Jul 30;211(5048):541–542. doi: 10.1038/211541a0. [DOI] [PubMed] [Google Scholar]
- MERIGAN T. C. PURIFIED INTERFERONS: PHYSICAL PROPERTIES AND SPECIES SPECIFICITY. Science. 1964 Aug 21;145(3634):811–813. doi: 10.1126/science.145.3634.811-a. [DOI] [PubMed] [Google Scholar]
- Osborn J. E., Medearis D. N., Jr Studies of relationship between mouse cytomegalovirus and interferon. Proc Soc Exp Biol Med. 1966 Mar;121(3):819–824. doi: 10.3181/00379727-121-30897. [DOI] [PubMed] [Google Scholar]
- RUIZ-GOMEZ J., ISAACS A. Optimal temperature for growth and sensitivity to interferon among different viruses. Virology. 1963 Jan;19:1–7. doi: 10.1016/0042-6822(63)90017-5. [DOI] [PubMed] [Google Scholar]
- Riley B. P., Toy S. T., Gifford G. E. Relative effects of interferon on virus plaque formation. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1142–1144. doi: 10.3181/00379727-122-31346. [DOI] [PubMed] [Google Scholar]
- Schonne E. Properties of rat-tumor interferon. Biochim Biophys Acta. 1966 Feb 28;115(2):429–439. doi: 10.1016/0304-4165(66)90441-7. [DOI] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Scott W. D., Sulkin S. E. Relative sensitivities of viruses to different species of interferon. J Virol. 1969 Aug;4(2):147–153. doi: 10.1128/jvi.4.2.147-153.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacore H., Youngner J. S. Cells persistently infected with newcastle disease virus: I. Properties of mutants isolated from persistently infected L cells. J Virol. 1969 Sep;4(3):244–251. doi: 10.1128/jvi.4.3.244-251.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAGNER R. R. Biological studies of interferon. I. Suppression of cellular infection with eastern equine encephalomyelitis virus. Virology. 1961 Mar;13:323–337. doi: 10.1016/0042-6822(61)90152-0. [DOI] [PubMed] [Google Scholar]
- WAGNER R. R., LEVEY A. H., SNYDER R. M., RATCLIFF G. A., Jr, HYATT D. F. BIOLOGIC PROPERTIES OF TWO PLAQUE VARIANTS OF VESICULAR STOMATITIS VIRUS (INDIANA SEROTYPE). J Immunol. 1963 Jul;91:112–122. [PubMed] [Google Scholar]
- Youngner J. S., Scott A. W., Hallum J. V., Stinebring W. R. Interferon production by inactivated Newcastle disease virus in cell cultures and in mice. J Bacteriol. 1966 Oct;92(4):862–868. doi: 10.1128/jb.92.4.862-868.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]



