Abstract
Objective
Many studies have shown that 5-HTTLPR genotype interacts with exposure to stress in conferring risk for psychopathology. However, the specific neural mechanisms through which this gene-by-environment interaction confers risk remain largely unknown, and no study to date has directly examined the modulatory effects of the 5-HTTLPR on corticolimbic circuit responses during exposure to acute stress.
Methods
An acute laboratory stressor was administered to 51 healthy women during BOLD fMRI scanning. In this task, electric shocks of uncertain intensity were threatened and unpredictably delivered to the wrist after a long anticipatory cue period of unpredictable duration.
Results
Relative to those carrying the L allele, SS homozygotes showed enhanced activation during threat anticipation in a network of regions including amygdala, hippocampus, anterior insula, thalamus, pulvinar, caudate, precuneus, anterior cingulate cortex, and medial prefrontal cortex. SS homozygotes also displayed enhanced positive coupling between medial prefrontal cortex activation and anxiety experience, whereas individuals carrying the L allele displayed enhanced negative coupling between insula activation and perceived success at regulating anxiety.
Conclusions
The present findings suggest that, when exposed to stress, SS homozygotes may preferentially engage neural systems which enhance fear and arousal, modulate attention toward threat, and perseverate on emotional salience of the threat. This may be one mechanism underlying risk for psychopathology conferred by the S allele upon exposure to life stressors.
INTRODUCTION
Diathesis-stress models suggest that psychopathology arises through the interplay of intrinsic biological factors, such as genetic variation, and extrinsic environmental factors, such as exposure to stressors (1). Capitalizing on this framework, numerous gene-by-environment interaction studies have now demonstrated that variation in specific genes interact with stress exposure to confer vulnerability for mood and anxiety disorders (e.g. (2–5)).
One of the best known demonstrations of gene-by-environment effects involves a functional promoter polymorphism (5-HTTLPR) in the serotonin transporter gene (SLC6A4) in which the short “S” allele interacts with exposure to stressful life events to predict risk for mood-related psychopathology (6, 7). This specific gene-by-environment interaction effect on risk for psychopathology has been widely replicated in human studies of both depression and anxiety (8, 9) and supported in non-human primate and rodent models (10–12). While there have been some failures at replication (13), a recent large-scale meta-analysis has provided strong support for this gene-by-environment interaction effect (7).
Imaging genetics studies, which leverage functional neuroimaging to uncover the neurobiological correlates of specific genetic variants (14), have found a robust association between the S allele and increased amygdala reactivity during implicit processing of pictures of negative facial expressions (15), but no changes to positive facial expressions (16, 17). While these studies clearly implicate neurobiological pathways involved in mediating sensitivity to environmental stress, the specific neural mechanisms through which this gene-by-environment interaction confers risk remain largely unknown. Indeed, few studies to date have directly examined the modulatory effects of the 5-HTTLPR on corticolimbic circuit responses during exposure to acute stress (18, 19).
To identify specific neurobiological mechanisms mediating 5-HTTLPR-by-stress effects on risk for psychopathology, it is critical to devise experimental paradigms that capture the unpredictability and aversiveness of environmental stressors in the laboratory. Such paradigms are valuable because they can overcome many limitations of currently utilized measures of stressful life events based on self-report including memory biases and limitations associated with retrospective assessment, self-presentation biases, mood-state effects, and substantial variability across instruments (20). Acute laboratory stressors also permit control over the timing and magnitude of stressors and examination of momentary changes in neurobiology to delineate the immediate effect of stressors on neurobiological systems.
In the current study, we administered an acute laboratory stressor to healthy adult women during blood oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI). Focusing on a healthy population allowed us to investigate the mechanism of risk without confound of current or past psychopathology, which makes it difficult to infer cause from consequence. In this paradigm, electric shocks of uncertain intensity were threatened and unpredictably delivered to the wrist after a long anticipatory cue period of unpredictable duration, allowing for robust responses to be generated (21). We obtained ratings of global anxiety experience and anxiety regulation success at the end of the task. We examined two hypotheses. First, based on existing epidemiological and neuroimaging research (8), we hypothesized an interaction between 5-HTTLPR genotype and stress such that in these healthy women the short allele would be associated with greater amygdala reactivity as well as greater activation in medial prefrontal cortex during stress exposure (22). Second, based on the brain regions identified in these analyses as well as extensive literature on neural responses to threat (23–25), we hypothesized that genotype would moderate the relationship between corticolimic BOLD responses and reports of anxiety and regulation success. Our specific focus was brain regions implicated in triggering central and peripheral responses to threat (amygdala) (23), interoceptive processing of threat (insula) (24) and cognitive appraisal of threat (medial prefrontal cortex) (25) and we hypothesized that S allele carriers would have greater brain-behavior coupling with anxiety experience, but lesser coupling with regulation success.
METHOD
Participants
Fifty-one right-handed healthy women (mean age 22 +/− 2.4) participated (21). All potential participants were screened using the Structured Clinical Interview (SCID) for DSM-IV (26). Eligible participants did not meet criteria for any psychiatric disorder within the past year, or for lifetime generalized anxiety, posttraumatic stress, bipolar, obsessive–compulsive, or psychotic disorders, and were not currently taking psychotropic medications. Only Caucasians were studied in order to minimize effects of genetic background and racial heterogeneity, and women were studied to maximize homogeneity of affective responses (27, 28). The study was approved by the Stanford University Institutional Review Board, and all subjects gave informed consent and were paid for participation.
Procedure
The procedure for the stress task has been reported previously (21). Briefly, the stress exposure task consisted of three conditions presented in a pseudorandom order: safe (12 trials), medium shock (13 trials), strong shock (13 trials). During safe trials, no shocks were administered. During shock trials, electric shocks were delivered to the left wrist above the median nerve with a Grass SD-9 stimulator (West Warick, RI) as described below.
In order to maximally induce stress and prevent habituation, instructions for the medium and strong trials featured three levels of unpredictability. Event unpredictability was implemented by indicating that shocks would be delivered during 85% of the trials. Temporal unpredictability was implemented by indicating that trials would last between 0–20 seconds and shocks could occur at any time (in actuality, trials ranged between 7–11s (avg 9s) and terminated with shock in 85% of the trials; two “quick” trials terminated with a shock at 3s in order to maintain the claim that shocks could occur at any time). Intensity unpredictability was implemented by leaving the exact strength of the shock unspecified within a 20% window; in medium trials the range was 40–60% of max voltage (MV) (in actuality shocks were always at 55% of MV) and strong trials the range was 70–90% of MV (in actuality shocks were always at 85% of MV). These levels of unpredictability have been shown to potentiate emotional reactivity (29) as well as autonomic (30) and neural responsiveness (31). At the end of each shock trial, participants provided anxiety ratings, using a scale of 1=not at all to 5=very much. Each trial was followed by an inter stimulus interval (ISI) which varied from 3 to 6s. At the end of the task, participants rated the overall anxiety they experienced due to the shocks as well as the success they had at reducing their anxiety during the shock trials. Analyses focused on these global ratings. The ratings for 3 subjects were not obtained due to software malfunction. Our group has shown that this task increases anxiety experience, skin conductance response and brain activity in a distributed corticolimbic network (21).
Brain responses of interest were responses during the anticipatory cue period, up to but not including the shock. This allowed us to focus our investigation on psychological factors associated with stress exposure rather than on physiology associated with receipt of physical stimulation. In order to examine potentially small effects of genotype, the current investigation focused on the most extreme contrast of safe vs. shock trials (medium and strong anticipation periods grouped together), rather than comparing the medium to strong trials, as our previous investigation demonstrated limited regional differences between these trials (21).
5-HTTLPR Genotyping
Tri-allelic 5-HTTLPR genotyping was carried out according to standard procedures described previously (32), which included genotyping for the presence of the A4G single nucleotide polymorphism within the L allele (33).
Control Variables
In order to ensure that genotype differences were not confounded with differential histories of life stress, we assessed exposure to life stressors. We choose an interview-based method (Early Trauma Inventory) to assess early life stress exposure (occurring up to age 18) in these five domains: general trauma and disasters, and physical, emotional and sexual abuse (34). Interviewers were blind to participant genotype. We also assessed exposure to recent life stress (within the last year) using the Life Events Scale for Students (35).
Imaging
Imaging was performed on a GE 3 Tesla Signa magnet (General Electric Medical System, Milwaukee, Wisconsin). BOLD signal was acquired with a T2*-weighted gradient echo spiral-in/out pulse sequence (36) and a custom-built quadrature “dome” elliptical bird cage head coil. Head movement was minimized using a bite-bar and head padding. 446 functional volumes were obtained during the functional run from 22 sequential axial slices (TR/TE=1500/30 msec, flip angle=70°, FOV=22 cm, matrix=64 × 64, single-shot, in-plane resolution = 3.438 mm2, and slice thickness=4.5 mm). Three-dimensional high-resolution anatomical scans were acquired using fast spin-echo spoiled gradient recalled (.859 × 1.2 mm; FOV=22 cm, frequency encoding=256).
fMRI Data Analysis
Functional data were analyzed with Analysis of Functional NeuroImages (AFNI) software (37). Preprocessing included coregistration, motion correction, 4 mm3 isotropic Gaussian spatial smoothing, high-pass filtering (.011 Hz), and linear detrending. Only volumes which demonstrated less than 1 mm of motion in the x, y, and z directions were included. Three participants exhibited volumes with motion above this threshold and were removed from all subsequent analyses, leaving 48 participants for analysis. There was no evidence of stimulus-correlated motion when conducting correlations of condition-specific reference functions and x, y, or z motion correction parameters (all ps > .05).
A multiple regression model implemented with AFNI 3dDeconvolve included baseline parameters to remove polynomial trends to the fifth order, as well as individual motion-related variance in the BOLD signal in six orientations. The model also included regressors for each separate condition coding for the anticipation period, shock period, and rating period, all of which were convolved with the gamma variate model of the hemodynamic response function. Contrasts were computed by weighting the appropriate columns in the design matrix. Statistical maps were resampled to 3.438 mm3 and converted to Talairach atlas space (38), and second-level statistical parametric maps were produced according to a random-effects analysis to enhance the generalizability of the results.
To correct for multiple comparisons, AlphaSim, a Monte Carlo simulation bootstrapping program in the AFNI library (37), was employed to estimate a joint probability distribution specifying a voxel-wise threshold and a minimum cluster volume threshold to establish a cluster-wise p-value that protects against false-positive detection of activation clusters (39). A voxel-wise threshold of p=.005 (t=2.946) resulted in a minimum cluster volume threshold of 257 mm3 (6 voxels) to protect against false-positive detection of clusters of activation at p < .05. All clusters cited in this paper survived this correction.
The relationship between tri-allelic 5-HTTLPR genotype and BOLD responses was investigated using a whole-brain regression analysis to examine linear dose-response (LL=−1, LS=0, SS=1) on the contrast ‘safe vs. shock anticipation’. Because a regression analysis is uninformative with respect to the role of the intermediate group, follow-up analyses examined the relationship between the three genotype groups in all functional clusters using ANOVA with post-hoc comparisons. Based on these results, a post-hoc whole-brain independent samples t-test analysis was conducted in AFNI comparing SSs and L carriers.
In order to investigate the relationship between 5-HTTLPR genotype, BOLD activation, and behavior, we used linear regression. Mean beta weights were extracted from the functional clusters identified in the group maps (SS vs. L carriers) for each subject. Interactions were analyzed, probed, and graphed according to the guidelines of Preacher et al. (40) using PASW (v.18, SPSS Inc.) on the brain regions of interest: amygdala, insula, medial prefrontal cortex. Brain activation values, genotype (SS vs. L carriers), and the brain-genotype interaction term were entered into a regression predicting behavioral ratings (41).
RESULTS
Sample
The genotype distribution in our final cohort of 48 women was consistent with prior reports and did not deviate from Hardy-Weinberg equilibrium: 11 LL (11 LaLa) 22 LS (20 LaS, 2 LaLg) 16 SS (6 LgS, 10 SS) (χ2 = .28, p=.87, df=2). The genotype groups did not differ with respect to age (p=.70) or IQ (p=.43). There were no significant differences between the three genotype groups on any index of life stress including childhood stress (Early Trauma Inventory, p=.16) or stress within the last year (Life Events Scale for Students, p=.33).
5-HTTLPR and Neural Responses to Stress
Regression analysis indicated that as the number of S alleles increased, activation increased in the amygdala, thalamus, putamen, caudate, middle temporal gyrus, middle frontal gyrus, fusiform gyrus, and precuneus (Figure 1; Supplementary Table 1). No regions showed the opposite effect (i.e., L alleles correlated with increased activation). We extracted mean beta weights from the functional clusters identified in this analysis for each subject and graphed them. This revealed a predominant pattern in which LL and LS individuals exhibited similar activation levels, while SS individuals showed increased activation (Figure 2). We thus conducted an ANOVA along with post-hoc comparisons to examine the genotype groups in a pairwise fashion across all significant clusters. Results indicated that LLs and LSs did not demonstrate significantly different activation in the majority of the clusters (Supplementary Table 2). Based on these results, LL and LS individuals were grouped into L carriers for the remaining analyses. A second, post-hoc whole-brain analysis was conducted comparing SSs and L carriers, as this model appears to fit the data more accurately. The results were similar to those with the linear regressor, but uncovered a number of additional regions which show a significant effect of genotype including hippocampus, anterior insula, pulvinar, anterior cingulate cortex, and medial prefrontal cortex (Figure 3; Supplementary Figure 1; Table 1). Subsequent analyses of brain-behavior relationship focused on functional clusters identified in the SS vs. L carriers analysis.
Figure 1.
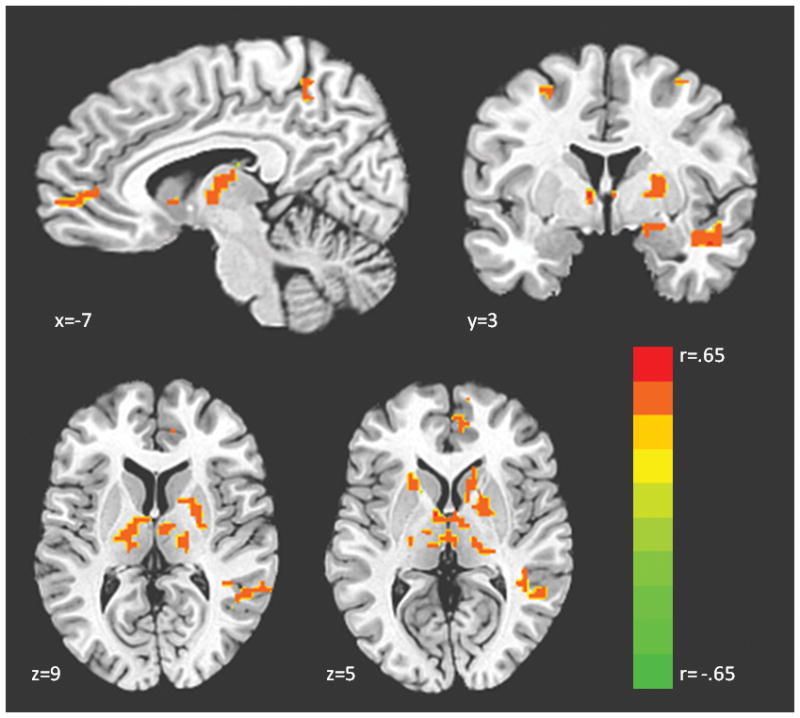
Regions showing an effect of 5-HTTLPR genotype in a whole brain regression (SS > LS > LL)a. aContrast displayed in neurological convention (left=left) at corrected p<.05, t>2.95, color bar indicates r statistic. Coordinates for all regions showing an effect of genotype are displayed in Table 1.
Figure 2.
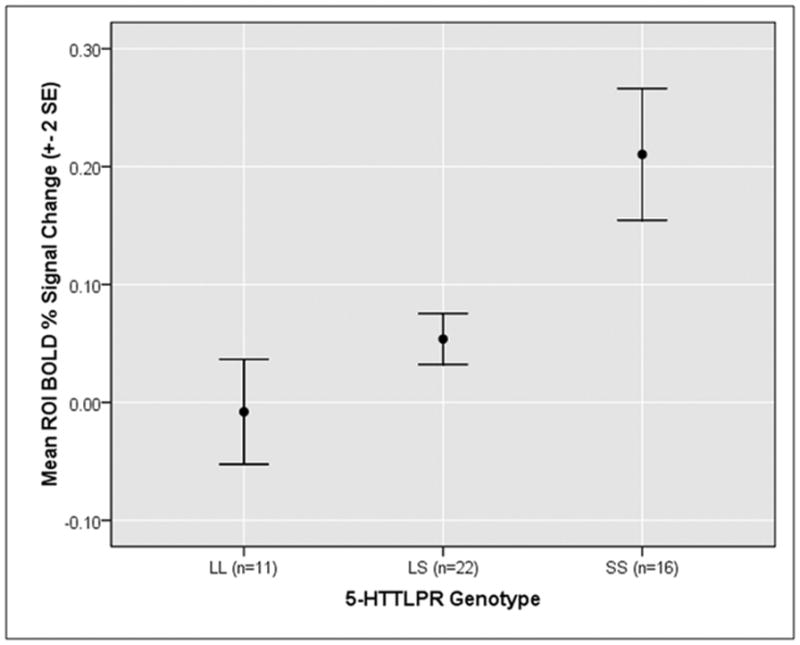
Mean single-subject activation values averaged across all clusters showing an effect of 5-HTTLPR genotype in a whole brain regressiona. aFunctional regions of interest were identified at corrected p < .05 and then extracted values were averaged across all significant clusters within each subject for display purposes. Follow-up post-hoc comparisons of LL vs. LS individuals for each cluster found that these groups did not significantly differ across the majority of the clusters.
Figure 3.
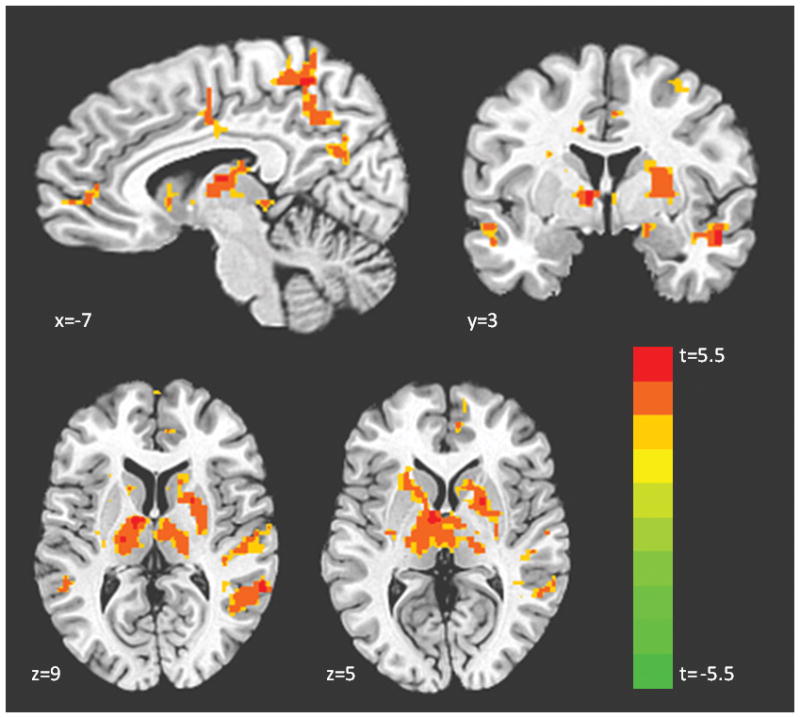
Regions showing an effect of genotype in a whole brain t-test (SS > L carriers)a. aContrast displayed in neurological convention (left=left) at corrected p<.05, t>2.95, color bar indicates t statistic. Coordinates for all regions showing an effect of genotype are displayed in Table 1.
Table 1.
Functional regions of interest from the whole brain t-test (SS > L carriers) identified at corrected p < .05, t>2.95. Coordinates indicate the peak of the cluster. Asterisk indicates a region which was identified as a supra-cluster, and the sub-cluster peaks are listed directly below it.
| Region | Side | BA | x | y | z | voxels | t |
|---|---|---|---|---|---|---|---|
| Thalamus (ventral anterior nucleus)* | L | - | −10 | −6 | 8 | 247 | 5.47 |
| Putamen | L | - | −21 | 11 | 1 | * | * |
| Pulvinar | L | - | −10 | −27 | 1 | * | * |
| Pulvinar | R | - | 17 | −30 | 1 | * | * |
| Precuneus** | R | 7 | 7 | −48 | 53 | 192 | 5.76 |
| Superior parietal lobule | R | 7 | 28 | −51 | 63 | ** | ** |
| Postcentral gyrus | R | 3 | 28 | −20 | 50 | ** | ** |
| Superior frontal gyrus | R | 6 | 24 | 7 | 53 | ** | ** |
| Precentral gyrus | R | 4 | 14 | −30 | 63 | ** | ** |
| Putamen*** | R | - | 21 | 4 | 5 | 116 | 4.75 |
| Caudate | R | - | 21 | −10 | 22 | *** | *** |
| Middle temporal gyrus | R | 39 | 55 | −51 | 12 | 75 | 4.82 |
| Insula | R | 41 | 34 | −31 | 15 | 64 | 4.31 |
| Cingulate gyrus | R | 24 | 10 | −10 | 36 | 52 | 4.9 |
| Fusiform gyrus | L | - | −28 | −61 | −12 | 44 | 4.48 |
| Supramarginal gyrus | L | 40 | −55 | −51 | 32 | 43 | 4.46 |
| Supramarginal gyrus | R | 39 | 41 | −55 | 29 | 36 | 4.22 |
| Superior Temporal gyrus | R | 38 | 52 | 1 | −9 | 33 | 5.55 |
| Precuneus | R | 31 | 10 | −61 | 22 | 30 | 4.55 |
| Middle frontal gyrus | R | 9 | 34 | 31 | 32 | 26 | 4.06 |
| Medial frontal gyrus | R | 10 | 10 | 56 | 1 | 20 | 4.25 |
| Middle temporal gyrus | L | 21 | −55 | −31 | −2 | 18 | 4.27 |
| Insula | L | 13 | −31 | −20 | 15 | 17 | 3.81 |
| Precuneus | L | 7 | −10 | −51 | 50 | 15 | 3.61 |
| Middle temporal gyrus | L | 21 | −52 | −3 | −9 | 14 | 4.21 |
| Middle temporal gyrus | R | 37 | 48 | −48 | −6 | 13 | 4.26 |
| Precuneus | L | 7 | −10 | −65 | 50 | 13 | 4.79 |
| Posterior cingulate | L | 23 | −3 | −55 | 15 | 12 | 3.66 |
| Superior frontal gyrus | L | 9 | −14 | 35 | 36 | 12 | 4.21 |
| Medial frontal gyrus | L | 9 | −14 | 28 | 32 | 10 | 3.44 |
| Superior parietal lobule | R | 7 | 34 | −48 | 63 | 10 | 3.6 |
| Amygdala | R | - | 17 | −3 | −12 | 9 | 3.52 |
| Superior temporal gyrus | R | - | 41 | −41 | 1 | 9 | 3.88 |
| Postcentral gyrus | L | 40 | −58 | −24 | 15 | 9 | 4.09 |
| Culmen | R | - | 28 | −48 | −23 | 8 | 4.1 |
| Hippocampus | R | - | 28 | −27 | −9 | 8 | 4.62 |
| Putamen | L | - | −17 | 1 | 15 | 8 | 3.71 |
| Medial frontal gyrus | L | 10 | 0 | 62 | 8 | 7 | 3.11 |
| Middle frontal gyrus | L | 10 | −34 | 49 | 19 | 7 | 3.67 |
| Putamen | L | - | −24 | −6 | 22 | 7 | 4.34 |
| Superior frontal gyrus | R | 9 | 14 | 49 | 32 | 7 | 3.84 |
| Cingulate gyrus | L | 24 | −10 | −6 | 36 | 7 | 3.75 |
| Precuneus | L | 7 | 0 | −72 | 46 | 7 | 4.38 |
| Superior temporal gyrus | L | 22 | −52 | 7 | −6 | 6 | 3.88 |
| Lingual gyrus | L | 18 | −17 | −82 | −6 | 6 | 3.55 |
| Middle temporal gyrus | L | 22 | −48 | −41 | 8 | 6 | 4.12 |
| Insula | R | 13 | 48 | −13 | 12 | 6 | 3.58 |
| Medial frontal gyrus | L | 10 | −3 | 56 | 19 | 6 | 3.27 |
| Inferior parietal lobule | R | 13 | 45 | −41 | 22 | 6 | 3.52 |
| Precentral gyrus | L | 6 | −31 | −10 | 53 | 6 | 3.31 |
5-HTTLPR, Neural Responses, and Behavior
On the basis of prior literature on threat processing, we selected three target regions of interest for an investigation of the links between 5-HTTLPR, neural responses, and behavior: amygdala, insula and medial prefrontal cortex. We examined whether genotype impacted the relationship between brain activation in these functional regions-of-interest and task-dependent ratings of anxiety and regulation success. Independent samples t-tests indicated that there was no difference between the genotype groups in ratings of anxiety (p=.64) or regulation success (p=.72), and anxiety and regulation success were not significantly correlated (r=−.25, p=.08).
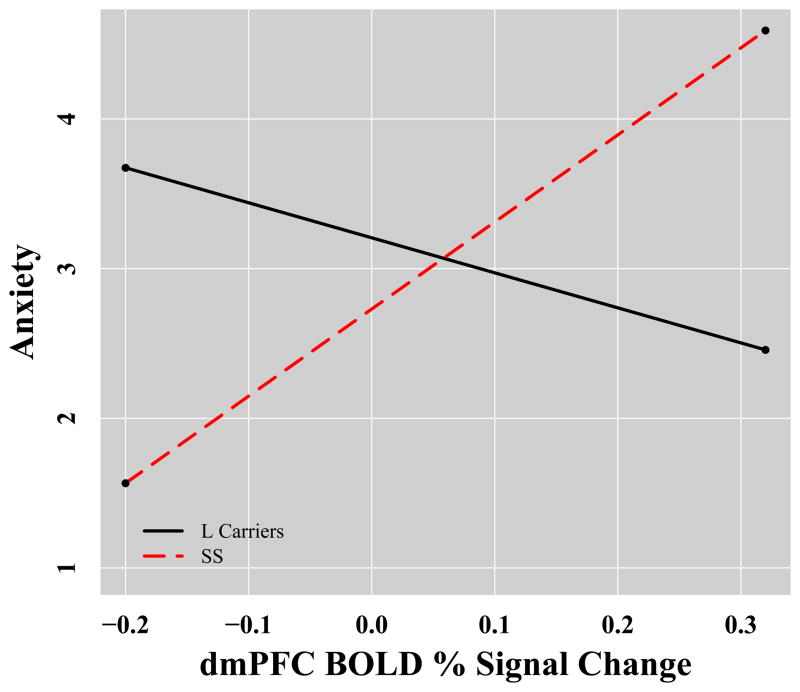
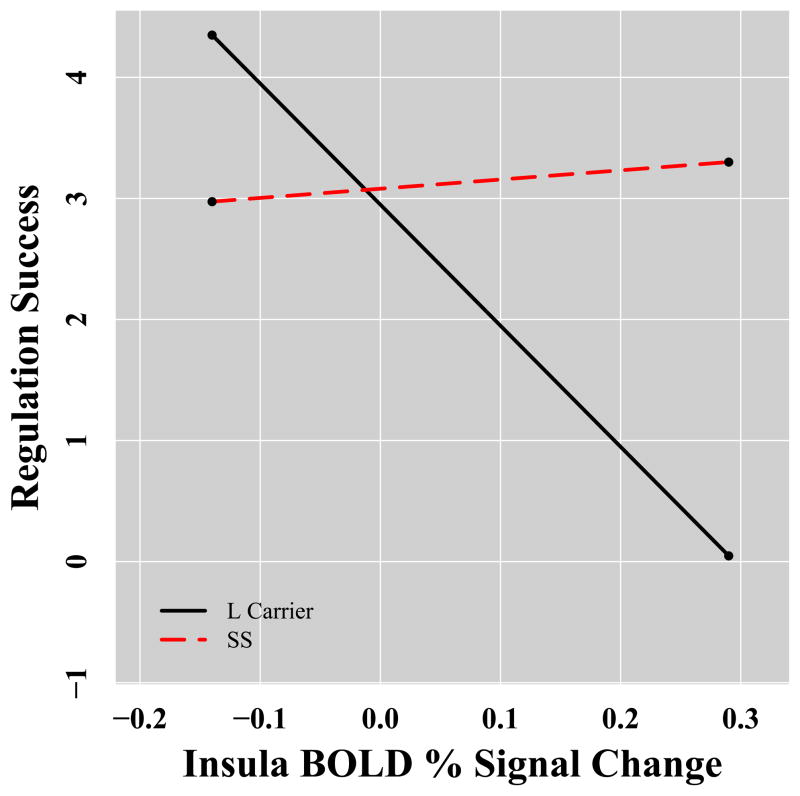
We did not find a moderating effect of genotype on the relationship between amygdala activation and anxiety or regulation success. However, we did find evidence that 5-HTTLPR genotype significantly moderated the relationship between medial prefrontal cortex activation and anxiety (β=.50, p=.04). Simple slope analyses indicated that SSs showed a significant positive correlation between medial prefrontal cortex activation and anxiety (simple slope=5.8, se=2.8, p=.046) whereas L carriers did not have a significant relationship between these variables (simple slope=−2.34, se=2.68, p=.39; see Figure 4). We also found evidence that 5-HTTLPR genotype significantly moderated the relationship between insula activation and regulation success (β=.58, p=.02). Simple slope analyses revealed that L carriers showed a significant negative correlation between insula and regulation success (simple slope=10.0, se=3.13, p=.003) whereas SSs did not have a significant relationship between these variables (simple slope=.76, se=3.00, p=.80; see Figure 4). See Supplementary Table 3 for more details.
Figure 4.
5-HTTLPR genotype predicts differential correlation between brain activation and anxiety and successful regulation in response to the taska. aA) Functional cluster in left medial prefrontal cortex (centered at x=−14, y=35, z=36 ) shows positive coupling with anxiety in SS homozygotes but not L carriers, B) A functional cluster in left insula (centered at x=−31, y=−20, z=−15 ) shows negative coupling with success at regulation anxiety in L carriers but not SS homozygotes.
DISCUSSION
In the present study, we examined neural correlates of genetic sensitivity to acute stress exposure conferred by the 5-HTTLPR short allele. Findings revealed that relative to L carriers, SS homozygotes displayed enhanced stress induced activation in the amygdala, hippocampus, anterior insula, thalamus, pulvinar, caudate, precuneus, anterior cingulate cortex, and medial prefrontal cortex. Notably, enhanced right amygdala reactivity seen in SS homozygotes was located in the dorsal region encompassing the central nucleus, which is known to drive behavioral and physiological arousal (42) through interactions with thalamus and cortex. The current study also shows substantial modulation of the thalamus by 5-HTTLPR genotype, particularly the dorsomedial nucleus and pulvinar. These regions are known to modulate emotion and mood; the thalamus gates sensory information to the amygdala and mediates the flow of information within the limbic system (43). Moreover, the pulvinar is anatomically connected to both the anterior cingulate cortex and the amygdala (44, 45) and relays emotionally salient information about the environment to the limbic system (46). Our findings are in line with previous research indicating that the thalamus contains one of the highest levels of serotonin transporters in the brain (47) and the pulvinar specifically is enlarged in S allele carriers (48, 49). Enhanced activation in the anterior insula, a region implicated in interoceptive processing, awareness of negative emotions, and anticipating pain (31, 50), provides further evidence of upregulated affective salience in SS homozyotes. In addition to bottom-up affective processing, it is possible that top-down attentional mechanisms are also driving sensitivity to threat. In SS homozygotes, increased activation in the dorsal anterior cingulate and bilateral precuneus is consistent with biased attention toward the danger of the upcoming shock and perhaps greater responsivity in the face of uncertainty (31). Lastly, activation in the medial prefrontal cortex has been consistently shown to be necessary for the generation of conscious appraisal of threat, and the increased activation seen here in SS individuals may reflect altered cognitive interpretation of the shock trials.
It is striking that when a more potent task is utilized than simple images, as with previous imaging genetics studies, genetic effects in a larger and more sophisticated network for processing environmental threat are unmasked. It is possible that neural alterations associated with the SS genotype result in upregulation of affective information entering the limbic system, via the thalamus and amygdala, which drives further salience and processing in a distributed cortical network. Taken together, the present findings suggest that, when exposed to acute stress, those carrying two S alleles engage neural systems which enhance fear and arousal, modulate attention toward threat, and perseverate on emotional salience of the threat. In turn, this may be a mechanism underlying risk for psychopathology conferred by the S allele upon exposure to life stressors, and may specifically speak to risk for anxiety disorders, which are characterized by chronic worry and anxiety about future events.
Using a task which generates visceral emotional responses allowed us to examine the relationship between psychological responses to stress, neural activity, and genotype. Results indicated that SS homozygotes exhibited a markedly increased positive relationship between medial prefrontal cortex activation and anxiety reactivity in response to the task. This interaction effect demonstrates that medial prefrontal cortex activation signals or triggers feelings of anxiety in SSs, suggesting that SSs may elaborate on the nature of the threat using different neural circuits than L carriers. We also found that L carriers exhibited a significant negative relationship between insula activation and regulation success in response to the task. In L carriers, perhaps efforts to regulate anxiety result in an extinguished response in the insula, a putatively more adaptive response. Interestingly, we did not find an association between 5-HTTLPR and the coupling of amygdala with anxiety or regulation success. One possible interpretation is that the amygdala functions to rapidly and often unconsciously alert higher brain systems to environmental threat, but those higher structures are required to elaborate on the threat in order to generate subjective experience of anxiety and successful regulation of that anxiety. Thus, the interactions shown appear to indicate that for SSs versus L carriers there are different circuit dynamics which translate differentially into behavior based on genotype. Notably, there were no direct associations between 5-HTTLPR genotype and behavioral phenotypes in our study. This is a common occurrence when working with relatively small samples, possibly reflecting the minimal effect that genotype has on any distal behavioral phenotype (14). The current results help us to appreciate the relevance of a network of structures beyond the amygdala that demonstrated effects of 5-HTTLPR in the face of stress exposure.
It is noteworthy that in this study the post-hoc analyses clearly indicated it was most appropriate to group individuals carrying the L allele. Consistent with past imaging genetics studies, our a priori model was one of co-dominance in which the S allele added a dose effect, and thus we conducted a whole-brain regression. However, a regression analysis is agnostic to the effect of the intermediate group, prompting us to extract the data from all of the significant clusters to explore the relationship between the three genotype groups graphically. The majority of the graphs showed a similar pattern of activation among the L carriers. While these results are unexpected, it is not clear that many other published studies of 5-HTTLPR have ever explored multiple analytic models or extracted the data to examine the fit of the model to the three genotype groups. Of the studies which have considered the genotype groups individually, some have found no differences between LL and LS individuals in HPA reactivity in response to a laboratory stressor (51) or likelihood of developing depression in response to moderately threatening life events (52). As suggested by Gotlib et al., a single laboratory stressor may be too transient to elicit significantly different physiological reactivity between LL and LS individuals. Along with the present findings, this suggests that SS homozygotes may have a lower threshold for stress sensitivity than their L allele counterparts. However, the fact that theses analyses were post-hoc is a limitation and future work is needed.
Recently, some controversy has arisen in the 5-HTTLPR-by-stress interaction literature, as not all studies have replicated this gene-by-environment effect (13, 53). Importantly, of those studies that haven’t replicated the effect, nearly every one has relied on retrospective questionnaire assessments of life stress exposure. Conversely, studies which have utilized interview based methods and rich multi-source objective data in carefully followed epidemiological cohorts have consistently found evidence of this gene-by-environment effect (7, 8, 54, 55). This further highlights the utility of employing laboratory-based stressors, particularly when examining neural mechanisms mediating this effect. Moreover, in contrast to previous imaging genetics studies which have relied on presentation of pictures that act as conditioned stimuli, the current task provides an immediate and robust psychological and bodily threat to subjects, which is a visceral model of real-world stressors. As the field moves forward, it will be important to shift to paradigms such as the one used here which can powerfully unmask genetic sensitivity to threat and stress.
Our results add to the mounting evidence, accumulated in animals and humans, which suggests that the S allele renders individuals stress sensitive by biasing neurobiological systems underlying threat reactivity and arousal.
Despite its promise, this study has several limitations. First, while we prioritized studying a relatively homogenous sample in order to decrease error variance, this sampling decision limits the generalizablity of the findings. Future studies should examine neural mechanisms of genetic sensitivity to acute stress in men and a wider range of genetic backgrounds. Second, while focusing on a healthy population allowed us to investigate the mechanism of risk without the confound of current or past psychopathology, it will be important for future studies to longitudinally follow subjects from this type of imaging genetics experiment to determine if these neural biomarkers result in the onset of psychopathology in the face of exposure to life stressors. Third, the small sample employed in this study limits to power to detect all effects of genotype, particularly any effect on the amygdala (15); future work in larger samples is needed. Fourth, while research suggests that recent stressors are the most potent predictor of increased risk for psychopathology (56) and hence this anxiogenic task provides a proxy for such acute “real-life” stressors, this design does not allow for investigation of the effects of chronic stressors or the full range of life stressors known to trigger psychopathology. Indeed, it may be the accumulation of chronic stress exposure over time which interacts with genotype to confer risk. Finally, our examination of brain-behavior relations is suggestive, but the directionality of these associations cannot be inferred; it could be that neural reactivity drives behavior or vice versa.
Supplementary Material
Regions showing an effect of genotype in a whole brain t-test (SS > L carriers)a. aContrast displayed in neurological convention (left=left) at corrected p<.05, t>2.95, color bar indicates t statistic. Coordinates for all regions showing an effect of genotype are displayed in Table 1.
Regions showing an effect of 5-HTTLPR genotype in a whole brain regression (SS > LS > LL)a. aAll clusters survived corrected p<.05, t>2.95. Coordinates indicate the peak of the cluster. Asterisk indicates a region which was identified as a supra-cluster, and the sub-cluster peaks are listed directly below it.
Pairwise comparison of individual genotype groupsa. a p-value obtained from pairwise post-hoc comparisons after ANOVA. Table shows that the majority of clusters did not show significant differences between LL and LS individuals, whereas SS individuals were significantly different from both LL and LS individuals.
Beta values obtained from interaction analysis of 5-HTTLPR, brain activation and responses to the taska. aFunctional clusters were obtained from SS > L carriers analysis. Interaction analyses examined amygdala (peak at x= 17, y=−3, z=−12), insula (peak at x=−31, y=−20, z=−15 ), dorsomedial prefrontal cortex (peak at x=−14, y=35, z=36 )
Acknowledgments
The authors thank Isabel Edge, Whitney Lynch, and Michael Mehler for help with participant recruitment, screening, and data collection. The authors also thank Gary Glover for adapting the Grass SD Stimulator to fit the specifications of the task and function compatibly with the MR environment.
Footnotes
Disclosures: The authors state no conflicts of interest. This research was supported by the National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award 34676 to Wiveka Ramel, the National Institutes of Health (NIH) R01 Grant MH58147 awarded to James Gross, and the National Defense Science and Engineering Graduate Fellowship (NDSEG) awarded to Emily Drabant.
This research was performed at Stanford University, Stanford, California, USA
References
- 1.Monroe SM, Simons AD. Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychol Bull. 1991;110(3):406–25. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- 2.van Winkel R, Henquet C, Rosa A, Papiol S, Fananas L, De Hert M, Peuskens J, van Os J, Myin-Germeys I. Evidence that the COMT(Val158Met) polymorphism moderates sensitivity to stress in psychosis: an experience-sampling study. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(1):10–7. doi: 10.1002/ajmg.b.30559. [DOI] [PubMed] [Google Scholar]
- 3.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Jama. 2008;299(11):1291–305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enoch MA, Hodgkinson CA, Yuan Q, Shen PH, Goldman D, Roy A. The influence of GABRA2, childhood trauma, and their interaction on alcohol, heroin, and cocaine dependence. Biol Psychiatry. 2010;67(1):20–7. doi: 10.1016/j.biopsych.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: new evidence and a meta-analysis. Mol Psychiatry. 2006;11(10):903–13. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- 6.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 7.Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68(5):444–54. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167(5):509–27. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klauke B, Deckert J, Reif A, Pauli P, Zwanzger P, Baumann C, Arolt V, Glockner-Rist A, Domschke K. Serotonin transporter gene and childhood trauma - a G x E effect on anxiety sensitivity. Depress Anxiety. 2011 doi: 10.1002/da.20840. [DOI] [PubMed] [Google Scholar]
- 10.Holmes A, Murphy DL, Crawley JN. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol Psychiatry. 2003;54(10):953–9. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Barr CS, Newman TK, Schwandt M, Shannon C, Dvoskin RL, Lindell SG, Taubman J, Thompson B, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. Proc Natl Acad Sci U S A. 2004;101(33):12358–63. doi: 10.1073/pnas.0403763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adamec R, Burton P, Blundell J, Murphy DL, Holmes A. Vulnerability to mild predator stress in serotonin transporter knockout mice. Behav Brain Res. 2006;170(1):126–40. doi: 10.1016/j.bbr.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. Jama. 2009;301(23):2462–71. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol Psychiatry. 2006;59(10):888–97. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63(9):852–7. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dannlowski U, Konrad C, Kugel H, Zwitserlood P, Domschke K, Schoning S, Ohrmann P, Bauer J, Pyka M, Hohoff C, Zhang W, Baune BT, Heindel W, Arolt V, Suslow T. Emotion specific modulation of automatic amygdala responses by 5-HTTLPR genotype. Neuroimage. 2010;53(3):893–8. doi: 10.1016/j.neuroimage.2009.11.073. [DOI] [PubMed] [Google Scholar]
- 17.Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Klein S, Grusser SM, Flor H, Schumann G, Mann K, Buchel C. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8(1):20–1. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- 18.Furmark T, Henningsson S, Appel L, Ahs F, Linnman C, Pissiota A, Faria V, Oreland L, Bani M, Pich EM, Eriksson E, Fredrikson M. Genotype over-diagnosis in amygdala responsiveness: affective processing in social anxiety disorder. J Psychiatry Neurosci. 2009;34(1):30–40. [PMC free article] [PubMed] [Google Scholar]
- 19.Furmark T, Tillfors M, Garpenstrand H, Marteinsdottir I, Langstrom B, Oreland L, Fredrikson M. Serotonin transporter polymorphism related to amygdala excitability and symptom severity in patients with social phobia. Neurosci Lett. 2004;362(3):189–92. doi: 10.1016/j.neulet.2004.02.070. [DOI] [PubMed] [Google Scholar]
- 20.Monroe SM, Reid MW. Gene-environment interactions in depression research: genetic polymorphisms and life-stress polyprocedures. Psychol Sci. 2008;19(10):947–56. doi: 10.1111/j.1467-9280.2008.02181.x. [DOI] [PubMed] [Google Scholar]
- 21.Drabant EM, Kuo JR, Ramel W, Blechert J, Edge MD, Cooper JR, Goldin PR, Hariri AR, Gross JJ. Experiential, autonomic, and neural responses during threat anticipation vary as a function of threat intensity and neuroticism. Neuroimage. 2011;55(1):401–10. doi: 10.1016/j.neuroimage.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8(6):828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 23.LeDoux J. The amygdala. Curr Biol. 2007;17(20):R868–74. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 25.Mechias ML, Etkin A, Kalisch R. A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. Neuroimage. 2010;49(2):1760–8. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 26.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, DC: 1995. [Google Scholar]
- 27.Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: sex differences in picture processing. Emotion. 2001;1(3):300–19. [PubMed] [Google Scholar]
- 28.Straube T, Schmidt S, Weiss T, Mentzel HJ, Miltner WH. Sex differences in brain activation to anticipated and experienced pain in the medial prefrontal cortex. Hum Brain Mapp. 2009;30(2):689–98. doi: 10.1002/hbm.20536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monat A, Averill JR, Lazarus RS. Anticipatory stress and coping reactions under various conditions of uncertainty. J Pers Soc Psychol. 1972;24(2):237–53. doi: 10.1037/h0033297. [DOI] [PubMed] [Google Scholar]
- 30.Geer JH, Maisel E. Evaluating the effects of the prediction-control confound. J Pers Soc Psychol. 1972;23(3):314–9. doi: 10.1037/h0033122. [DOI] [PubMed] [Google Scholar]
- 31.Carlsson K, Andersson J, Petrovic P, Petersson KM, Ohman A, Ingvar M. Predictability modulates the affective and sensory-discriminative neural processing of pain. Neuroimage. 2006;32(4):1804–14. doi: 10.1016/j.neuroimage.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 32.Fredericks CA, Drabant EM, Edge MD, Tillie JM, Hallmayer J, Ramel W, Kuo JR, Mackey S, Gross JJ, Dhabhar FS. Healthy young women with serotonin transporter SS polymorphism show a pro-inflammatory bias under resting and stress conditions. Brain Behav Immun. 2009 doi: 10.1016/j.bbi.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11(3):224–6. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- 34.Bremner JD, Vermetten E, Mazure CM. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the Early Trauma Inventory. Depress Anxiety. 2000;12(1):1–12. doi: 10.1002/1520-6394(2000)12:1<1::AID-DA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 35.Clements K, Turpin G. The life events scale for students: validation for use with British samples. Pers Indiv Differ. 1996;20(6):747–51. [Google Scholar]
- 36.Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46(3):515–22. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- 37.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 38.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 39.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 40.Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. J Edu Behav Stat. 2006;31:437–48. [Google Scholar]
- 41.Hyde LW, Gorka A, Manuck SB, Hariri AR. Perceived social support moderates the link between threat-related amygdala reactivity and trait anxiety. Neuropsychologia. 2011 doi: 10.1016/j.neuropsychologia.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis FC, Johnstone T, Mazzulla EC, Oler JA, Whalen PJ. Regional response differences across the human amygdaloid complex during social conditioning. Cereb Cortex. 2010;20(3):612–21. doi: 10.1093/cercor/bhp126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 44.Jones EG, Burton H. A projection from the medial pulvinar to the amygdala in primates. Brain Res. 1976;104(1):142–7. doi: 10.1016/0006-8993(76)90654-5. [DOI] [PubMed] [Google Scholar]
- 45.Romanski LM, Giguere M, Bates JF, Goldman-Rakic PS. Topographic organization of medial pulvinar connections with the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1997;379(3):313–32. [PubMed] [Google Scholar]
- 46.Ohman A. The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology. 2005;30(10):953–8. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 47.Frankle WG, Huang Y, Hwang DR, Talbot PS, Slifstein M, Van Heertum R, Abi-Dargham A, Laruelle M. Comparative evaluation of serotonin transporter radioligands 11C-DASB and 11C-McN 5652 in healthy humans. J Nucl Med. 2004;45(4):682–94. [PubMed] [Google Scholar]
- 48.Young KA, Bonkale WL, Holcomb LA, Hicks PB, German DC. Major depression, 5HTTLPR genotype, suicide and antidepressant influences on thalamic volume. Br J Psychiatry. 2008;192(4):285–9. doi: 10.1192/bjp.bp.107.039180. [DOI] [PubMed] [Google Scholar]
- 49.Young KA, Holcomb LA, Bonkale WL, Hicks PB, Yazdani U, German DC. 5HTTLPR polymorphism and enlargement of the pulvinar: unlocking the backdoor to the limbic system. Biol Psychiatry. 2007;61(6):813–8. doi: 10.1016/j.biopsych.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 50.Ogino Y, Nemoto H, Inui K, Saito S, Kakigi R, Goto F. Inner experience of pain: imagination of pain while viewing images showing painful events forms subjective pain representation in human brain. Cereb Cortex. 2007;17(5):1139–46. doi: 10.1093/cercor/bhl023. [DOI] [PubMed] [Google Scholar]
- 51.Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol Psychiatry. 2008;63(9):847–51. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry. 2005;62(5):529–35. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- 53.Bouma E, Riese H, Nederhof E, Ormel J, Oldehinkel A. No replication of genotype effect of 5-HTTLPR on cortisol response to social stress in larger adolescent sample. Biol Psychiatry. 2011;68(11):e33–4. doi: 10.1016/j.biopsych.2010.04.041. [DOI] [PubMed] [Google Scholar]
- 54.Mueller A, Armbruster D, Moser DA, Canli T, Lesch KP, Brocke B, Kirschbaum C. Interaction of serotonin transporter gene-linked polymorphic region and stressful life events predicts cortisol stress response. Neuropsychopharmacology. 2011;36(7):1332–9. doi: 10.1038/npp.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wankerl M, Wust S, Otte C. Current developments and controversies: does the serotonin transporter gene-linked polymorphic region (5-HTTLPR) modulate the association between stress and depression? Curr Opin Psychiatry. 2010;23(6):582–7. doi: 10.1097/YCO.0b013e32833f0e3a. [DOI] [PubMed] [Google Scholar]
- 56.Brown GW, Harris TO. Social origins of depression: A study of psychiatric disorder in women. New York: Free Press; 1978. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Regions showing an effect of genotype in a whole brain t-test (SS > L carriers)a. aContrast displayed in neurological convention (left=left) at corrected p<.05, t>2.95, color bar indicates t statistic. Coordinates for all regions showing an effect of genotype are displayed in Table 1.
Regions showing an effect of 5-HTTLPR genotype in a whole brain regression (SS > LS > LL)a. aAll clusters survived corrected p<.05, t>2.95. Coordinates indicate the peak of the cluster. Asterisk indicates a region which was identified as a supra-cluster, and the sub-cluster peaks are listed directly below it.
Pairwise comparison of individual genotype groupsa. a p-value obtained from pairwise post-hoc comparisons after ANOVA. Table shows that the majority of clusters did not show significant differences between LL and LS individuals, whereas SS individuals were significantly different from both LL and LS individuals.
Beta values obtained from interaction analysis of 5-HTTLPR, brain activation and responses to the taska. aFunctional clusters were obtained from SS > L carriers analysis. Interaction analyses examined amygdala (peak at x= 17, y=−3, z=−12), insula (peak at x=−31, y=−20, z=−15 ), dorsomedial prefrontal cortex (peak at x=−14, y=35, z=36 )