Abstract
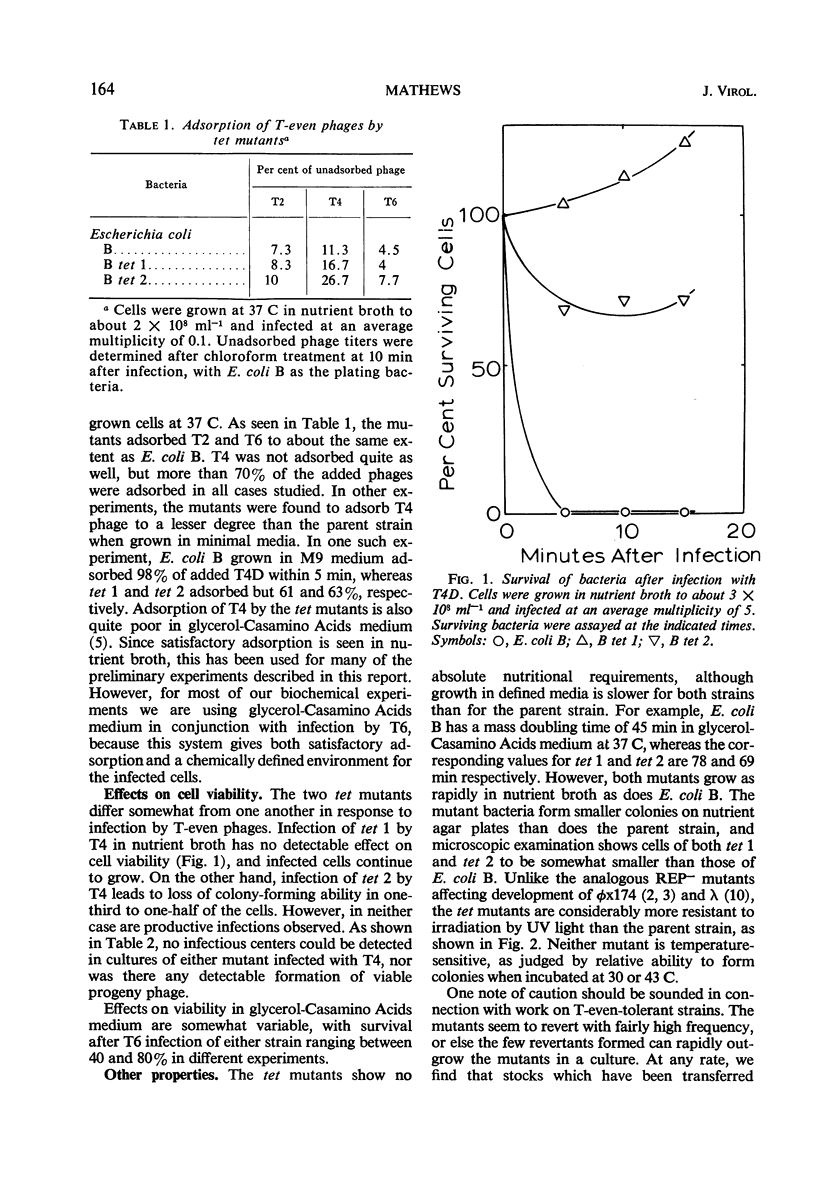
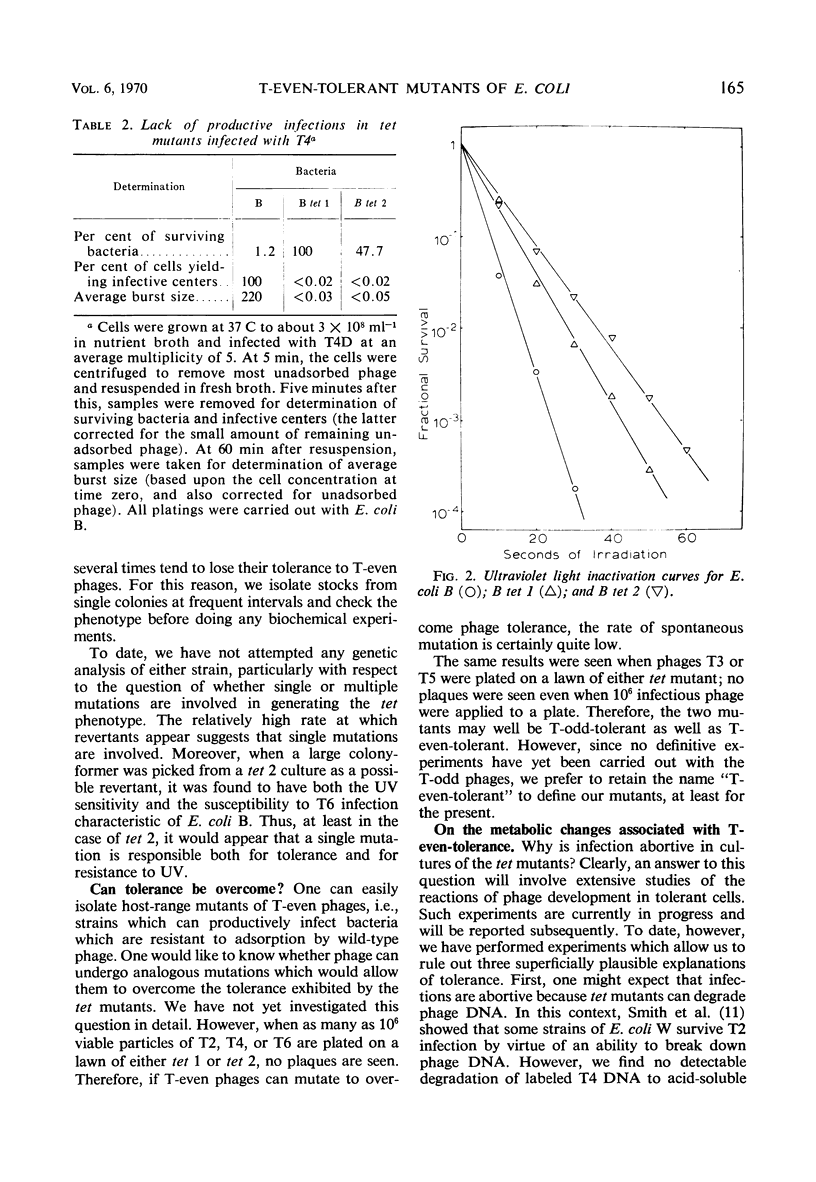
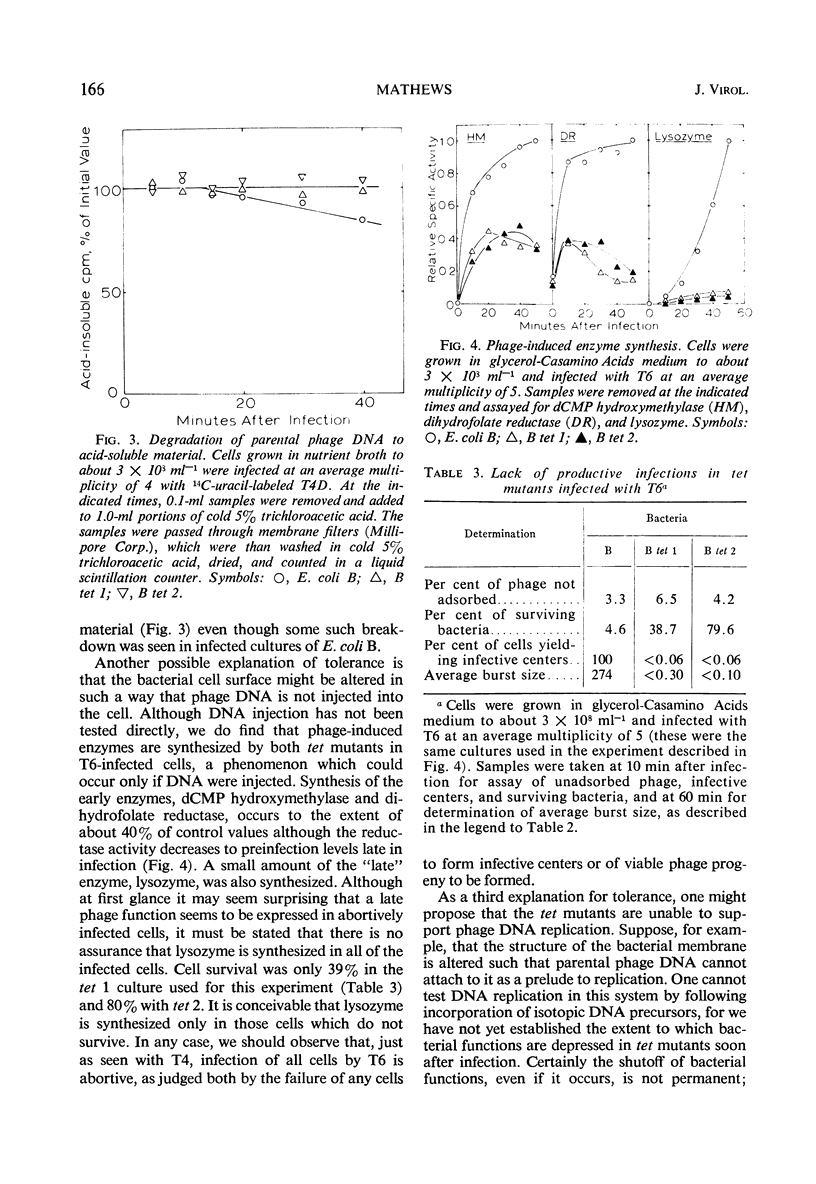
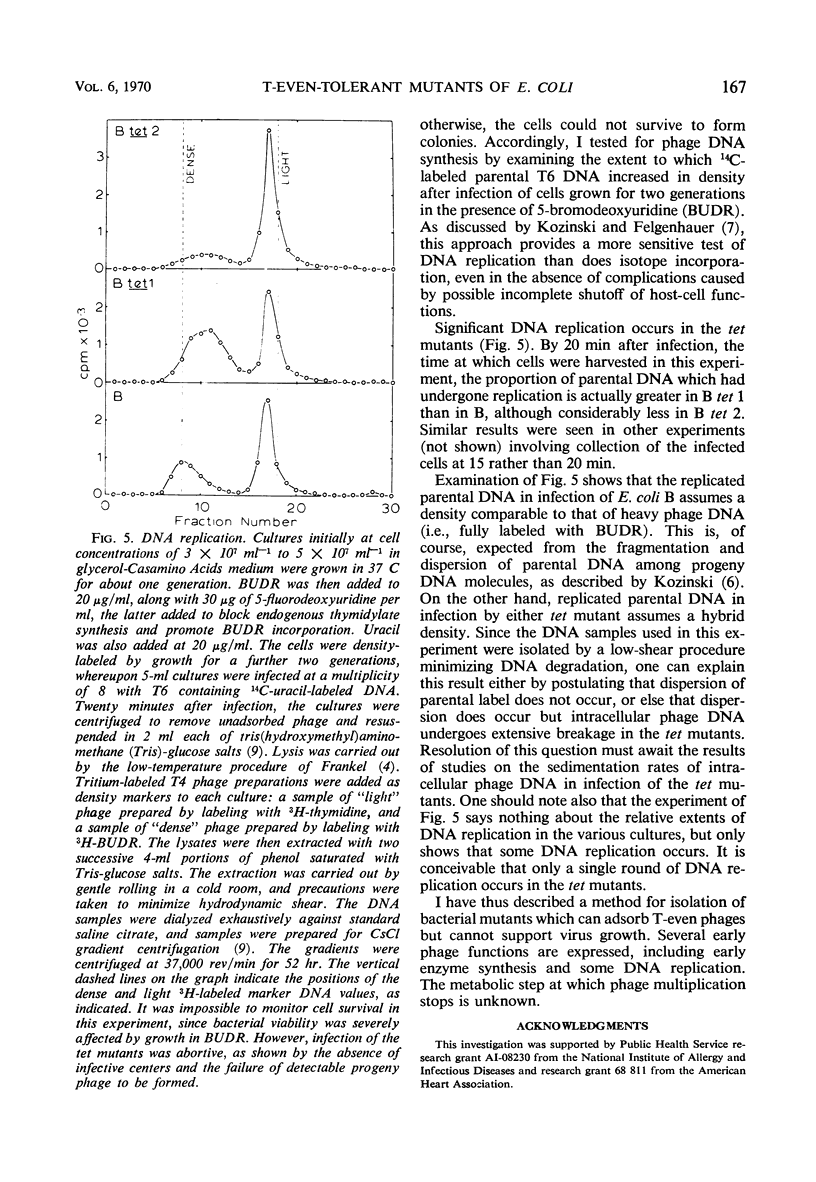
A general procedure is described for isolation of T-even phage-tolerant mutants of Escherichia coli. Two such mutants of E. coli B have been examined in some detail. These mutants adsorb T-even phages but are unable to release viable progeny. Under certain conditions, viability of the cells is completely unaffected by phage infection in one mutant, and there is but a slight decrease in colony-forming ability in the other. Phage deoxyribonucleic acid (DNA) is injected into these cells, as shown by the formation of phage-specific enzymes, but it is not degraded to acid-soluble material. Some phage DNA replication occurs in both strains. The mutants are both more resistant to ultraviolet light than is the parent strain.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calendar R., Lindqvist B., Sironi G., Clark A. J. Characterization of REP- mutants and their interaction with P2 phage. Virology. 1970 Jan;40(1):72–83. doi: 10.1016/0042-6822(70)90380-6. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T., Dressler D. H., Hathaway A. THE ABORTIVE REPLICATION OF PhiX174 DNA IN A RECOMBINATION-DEFICIENT MUTANT OF Escherichia coli. Proc Natl Acad Sci U S A. 1967 Mar;57(3):813–820. doi: 10.1073/pnas.57.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- Frankel F. R. Evidence for long DNA strands in the replicating pool after T4 infection. Proc Natl Acad Sci U S A. 1968 Jan;59(1):131–138. doi: 10.1073/pnas.59.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOZINSKI A. W. Fragmentary transfer of P32-labeled parental DNA to progeny phage. Virology. 1961 Jan;13:124–134. doi: 10.1016/0042-6822(61)90039-3. [DOI] [PubMed] [Google Scholar]
- Kozinski A. W., Felgenhauer Z. Z. Molecular recombination in T4 bacteriophage deoxyribonucleic acid. II. Single-strand breaks and exposure of uncomplemented areas as a prerequisite for recombination. J Virol. 1967 Dec;1(6):1193–1202. doi: 10.1128/jvi.1.6.1193-1202.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews C. K. Deoxyribonucleic acid metabolism and virus-induced enzyme synthesis in a thymine-requiring bacterium infected by a thymine-requiring bacteriophage. Biochemistry. 1966 Jun;5(6):2092–2100. doi: 10.1021/bi00870a042. [DOI] [PubMed] [Google Scholar]
- Murray R. E., Mathews C. K. Addition of nucleotides to parental DNA early in infection by bacteriophage T4. J Mol Biol. 1969 Sep 14;44(2):233–248. doi: 10.1016/0022-2836(69)90172-7. [DOI] [PubMed] [Google Scholar]
- Naha P. M. Bacterial control of lambda replication. I. Initial characterization of a rep mutation of Escherichia coli K12. Virology. 1968 Nov;36(3):434–441. doi: 10.1016/0042-6822(68)90168-2. [DOI] [PubMed] [Google Scholar]
- Smith H. S., Miovic M., Pizer L. I. Correlation between degradation of bacteriophage T2 deoxyribonucleic acid and the resistance of Escherichia coli to infection. J Virol. 1969 Aug;4(2):195–196. doi: 10.1128/jvi.4.2.195-196.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W. Bacterial viruses; with particular reference to adsorption/penetration. Annu Rev Microbiol. 1958;12:27–48. doi: 10.1146/annurev.mi.12.100158.000331. [DOI] [PubMed] [Google Scholar]



